Genetic Diversity of Merozoite Surface Antigens in Global Babesia bovis Populations
Abstract
1. Introduction
2. Methods
2.1. Collection of B. bovis-MSA Nucleotide Sequences
2.2. Phylogenetic and Population Structure Analyses
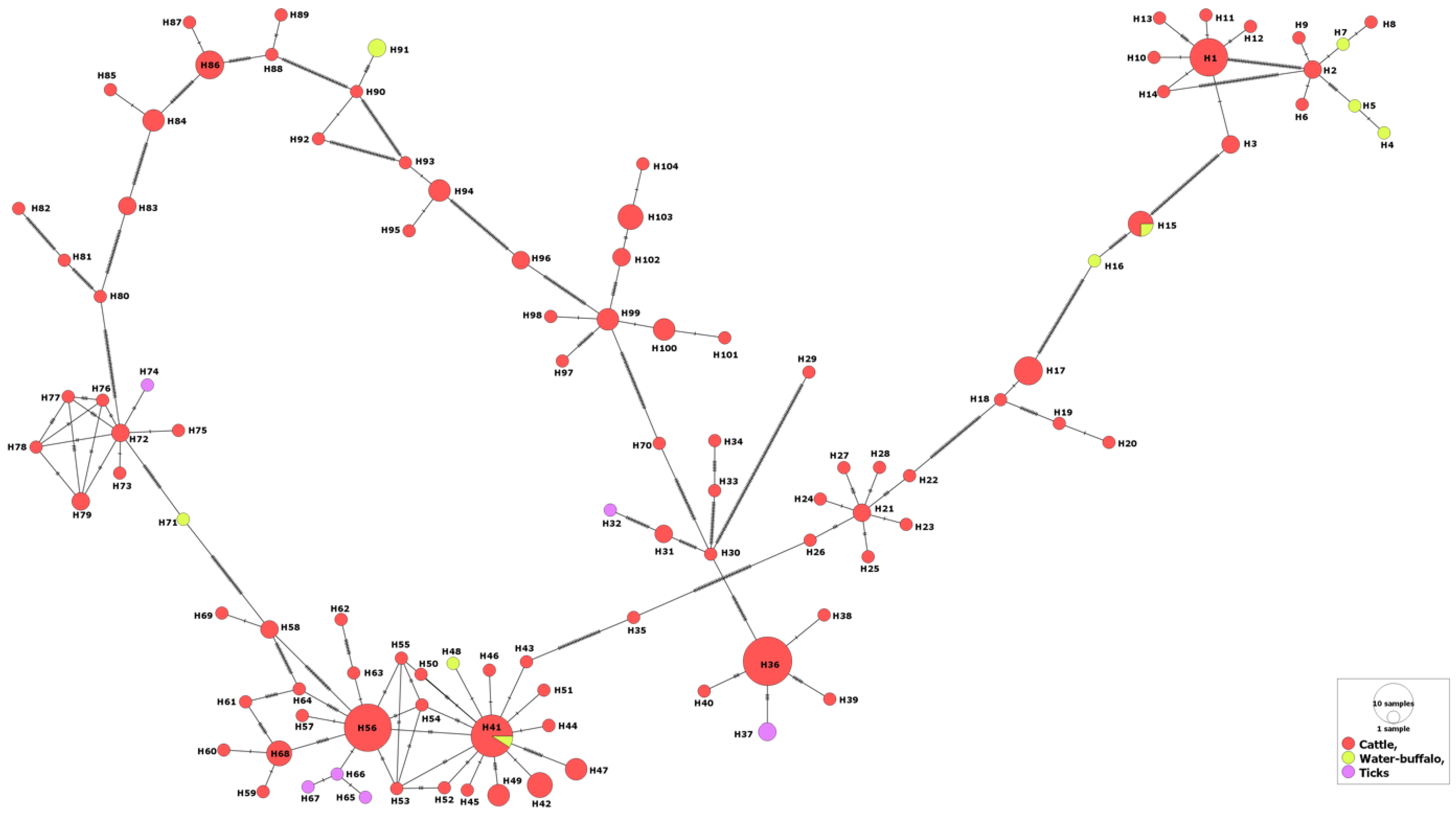
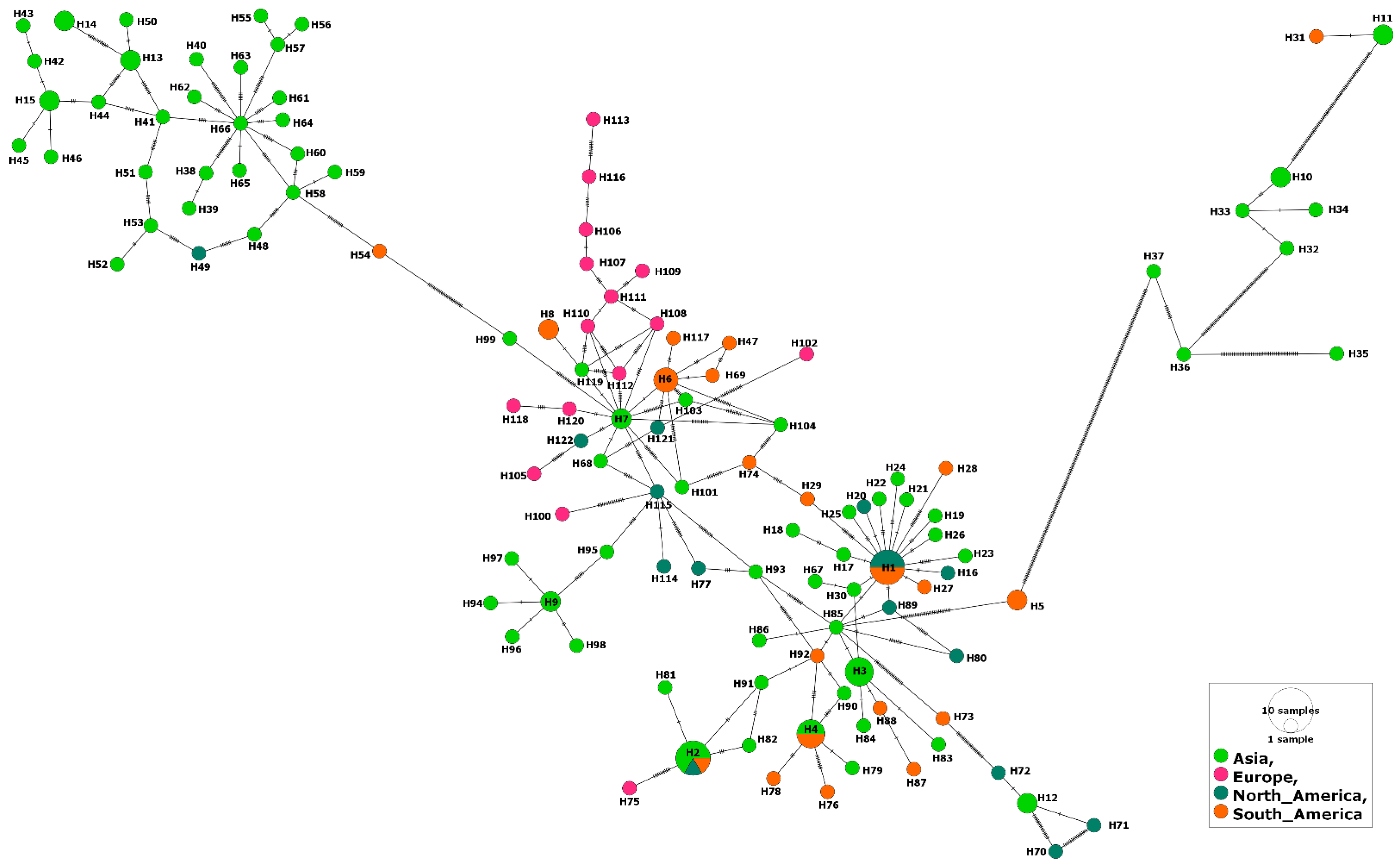
3. Results
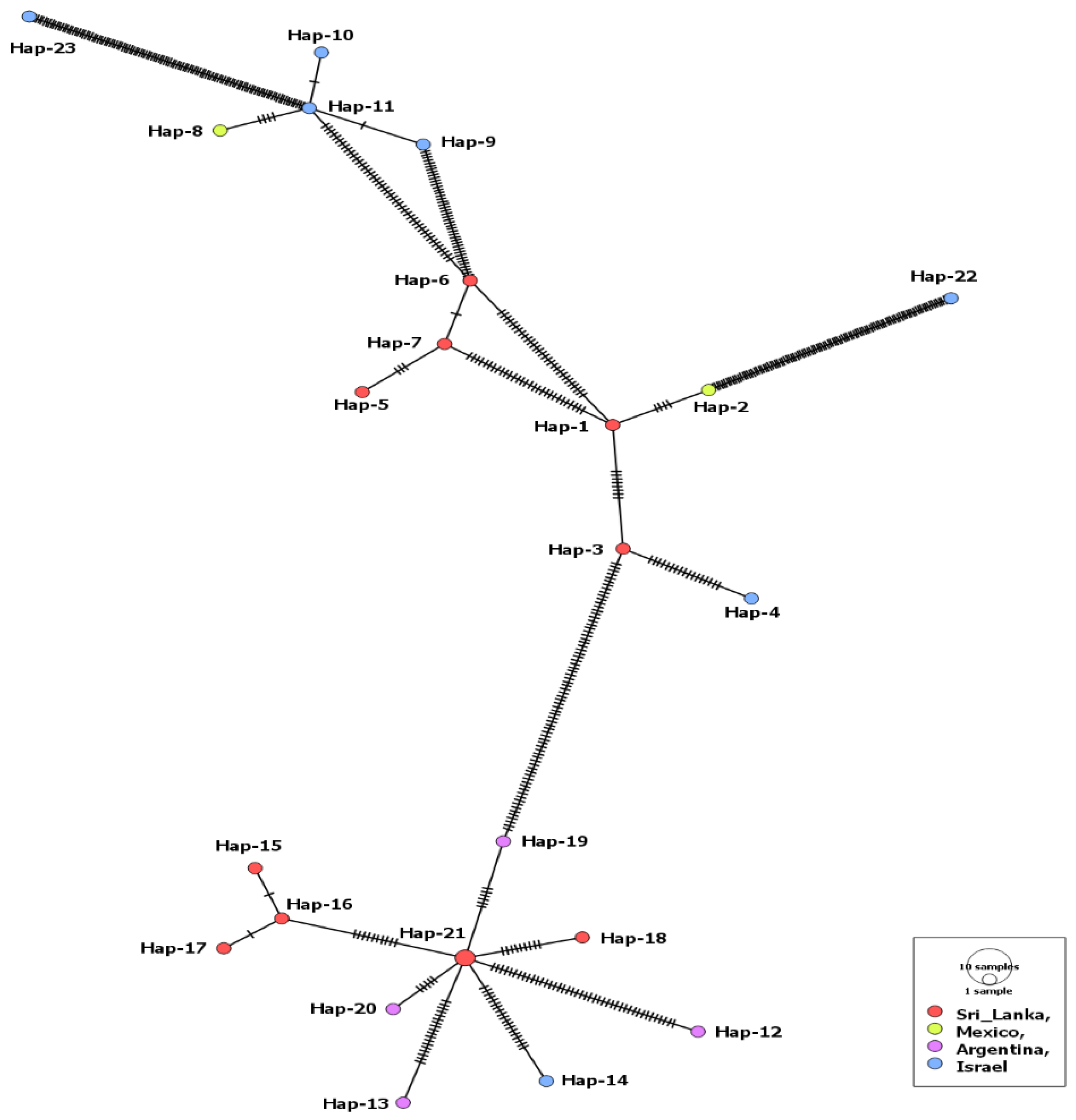
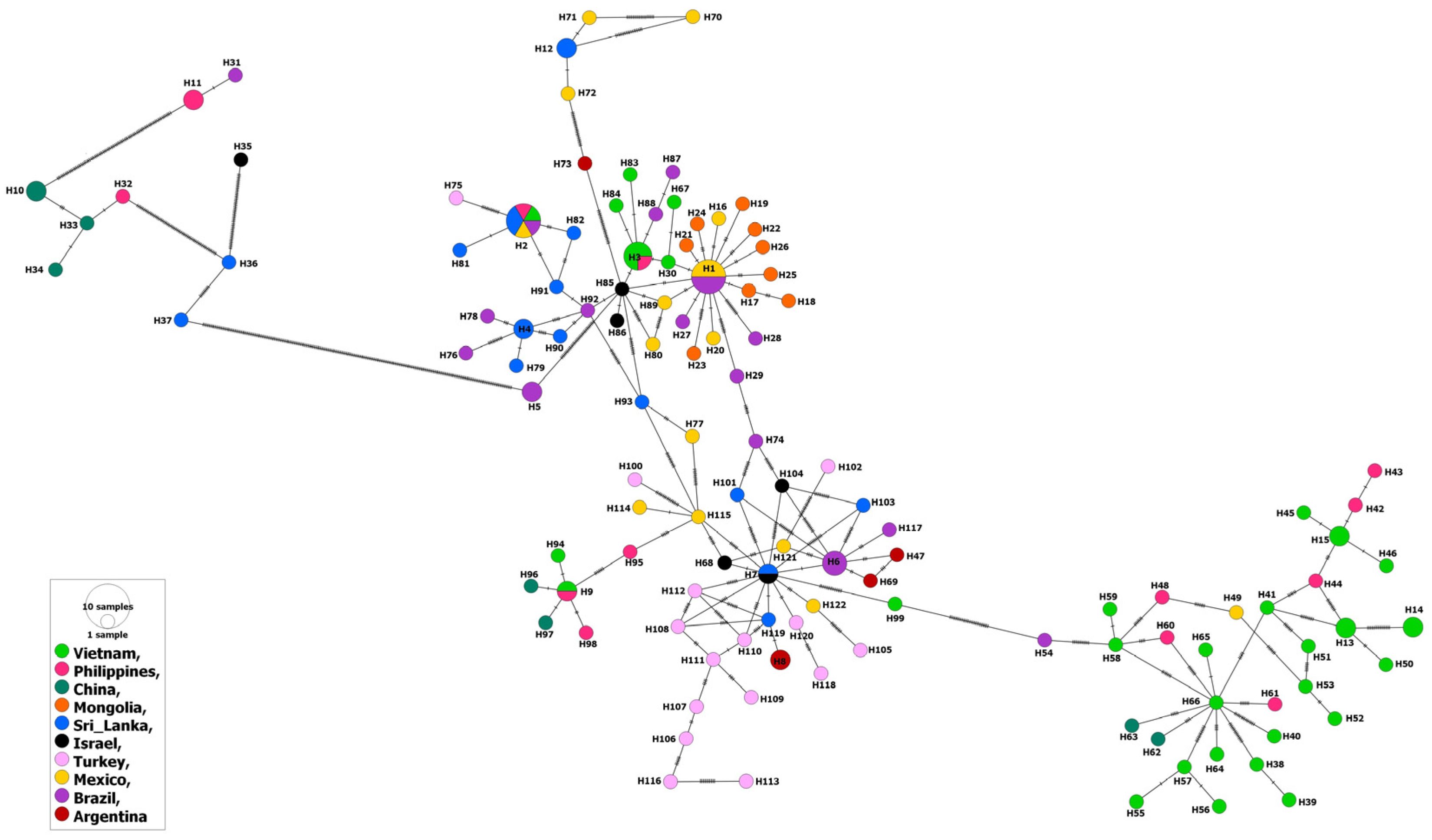
3.1. MSA-1 Isolates
3.2. MSA-2a1 Isolates
3.3. MSA-2b Isolates
3.4. MSA-2c Isolates
3.5. Detecting the Conserved Regions in the Aligned Amino Acid Sequences of Various MSA Gene Fragments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Weakley, M.; Do, T.; Mir, S. Current and future molecular diagnostics of tick-borne diseases in cattle. Vet. Sci. 2022, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. IJP-PAW 2015, 4, 104–118. [Google Scholar] [CrossRef]
- Schnittger, L.; Ganzinelli, S.; Bhoora, R.; Omondi, D.; Nijhof, A.M.; Florin-Christensen, M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022, 121, 1207–1245. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129 (Suppl. S1), S247–S269. [Google Scholar] [CrossRef]
- Gohil, S.; Herrmann, S.; Günther, S.; Cooke, B.M. Bovine babesiosis in the 21st century: Advances in biology and functional genomics. Int. J. Parasitol. 2013, 43, 125–132. [Google Scholar] [CrossRef]
- Suarez, C.E.; Alzan, H.F.; Silva, M.G.; Rathinasamy, V.; Poole, W.A.; Cooke, B.M. Unravelling the cellular and molecular pathogenesis of bovine babesiosis: Is the sky the limit? Int. J. Parasitol. 2019, 49, 183–197. [Google Scholar] [CrossRef]
- Hines, S.A.; McElwain, T.F.; Buening, G.M.; Palmer, G.H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol. Biochem. Parasitol. 1989, 37, 1–9. [Google Scholar] [CrossRef]
- Hines, S.A.; Palmer, G.H.; Jasmer, D.P.; McGuire, T.C.; McElwain, T.F. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 1992, 55, 85–94. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Suarez, C.E.; Hines, S.A.; Palmer, G.H.; Brown, W.C.; McElwain, T.F. The Babesia bovis merozoite surface antigen 2 locus contains four tandemly arranged and expressed genes encoding immunologically distinct proteins. Infect. Immun. 