Heterologous Expression of Platycodon grandiflorus PgF3′5′H Modifies Flower Color Pigmentation in Tobacco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Plant Expression Vector and Tobacco Transformation
2.3. qRT-PCR Analysis for PgF3′5′H Expression in Transformed Tobacco
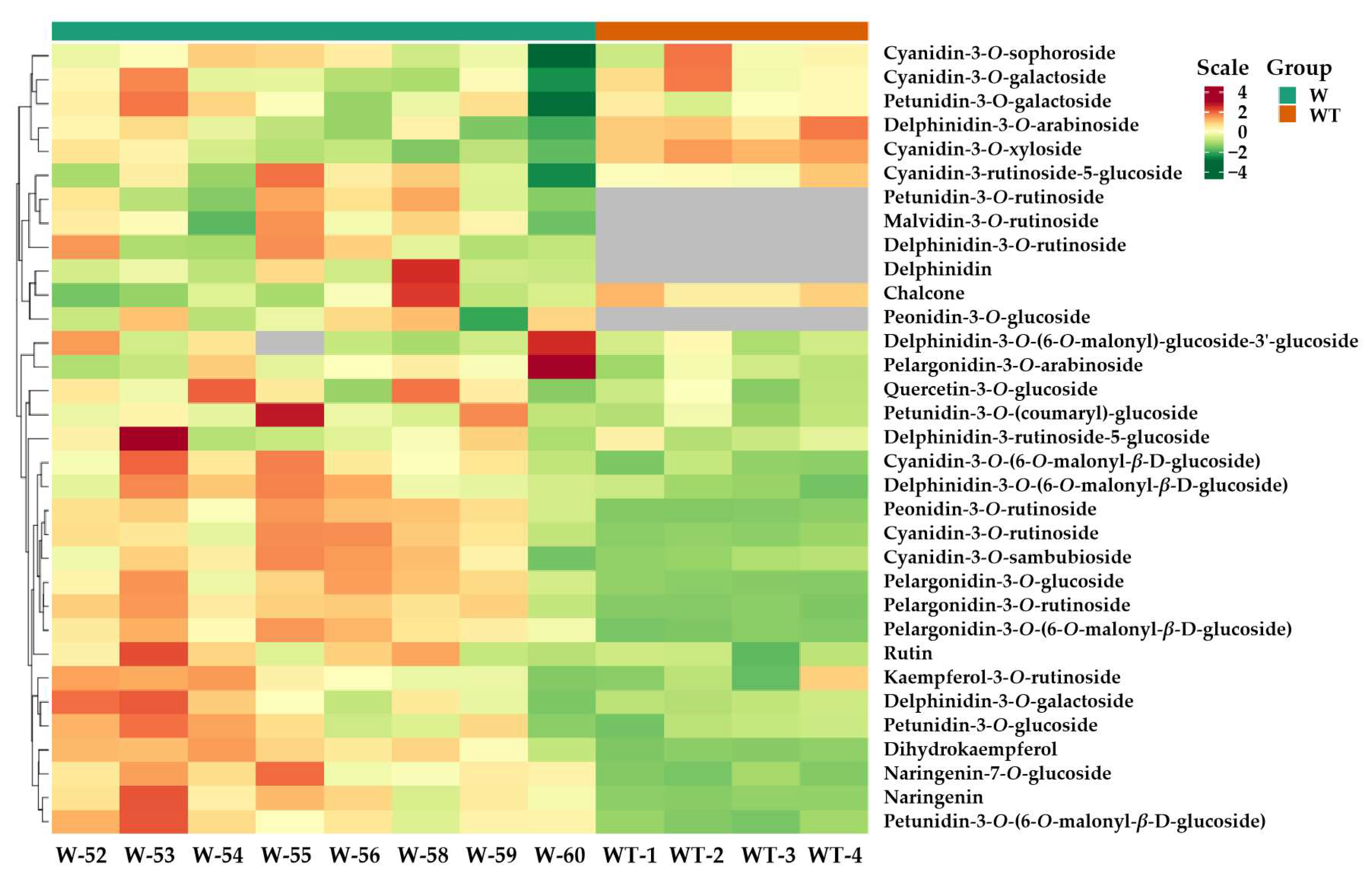
2.4. Extraction and Analysis of Anthocyanins in Transformed Tobacco
3. Results
3.1. Phenotype Comparison of Transgenic T0 Generation Tobaccos
3.2. Heterologous Expression of PgF3′5′H in Transformed Tobacco
3.3. Comparative Analysis of Anthocyanins in Transformed and Wild-Type Tobacco
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Kalc, G.; Senior, M.; Dyson, B.; Nakamura, N.; Katsumoto, Y.; Chandler, S. Flower color modification by engineering of the flavonoid biosynthetic pathway: Practical perspectives. Biosci. Biotechnol. Biochem. 2010, 74, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, L.J. Allele-specific marker development and selection efficiencies for both flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes in soybean subgenus soja. Theor. Appl. Genet. 2013, 126, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Brugliera, F. Flower colour and cytochromes P450. Philos. Trans. R. Soc. B 2013, 368, 20120432. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Ishiguro, K.; Taniguchi, M.; Tanaka, Y. Functional analysis of Antirrhinum kelloggii flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes; critical role in flower color and evolution in the genus Antirrhinum. J. Plant Res. 2012, 125, 451–456. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins from chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Shimada, Y.; Ohbayashi, M.; Nakano-Shimada, R.; Okinaka, Y.; Kiyokawa, S.; Kikuchi, Y. Genetic engineering of the anthocyanin biosynthetic pathway with flavonoid-3′,5′-hydroxylase: Specific switching of the pathway in petunia. Plant Cell Rep. 2001, 20, 456–462. [Google Scholar] [CrossRef]
- Nakamura, N.; Fukuchi-Mizutani, M.; Fukui, Y.; Ishiguro, K.; Suzuki, K.; Suzuki, H.; Okazaki, K.; Shibata, D.; Tanaka, Y. Generation of pink flower varieties from blue Torenia hybrida by redirecting the flavonoid biosynthetic pathway from delphinidin to pelargonidin. Plant Biotechnol. 2010, 27, 375–383. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xu, Y.J.; Gao, L.P.; Yu, O.; Wang, X.Z.; He, X.J.; Jiang, X.L.; Liu, Y.J.; Xia, T. Functional analysis of Flavonoid 3′,5′ -hydroxylase from Tea plant (Camellia sinensis): Critical role in the accumulation of catechins. BMC Plant Biol. 2014, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Fukui, Y.; Nakamura, N.; Katsumoto, Y.; Yonekura-Sakakibara, K.; Fukuchi-Mizutani, M.; Ohira, K.; Ueyama, Y.; Ohkawa, H.; Holton, T.A.; et al. Flower color modification of Petunia hybrida commercial varieties by metabolic engineering. Plant Biotechnol. 2004, 21, 377–386. [Google Scholar] [CrossRef]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef]