2002, 70, 3566–3575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suarez, C.E.; Florin-Christensen, M.; Hines, S.A.; Palmer, G.H.; Brown, W.C.; McElwain, T.F. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 2000, 68, 6865–6870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmer, G.H.; McElwain, T.F.; Perryman, L.E.; Davis, W.C.; Reduker, D.R.; Jasmer, D.P.; Shkap, V.; Pipano, E.; Goff, W.L.; McGuire, T.C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect. Immun. 1991, 59, 3340–3342. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda, J.; McElwain, T.F.; Palmer, G.H. Babesia bovis merozoite surface antigen 2 proteins are expressed on the merozoite and sporozoite surface, and specific antibodies inhibit attachment and invasion of erythrocytes. Infect. Immun. 2002, 70, 6448–6455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilkowsky, S.E.; Farber, M.; Echaide, I.; De Echaide, S.T.; Zamorano, P.I.; Dominguez, M.; Suarez, C.E.; Florin-Christensen, M. Babesia bovis merozoite surface protein-2c (MSA-2c) contains highly immunogenic, conserved B-cell epitopes that elicit neutralization-sensitive antibodies in cattle. Mol. Biochem. Parasitol. 2003, 127, 133–141. [Google Scholar] [CrossRef]
- Yokoyama, N.; Okamura, M.; Igarashi, I. Erythrocyte invasion by Babesia parasites: Current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage. Vet. Parasitol. 2006, 138, 22–32. [Google Scholar] [CrossRef]
- Carcy, B.; Précigout, E.; Schetters, T.; Gorenflot, A. Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity. Vet. Parasitol. 2006, 138, 33–49. [Google Scholar] [CrossRef]
- Mendes, N.S.; de Souza Ramos, I.A.; Herrera, H.M.; Campos, J.B.V.; de Almeida Alves, J.V.; de Macedo, G.C.; Machado, R.Z.; André, M.R. Genetic diversity of Babesia bovis in beef cattle in a large wetland in Brazil. Parasitol. Res. 2019, 118, 2027–2040. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Maddison, D.R.; Swofford, D.L.; Maddison, W.P. NEXUS: An extensible file format for systematic information. Syst. Biol. 1997, 46, 590–621. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Wilkowsky, S.E.; Moretta, R.; Mosqueda, J.; Gil, G.; Echaide, I.; Lía, V.; Falcon, A.; Christensen, M.F.; Farber, M. A new set of molecular markers for the genotyping of Babesia bovis isolates. Vet. Parasitol. 2009, 161, 9–18. [Google Scholar] [CrossRef]
- Simas, P.V.M.; Bassetto, C.C.; Giglioti, R.; Okino, C.H.; de Oliveira, H.N.; de Sena Oliveira, M.C. Use of molecular markers can help to understand the genetic diversity of Babesia bovis. Infect. Genet. Evol. 2020, 79, 104161. [Google Scholar] [CrossRef]
- Gunasekara, E.; Sivakumar, T.; Kothalawala, H.; Abeysekera, T.S.; Weerasingha, A.S.; Vimalakumar, S.C.; Kanagaratnam, R.; Yapa, P.R.; Zhyldyz, A.; Igarashi, I.; et al. Epidemiological survey of hemoprotozoan parasites in cattle from low-country wet zone in Sri Lanka. Parasitol. Int. 2019, 71, 5–10. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Z.; Yu, P.; Yang, J.; Abdallah, M.O.; Guan, G.; Liu, G.; Luo, J.; Yin, H. Genetic characterization and molecular survey of Babesia bovis, Babesia bigemina and Babesia ovata in cattle, dairy cattle and yaks in China. Parasites Vectors 2015, 8, 518. [Google Scholar] [CrossRef]
- Altangerel, K.; Sivakumar, T.; Battsetseg, B.; Battur, B.; Ueno, A.; Igarashi, I.; Yokoyama, N. Phylogenetic relationships of Mongolian Babesia bovis isolates based on the merozoite surface antigen (MSA)-1, MSA-2b, and MSA-2c genes. Vet. Parasitol. 2012, 184, 309–316. [Google Scholar] [CrossRef]
- Borgonio, V.; Mosqueda, J.; Genis, A.D.; Falcon, A.; Alvarez, J.A.; Camacho, M.; Figueroa, J.V. msa-1 and msa-2c gene analysis and common epitopes assessment in Mexican Babesia bovis isolates. Ann. N. Y. Acad. Sci. 2008, 1149, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Byaruhanga, C.; Makgabo, S.M.; Choopa, C.N.; Mulandane, F.C.; Vorster, I.; Troskie, M.; Chaisi, M.E.; Collins, N.E. Genetic diversity in Babesia bovis from southern Africa and estimation of B. bovis infection levels in cattle using an optimised quantitative PCR assay. Ticks Tick-Borne Dis. 2023, 14, 102084. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Echaide, I.; de Echaide, S.T.; Mosqueda, J.; Cetrá, B.; Suarez, C.E.; Florin-Christensen, M. In silico predicted conserved B-cell epitopes in the merozoite surface antigen-2 family of B. bovis are neutralization sensitive. Vet. Parasitol. 