- Ueyama, Y.; Suzuki, K.; Fukuchi-Mizutani, M.; Fukui, Y.; Miyazaki, K.; Ohkawa, H.; Kusumi, T.; Tanaka, Y. Molecular and biochemical characterization of torenia flavonoid 3′-hydroxylase and flavone synthase II and modification of flower color by modulating the expression of these genes. Plant Sci. 2002, 163, 253–263. [Google Scholar] [CrossRef]
- Ueyama, Y.; Katsumoto, Y.; Fukui, Y.; Fukuchi-Mizutani, M.; Ohkawa, H.; Kusumi, T.; Iwashita, T.; Tanaka, Y. Molecular characterization of the flavonoid biosynthetic pathway and flower color modification of Nierembergia sp. Plant Biotechnol. 2006, 23, 19–24. [Google Scholar] [CrossRef]
- Fukui, Y.; Tanaka, Y.; Kusumi, T.; Iwashita, T.; Nomoto, K. A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 2003, 63, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Flower colour and cytochromes P450. Phytochem. Rev. 2006, 5, 283–291. [Google Scholar] [CrossRef]
- Vetten, N.D.; Horst, J.T.; Schaik, H.P.V.; de Boer, A.D.; Mol, J.; Koes, R. A cytochrome b5 is required for full activity of flavonoid 3′,5′-hydroxylase, a cytochrome P450 involved in the formation of blue flower colors. Proc. Natl. Acad. Sci. USA 1999, 96, 778–783. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Schlangen, K.; Forkmann, G.; Halbwirth, H. Multiple evolution of flavonoid 3′,5′-hydroxylase. Planta 2015, 242, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Holton, T.A.; Tanaka, Y. Blue roses—A pigment of our imagination? Trends Biotechnol. 1994, 12, 40–42. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, H.; Meng, L.; Hu, K.; Dai, S.L. Isolation and functional analysis of a homolog of flavonoid 3′,5′-hydroxylase gene from Pericallis × hybrida. Physiol. Plant. 2013, 149, 151–159. [Google Scholar] [CrossRef]
- Holton, T.A.; Brugliera, F.; Lester, D.R.; Tanaka, Y.; Hyland, C.D.; Menting, J.G.T.; Lu, C.; Farcy, E.; Stevenson, T.W.; Cornish, E.C. Cloning and expression of cytochrome P450 genes controlling flower colour. Nature 1993, 366, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Okinaka, Y.; Shimada, Y.; Nakano-Shimada, R.; Ohbayashi, M.; Kiyokawa, S.; Kikuchi, Y. Selective accumulation of delphinidin derivatives in tobacco using a putative flavonoid 3′,5′-hydroxylase cDNA from Campanula medium. Biosci. Biotechnol. Biochem. 2003, 67, 161–165. [Google Scholar] [CrossRef]
- Mori, S.; Kobayashi, H.; Hoshi, Y.; Kondo, M.; Nakano, M. Heterologous expression of the flavonoid 3′,5′-hydroxylase gene of Vinca major alters flower color in transgenic Petunia hybrida. Plant Cell Rep. 2004, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Togami, J.; Tamura, M.; Ishiguro, K.; Hirose, C.; Okuhara, H.; Ueyama, Y.; Nakamura, N.; Yonekura-Sakakibara, K.; Fukuchi-Mizutani, M.; Suzuki, K.; et al. Molecular characterization of the flavonoid biosynthesis of Verbena hybrida and the functional analysis of verbena and Clitoria ternatea F3′5′H genes in transgenic verbena. Plant Biotechnol. 2006, 23, 5–11. [Google Scholar] [CrossRef]