2010, 167, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Düzlü, Ö.; Yildirim, A.; İnci, A.; Avcioğlu, H.; Balkaya, I. Babesia bovis ve Babesia bigemina’nın Real-Time PCR ile araştırılması ve izolatların moleküler karakterizasyonu. Ank. Üniv. Vet. Fakültesi Derg. 2015, 62, 27–35. [Google Scholar]
- Düzlü, Ö.; İnci, A.; Yildirim, A. Molecular Characterization of Babesia bovis Isolates Collected from Cattle in Black Sea Region. Saglik Bilim. Derg. 2011, 20, 18–29. [Google Scholar]
- Genis, A.D.; Mosqueda, J.J.; Borgonio, V.M.; Falcon, A.; Alvarez, A.; Camacho, M.; Munoz, M.L.; Figueroa, J.V. Phylogenetic analysis of Mexican Babesia bovis isolates using msa and ssrRNA gene sequences. Ann. N. Y. Acad. Sci. 2008, 1149, 121–125. [Google Scholar] [CrossRef]
- Genis, A.D.; Perez, J.; Mosqueda, J.J.; Alvarez, A.; Camacho, M.; de Lourdes Munoz, M.; Rojas, C.; Figueroa, J.V. Using msa-2b as a molecular marker for genotyping Mexican isolates of Babesia bovis. Infect. Genet. Evol. 2009, 9, 1102–1107. [Google Scholar] [CrossRef]
- LeRoith, T.; Brayton, K.A.; Molloy, J.B.; Bock, R.E.; Hines, S.A.; Lew, A.E.; McElwain, T.F. Sequence variation and immunologic cross-reactivity among Babesia bovis merozoite surface antigen 1 proteins from vaccine strains and vaccine breakthrough isolates. Infect. Immun. 2005, 73, 5388–5394. [Google Scholar] [CrossRef]
- Matos, C.A.; Gonçalves, L.R.; Alvarez, D.O.; Freschi, C.R.; Silva, J.B.D.; Val-Moraes, S.P.; Mendes, N.S.; André, M.R.; Machado, R.Z. Longitudinal evaluation of humoral immune response and merozoite surface antigen diversity in calves naturally infected with Babesia bovis, in São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 479–490. [Google Scholar] [CrossRef]
- Matos, C.A.; Silva, J.B.D.; Gonçalves, L.R.; Mendes, N.S.; Alvarez, D.O.; André, M.R.; Machado, R.Z. Genetic diversity of Babesia bovis studied longitudinally under natural transmission conditions in calves in the state of Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e021220. [Google Scholar] [CrossRef]
- Molad, T.; Fleiderovitz, L.; Leibovich, B.; Wolkomirsky, R.; Erster, O.; Roth, A.; Mazuz, M.L.; Markovics, A.; Shkap, V. Genetic polymorphism of Babesia bovis merozoite surface antigens-2 (MSA-2) isolates from bovine blood and Rhipicephalus annulatus ticks in Israel. Vet. Parasitol. 2014, 205, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Nagano, D.; Sivakumar, T.; De De Macedo, A.C.C.; Inpankaew, T.; Alhassan, A.; Igarashi, I.; Yokoyama, N. The genetic diversity of merozoite surface antigen 1 (MSA-1) among Babesia bovis detected from cattle populations in Thailand, Brazil and Ghana. J. Vet. Med. Sci 2013, 75, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Simking, P.; Saengow, S.; Bangphoomi, K.; Sarataphan, N.; Wongnarkpet, S.; Inpankaew, T.; Jittapalapong, S.; Munkhjargal, T.; Sivakumar, T.; Yokoyama, N.; et al. The molecular prevalence and MSA-2b gene-based genetic diversity of Babesia bovis in dairy cattle in Thailand. Vet. Parasitol. 2013, 197, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Okubo, K.; Igarashi, I.; de Silva, W.K.; Kothalawala, H.; Silva, S.S.P.; Vimalakumar, S.C.; Meewewa, A.S.; Yokoyama, N. Genetic diversity of merozoite surface antigens in Babesia bovis detected from Sri Lankan cattle. Infect. Genet. Evol. 2013, 19, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tattiyapong, M.; Sivakumar, T.; Ybanez, A.P.; Ybanez, R.H.D.; Perez, Z.O.; Guswanto, A.; Igarashi, I.; Yokoyama, N. Diversity of Babesia bovis merozoite surface antigen genes in the Philippines. Parasitol. Int. 2014, 63, 57–63. [Google Scholar] [CrossRef]
- Tattiyapong, M.; Sivakumar, T.; Takemae, H.; Simking, P.; Jittapalapong, S.; Igarashi, I.; Yokoyama, N. Genetic diversity and antigenicity variation of Babesia bovis merozoite surface antigen-1 (MSA-1) in Thailand. Infect. Genet. Evol. 2016, 41, 255–261. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Gao, S.; Wang, X.; Sun, H.; Lv, Z.; Li, Y.; Liu, A.; Liu, J.; Luo, J.; et al. Genetic Diversity of Babesia bovis MSA-1, MSA-2b and MSA-2c in China. Pathogens 2020, 9, 473. [Google Scholar] [CrossRef]
- Yavuz, A.; İnci, A.; Düzlü, Ö.; Bişkin, Z.; Yıldırım, A. Molecular Characterization of Babesia bovis msa-2c Gene. Turk. Parazitol. Derg. 2011, 35, 140–144. [Google Scholar] [CrossRef]