- Qi, Y.; Lou, Q.; Quan, Y.; Liu, Y.; Wang, Y. Flower-specific expression of the Phalaenopsis flavonoid 3′,5′-hydoxylase modifies flower color pigmentation in Petunia and Lilium. Plant Cell Tiss. Organ Cult. 2013, 115, 263–273. [Google Scholar] [CrossRef]
- Wang, J.; Ming, F.; Han, Y.; Shen, D. Flavonoid-3′,5′-hydroxylase from Phalaenopsis: A novel member of cytochrome P450s, its cDNA cloning, endogenous expression and molecular modeling. Biotechnol. Lett. 2006, 28, 327–334. [Google Scholar] [CrossRef]
- Takahashi, R.; Dubouzet, J.G.; Matsumura, H.; Yasuda, K.; Iwashina, T. A new allele of flower color gene W1 encoding flavonoid 3′,5′ -hydroxylase is responsible for light purple flowers in wild soybean Glycine soja. BMC Plant Biol. 2010, 10, 155. [Google Scholar] [CrossRef]
- Boase, M.R.; Lewis, D.H.; Davies, K.M.; Marshall, G.B.; Deroles, S.C. Isolation and antisense suppression of flavonoid 3′,5′-hydroxylase modifies flower pigments and colour in cyclamen. BMC Plant Biol. 2010, 10, 107. [Google Scholar] [CrossRef]
- Whang, S.S.; Um, W.S.; Song, I.J.; Lim, P.O.; Choi, K.; Park, K.W.; Kang, K.W.; Choi, M.S.; Koo, J.C. Molecular analysis of anthocyanin biosynthetic genes and control of flower coloration by flavonoid 3′,5′-hydroxylase (F3′5′H) in Dendrobium moniliforme. J. Plant Biol. 2011, 54, 209–218. [Google Scholar] [CrossRef]
- Huang, W.; Sun, W.; Wang, Y. Isolation and molecular characterisation of flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes from a traditional Chinese medicinal plant, Epimedium sagittatum. Gene 2012, 497, 125–130. [Google Scholar] [CrossRef]
- Nguyen, T.N.L.; Hoang, T.T.H.; Nguyen, H.Q.; Tu, Q.T.; Tran, T.H.; Lo, T.M.T.; Vu, T.T.T.; Chu, H.M. Agrobacterium tumefaciens–mediated genetic transformation and overexpression of the flavonoid 3′5′-hydroxylase gene increases the flavonoid content of the transgenic Aconitum carmichaelii Debx. plant. In Vitro Cell. Dev. Biol. Plant 2022, 58, 93–102. [Google Scholar] [CrossRef]
- Nguyen, Y.T.H.; Hoang, H.T.T.; Mai, A.T.H.; Nguyen, L.T.N.; Nguyen, Q.H.; Pham, N.T.T.; Sy, T.D.; Chu, M.H. The Aconitum carmichaelii F3′5′H gene overexpression increases flavonoid accumulation in transgenic tobacco plants. Horticulturae 2021, 7, 384. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandifloras—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef]
- Tamura, K.; Teranishi, Y.; Ueda, S.; Suzuki, H.; Kawano, N.; Yoshimatsu, K.; Saito, K.; Kawahara, N.; Muranaka, T.; Seki, H. Cytochrome P450 monooxygenase CYP716A141 is a unique β-Amyrin C-16β oxidase involved in triterpenoid saponin biosynthesis in Platycodon grandiflorus. Plant Cell Physiol. 2017, 58, 874–884. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.H.; Park, S.G.; Yang, T.J.; Lee, Y.; Kim, O.T.; Chung, O.; Lee, J.; Choi, J.P.; Kwon, S.J.; et al. Whole-genome, transcriptome, and methylome analyses provide insights into the evolution of platycoside biosynthesis in Platycodon grandiflorus, a medicinal plant. Hortic. Res. 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Jia, W.J.; Duan, Q.; Wang, X.N.; Jiang, Y.L.; Guo, Y.; Li, J.K.; Wang, J.H. Clone and expression analysis of PgF3′5′H gene in Platycodon grandiflorus. Acta Bot. Boreal. Occident. Sin. 2014, 34, 40–46. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef]