- Yokoyama, N.; Sivakumar, T.; Tuvshintulga, B.; Hayashida, K.; Igarashi, I.; Inoue, N.; Long, P.T.; Lan, D.T.B. Genetic variations in merozoite surface antigen genes of Babesia bovis detected in Vietnamese cattle and water buffaloes. Infect. Genet. Evol. 2015, 30, 288–295. [Google Scholar] [CrossRef]
- Lau, A.O.; Cereceres, K.; Palmer, G.H.; Fretwell, D.L.; Pedroni, M.J.; Mosqueda, J.; McElwain, T.F. Genotypic diversity of merozoite surface antigen 1 of Babesia bovis within an endemic population. Mol. Biochem. Parasitol. 2010, 172, 107–112. [Google Scholar] [CrossRef]
- Liyanagunawardena, N.; Sivakumar, T.; Kothalawala, H.; Silva, S.S.P.; Battsetseg, B.; Lan, D.T.B.; Inoue, N.; Igarashi, I.; Yokoyama, N. Type-specific PCR assays for Babesia bovis msa-1 genotypes in Asia: Revisiting the genetic diversity in Sri Lanka, Mongolia, and Vietnam. Infect. Genet. Evol. 2016, 37, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Palmer, G.H.; Mcelwain, T.F.; Hines, S.A.; Dobbelaere, D.A.E. Babesia bovis: Characterization of the T helper cell response against the 42-kDa merozoite surface antigen (MSA-1) in cattle. Exp. Parasitol. 1993, 77, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Shkap, V.; Pipano, E.; McElwain, T.F.; Herzberg, U.; Krigel, Y.; Fish, L.; Palmer, G.H. Cross-protective immunity induced by Babesia bovis clones with antigenically unrelated variable merozoite surface antigens. Vet. Immunol. Immunopathol. 1994, 41, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hines, S.A.; Palmer, G.H.; Jasmer, D.P.; Goff, W.L.; McElwain, T.F. Immunization of cattle with recombinant Babesia bovis merozoite surface antigen-1. Infect. Immun. 1995, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Spiridonov, N.A.; Kashina, A. Sounds of silence: Synonymous nucleotides as a key to biological regulation and complexity. Nucleic Acids Res. 2013, 41, 2073–2094. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A gene-based positive selection detection approach to identify vaccine candidates using Toxoplasma gondii as a test case protozoan pathogen. Front. Genet. 2018, 9, 332. [Google Scholar] [CrossRef]
- Escalante, A.A.; Cornejo, O.E.; Rojas, A.; Udhayakumar, V.; Lal, A.A. Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 2004, 20, 388–395. [Google Scholar] [CrossRef]
- Iriko, H.; Kaneko, O.; Otsuki, H.; Tsuboi, T.; Su, X.Z.; Tanabe, K.; Torii, M. Diversity and evolution of the rhoph1/clag multigene family of Plasmodium falciparum. Mol. Biochem. Parasitol. 2008, 58, 11–21. [Google Scholar] [CrossRef]
- Aguileta, G.; Refregier, G.; Yockteng, R.; Fournier, E.; Giraud, T. Rapidly evolving genes in pathogens: Methods for detecting positive selection and examples among fungi, bacteria, viruses and protists. Infect. Genet. Evol. 2009, 9, 9656–9670. [Google Scholar] [CrossRef]
- Cuy-Chaparro, L.; Ricaurte-Contreras, L.A.; Bohórquez, M.D.; Arévalo-Pinzón, G.; Barreto-Santamaria, A.; Pabón, L.; Reyes, C.; Moreno-Pérez, D.A.; Patarroyo, M.A. Identification of Babesia bovis MSA-1 functionally constraint regions capable of binding to bovine erythrocytes. Vet. Parasitol. 2022, 312, 109834. [Google Scholar] [CrossRef]
- Berens, S.J.; Brayton, K.A.; Molloy, J.B.; Bock, R.E.; Lew, A.E.; McElwain, T.F. Merozoite surface antigen 2 proteins of Babesia bovis vaccine breakthrough isolates contain a unique hypervariable region composed of degenerate repeats. Infect. Immun. 2005, 73, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruiz, M.; Mejia-López, S.; Pérez-Serrano, R.M.; de Larrea, G.Z.L.; Ganzinelli, S.; Florin-Christensen, M.; Suarez, C.E.; Hernández-Ortiz, R.; Mercado-Uriostegui, M.A.; Rodríguez-Torres, A.; et al. Babesia bovis AMA-1, MSA-2c and RAP-1 contain conserved B and T-cell epitopes, which generate neutralizing antibodies and a long-lasting Th1 immune response in vaccinated cattle. Vaccine 2022, 40, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.F.; Mangold, A.J.; Baravalle, M.E.; Valentini, B.S.; Thompson, C.S.; Wilkowsky, S.E.; Echaide, I.E.; Farber, M.D.; de Echaide, S.M.T. Efficiency of a recombinant MSA-2c-based ELISA to establish the persistence of antibodies in cattle vaccinated with Babesia bovis. Vet. Parasitol. 2008, 157, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.J.; Lopez, U.; Rojas, C.; Borgonio, V.M.; Sanchez, V.; Castañeda, R.; Vargas, P.; Figueroa, J.V. Immunization of Bos taurus steers with Babesia bovis recombinant antigens MSA-1, MSA-2c and 12D3. Transbound. Emerg. Dis. 2010, 57, 87–90. [Google Scholar] [CrossRef] [PubMed]