- Jiao, F.; Zhao, L.; Wu, X.; Song, Z.; Li, Y. Metabolome and transcriptome analyses of the molecular mechanisms of flower color mutation in tobacco. BMC Genom. 2020, 21, 611. [Google Scholar] [CrossRef]
- Kanani, P.; Shukla, Y.M.; Modi, A.R.; Subhash, N.; Kumar, S. Standardization of an efficient protocol for isolation of RNA from Cuminum cyminum. J. King Saud Univ. Sci. 2019, 31, 1202–1207. [Google Scholar] [CrossRef]
- Ding, C.; Lu, Y.; Song, Y.; Jia, R.; Jin, W.; Guo, H. Cloning and expression analysis of hydroperoxide lyase gene in Nicotiana tabacum. Life Sci. J. 2019, 16, 23–27. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xun, H.; Yi, G.; Li, T.; Yao, X.; Tang, F. Integrated metabolomic and transcriptomic analysis reveals the effect of artificial shading on reducing the bitter taste of bamboo shoots. Horticulturae 2022, 8, 594. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yonekura, K.; Fukuchimizutani, M.; Fukui, Y.; Fujiwara, H.; Ashikari, T.; Kusumi, T. Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora. Plant Cell Physiol. 1996, 37, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Hehn, A.; Jugdé, H.; Slimestad, R.; Larbat, R.; Bourgaud, F.; Lillo, C. Identification and characterisation of CYP75A31, a new flavonoid 3′5′-hydroxylase, isolated from Solanum lycopersicum. BMC Plant Biol. 2010, 10, 21. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Forkmann, G. Identification of the molecular basis for the functional difference between flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase. FEBS Lett. 2007, 581, 3429–3434. [Google Scholar] [CrossRef]
- Shimada, Y.; Nakano-Shimada, R.; Ohbayashi, M.; Okinaka, Y.; Kiyokawa, S.; Kikuchi, Y. Expression of chimeric P450 genes encoding flavonoid-3′,5′-hydroxylase in transgenic tobacco and petunia plants. FEBS Lett. 1999, 461, 241–245. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Chandler, S. Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 2009, 10, 5350–5369. [Google Scholar] [CrossRef]
- Noda, N. Recent advances in the research and development of blue flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef]
- Kaltenbach, M.; Schröder, G.; Schmelzer, E.; Lutz, V.; Schröder, J. Flavonoid hydroxylase from Catharanthus roseus: cDNA, heterologous expression, enzyme properties and cell-type specific expression in plants. Plant J. 1999, 19, 183–193. [Google Scholar] [CrossRef] [PubMed]



| Samples | WT-1 | WT-2 | WT-3 | WT-4 | W-52 | W-53 | W-54 | W-55 | W-56 | W-58 | W-59 | W-60 | VIP | Fold_Change | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHM | N.d | N.d | N.d | N.d | 0.026 | 0.019 | 0.018 | 0.031 | 0.016 | 0.021 | 0.016 | 0.018 | 1.117 | Inf | / |