| Gene Fragment | n | L | S | Eta | K | π | H | Hd | C | Fu’s Fs Statistic | Tajima’s D | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Significance | |||||||||||
| MSA-1 | 199 | 1014 | 505 | 1014 | 219.74255 | 0.40246 | 107 | 0.986 | 0.078 | 18.416 | 0.87536 | >0.10 |
| MSA-2a1 | 24 | 723 | 283 | 320 | 82.93478 | 0.11471 | 23 | 0.996 | 0.609 | −1.358 | −0.12975 | >0.10 |
| MSA-2b | 193 | 408 | 283 | 409 | 71.93469 | 0.18074 | 104 | 0.981 | 0.287 | −2.543 | 0.08555 | >0.10 |
| MSA-2c | 148 | 618 | 409 | 546 | 59.23727 | 0.09648 | 122 | 0.995 | 0.333 | −39.751 | −1.29882 | >0.10 |
| Gene Fragment | Neutral Selection (HA: dN ≠ dS) | Positive Selection (HA: dN > dS) | Negative Selection (HA: dN < dS) | |||
|---|---|---|---|---|---|---|
| Value | p-Value | Value | p-Value | Value | p-Value | |
| Msa-1 | 8.29 | 0.00 | 8.01 | 0.00 | −8.19 | 1.00 |
| Msa-2a1 | −2.24 | 0.03 | −2.25 | 1.00 | 2.27 | 0.01 |
| Msa-2b | 1.62 | 0.11 | 1.59 | 0.06 | −1.59 | 1.00 |
| Msa-2c | −3.21 | 0.00 | −3.21 | 1.00 | 3.15 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Alfy, E.-S.; Abbas, I.; Elseadawy, R.; El-Sayed, S.A.E.-S.; Rizk, M.A. Genetic Diversity of Merozoite Surface Antigens in Global Babesia bovis Populations. Genes 2023, 14, 1936. https://doi.org/10.3390/genes14101936
El-Alfy E-S, Abbas I, Elseadawy R, El-Sayed SAE-S, Rizk MA. Genetic Diversity of Merozoite Surface Antigens in Global Babesia bovis Populations. Genes. 2023; 14(10):1936. https://doi.org/10.3390/genes14101936
Chicago/Turabian StyleEl-Alfy, El-Sayed, Ibrahim Abbas, Rana Elseadawy, Shimaa Abd El-Salam El-Sayed, and Mohamed Abdo Rizk. 2023. "Genetic Diversity of Merozoite Surface Antigens in Global Babesia bovis Populations" Genes 14, no. 10: 1936. https://doi.org/10.3390/genes14101936
APA StyleEl-Alfy, E.-S., Abbas, I., Elseadawy, R., El-Sayed, S. A. E.-S., & Rizk, M. A. (2023). Genetic Diversity of Merozoite Surface Antigens in Global Babesia bovis Populations. Genes, 14(10), 1936. https://doi.org/10.3390/genes14101936