| Kaempferol | 0.072 | 0.066 | 0.070 | 0.067 | 0.107 | 0.105 | 0.108 | 0.119 | 0.109 | 0.088 | 0.117 | 0.130 | 1.062 | 1.607 | Insig |
| Luteolin | 0.029 | 0.028 | 0.028 | 0.028 | 0.174 | 0.148 | 0.134 | 0.148 | 0.120 | 0.156 | 0.116 | 0.121 | 1.122 | 4.973 | Up |
| DHQ | 1.776 | 1.737 | 1.746 | 1.765 | 69.367 | 39.973 | 50.996 | 55.477 | 38.780 | 68.377 | 35.011 | 42.298 | 1.063 | 28.499 | Up |
| Apigenin | 0.073 | 0.073 | 0.072 | 0.076 | 0.048 | 0.052 | 0.216 | 0.043 | 0.056 | 0.040 | 0.037 | 0.047 | 0.070 | 0.917 | Insig |
| Eriodictyol | 0.291 | 0.278 | 0.279 | 0.294 | 12.239 | 8.516 | 6.993 | 5.814 | 3.715 | 8.026 | 4.906 | 5.361 | 0.997 | 24.346 | Insig * |
| DHK | 1.814 | 1.846 | 1.831 | 1.856 | 4.429 | 4.008 | 4.447 | 3.892 | 3.757 | 4.566 | 3.381 | 2.671 | 1.034 | 2.120 | Up |
| Naringenin | 0.861 | 0.850 | 0.849 | 0.868 | 1.248 | 1.378 | 1.251 | 1.408 | 1.409 | 1.034 | 1.322 | 1.224 | 1.081 | 1.498 | Insig |
| Tricetin | N.d | N.d | N.d | N.d | N.d | N.d | N.d | N.d | N.d | N.d | N.d | N.d | / | / | / |
| Samples | WT-1 | WT-2 | WT-3 | WT-4 | W-52 | W-53 | W-54 | W-55 | W-56 | W-58 | W-59 | W-60 | VIP | Fold_Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pel-3-O-glu | 0.043 | 0.040 | 0.039 | 0.039 | 0.097 | 0.138 | 0.080 | 0.114 | 0.134 | 0.121 | 0.112 | 0.067 | 1.225 | 2.671 |
| Pel-3-O-rut | 5.606 | 5.534 | 5.937 | 5.182 | 18.993 | 22.351 | 16.427 | 18.806 | 19.151 | 17.274 | 18.770 | 9.389 | 1.252 | 3.171 |
| Pel-3-O-(6-O-mal)-glu | 0.078 | 0.081 | 0.087 | 0.084 | 0.187 | 0.230 | 0.171 | 0.242 | 0.225 | 0.192 | 0.184 | 0.155 | 1.321 | 2.403 |
| Peo-3-O-rut | 0.116 | 0.114 | 0.117 | 0.130 | 0.419 | 0.453 | 0.329 | 0.538 | 0.477 | 0.473 | 0.425 | 0.241 | 1.274 | 3.524 |
| DHK | 1.351 | 1.511 | 1.464 | 1.558 | 4.779 | 4.713 | 5.197 | 4.371 | 3.935 | 4.368 | 3.504 | 2.303 | 1.213 | 2.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Jia, W.; Duan, Q.; Du, W.; Li, X.; Cui, G.; Wang, X.; Wang, J. Heterologous Expression of Platycodon grandiflorus PgF3′5′H Modifies Flower Color Pigmentation in Tobacco. Genes 2023, 14, 1920. https://doi.org/10.3390/genes14101920
Ma L, Jia W, Duan Q, Du W, Li X, Cui G, Wang X, Wang J. Heterologous Expression of Platycodon grandiflorus PgF3′5′H Modifies Flower Color Pigmentation in Tobacco. Genes. 2023; 14(10):1920. https://doi.org/10.3390/genes14101920
Chicago/Turabian StyleMa, Lulin, Wenjie Jia, Qing Duan, Wenwen Du, Xiang Li, Guangfen Cui, Xiangning Wang, and Jihua Wang. 2023. "Heterologous Expression of Platycodon grandiflorus PgF3′5′H Modifies Flower Color Pigmentation in Tobacco" Genes 14, no. 10: 1920. https://doi.org/10.3390/genes14101920
APA StyleMa, L., Jia, W., Duan, Q., Du, W., Li, X., Cui, G., Wang, X., & Wang, J. (2023). Heterologous Expression of Platycodon grandiflorus PgF3′5′H Modifies Flower Color Pigmentation in Tobacco. Genes, 14(10), 1920. https://doi.org/10.3390/genes14101920




