Abstract
Background: β-lactamase-producing Escherichia coli are a widely distributed source of antimicrobial resistance for animals and humans. Little is known about the susceptibility profile and genetic characteristics of E. coli strains isolated from domestic dogs in Latin America. Methods: We report on a cross-sectional study that evaluated E. coli strains isolated from fecal samples of domestic dogs in central Panama. The extended-spectrum β-lactamase (ESBL), AmpC genes, and plasmid-mediated quinolone resistance were investigated. Molecular typing using Pasteur’s multilocus sequence typing (MLST) was conducted. Results: A total of 40 E. coli isolates were obtained, of which 80% (32/40) were resistant to at least one of the antibiotics tested, while 20% (8/40) were sensitive to all antibiotics analyzed in this study (p < 0.001). Forty percent of the strains were resistant to three or more antibiotics. The most common resistance was to tetracycline (45%) and ampicillin (30%) while 2.5% showed an ESBL phenotype. Antibiotic resistance genes were detected for one β-lactamase (blaTEM-1) and two plasmid-mediated quinolone resistance (PMQR) enzymes (qnrS and qnrB). In addition, mutations in the chromosomal AmpC gene were observed at positions −35, −28, −18, −1, and +58. Fourteen different sequence types (STs) were identified; the most frequent were ST399 and ST425 (12% each). ST3 and ST88, which have been previously identified in human clinical isolates, were also evidenced. Three new STs were found for the first time: ST1015, ST1016 (carrier of the blaTEM-1 gene), and ST1017 (carrier of the blaTEM-1, qnrS, and qnrB genes). Conclusions: In the intestinal strains of E. coli isolated from domestic dogs, there was a high frequency of resistance to antibiotics. The presence of genes from plasmids and chromosomal mutations that conferred antibiotic resistance, the identification of isolates previously reported in humans, and the genetic diversity of STs (including three that were newly identified) confirmed the determinants of resistance to antibiotics in the domestic dogs from central Panama.
1. Introduction
Escherichia coli is a commensal bacterium of the intestinal tract. While some strains of E. coli can cause intestinal infections, most strains can potentially cause extra-intestinal infections in many cases resistant to antibiotics, especially in the urinary tract. Therefore, under conditions favoring pathogenicity, E. coli is responsible for many infections in both humans and in animals [1]. These infections are mainly treated with β-lactam and fluoroquinolone antibiotics. However, resistance to these antibiotic groups has increased worldwide, which represents a major public health problem [2,3].
A study conducted in Latin America reported that the antibiotics most used for the treatment of companion animal infections were critically important and included β-lactams and quinolones, which were prescribed in 65.3% and 36.2% of infections, respectively [4]. Indiscriminate use of antibiotics in both human and veterinary medicine has resulted in bacterial resistance to antibiotics used in humans and their companion animals [5]. The exchange of bacterial serotypes between humans and domestic dogs has been reported [6,7,8,9,10,11].
Extended-spectrum β-lactamase-producing (ESBL) Enterobacteriaceae are a widely distributed source of antimicrobial resistance (AMR) in both humans and animals [12]. ESBL enzymes confer resistance to third- and fourth-generation cephalosporins and aztreonam. According to a systematic review conducted in South America, the prevalence of animal-origin ESBL-producing E. coli was estimated at 18.1% [13]. In addition, the prevalence of human-origin ESBL-producing E. coli in South America was estimated at 30% [14]. These results were similar to those observed in Tanzania, where ESBLs from animal sources showed a prevalence comparable to that of human isolates [13]. Thus, although many of the resistance mechanisms of E. coli are acquired in the human hospital environment, in several regions of the world the prevalence of ESBL-producing E. coli in animal sources can be considered a major problem. The latter may be due to the poor regulation of antibiotic use in these settings.
ESBL enzymes have been commonly detected in E. coli strains from healthy companion animals; the blaCTX-M-type, blaSHV, and blaTEM genes are the most identified on all continents [15]. The prevalence of the ESBL phenotype detected in E. coli isolates from dogs varies by region. For example, prevalence-rate estimates of 6% in Mexico [16], 9% in New Zealand [17], 9.2% in Brazil [18], 24% in Chile [19], and 24.9% in Republic of Korea [20] have been reported. Studies in the South American region have identified ESBLs such as CTX-M, TEM, SHV, PER-2, and AmpC in animals; the CTX-M-1, CTX-M-2, and CTX-M-8 enzymes are the most prevalent [13]. AmpCs are clinically important β-lactamases that hydrolyze first- and second-generation cephalosporins. These have been identified in intestinal E. coli isolates from small pets in which chromosomal mutations led to an enzyme overexpression responsible for AmpC resistance mechanisms [21,22]. Plasmid-mediated AmpC-producing (pAmpC) E. coli isolates have been identified in healthy dogs [16].
Carbapenemases, which are enzymes that hydrolyze carbapenems, have been identified in pet dogs; the main classes that have been described are: KPC, NDM, VIM, IMP, OXA-L48, and OXA-23 [23]. In companion animals as well as in humans, resistance to quinolones has been described more frequently in enterobacteria that are resistant to β-lactams. Multiple studies have shown the frequent association between genes that encode β-lactamases and several variants of genes that encode plasmid-mediated quinolone resistance (PMQR) such as the qnrA, qnrB, and qnrS genes [24,25].
Around 171 different sequence types (STs) have been reported in companion animals; ST38 and ST131 are found on all continents [26]. Various human-associated STs have been identified in domestic dogs [6,27,28,29,30]. Moreover, bacterial strains conduct intra- and inter-species genetic exchanges that include exchanges between dogs and their human owners [31,32,33]. Companion animals have been hypothesized as potential reservoirs of AMR bacteria, but there is a paucity of hard data. Studies conducted in Latin America showed high AMR to multiple antibiotics in intestinal strains of E. coli from small pets [34,35].
The purpose of the present study was the characterization of E. coli strains isolated from fecal samples of domestic dogs from the central region of Panama to investigate the AMR phenotype and molecular typing using the multilocus sequence typing (MLST) technique.
2. Materials and Methods
2.1. Strain Isolation
A cross-sectional study was conducted in August 2022. Fecal samples were obtained via rectal swabbing of domestic dogs from the central provinces of Herrera and Los Santos in the Republic of Panama with the informed consent of their owners. Samples were collected and transported in Cary-Blair medium (COPAN Diagnostics Inc.; Murrieta, CA, USA). Each sample was cultured on MacConkey agar (Merck Millipore; Darmstadt, Germany) and Chromocult® (Merck Millipore; Darmstadt, Germany). Biochemical tests were conducted to identify E. coli strains using triple sugar iron agar, Simmons citrate agar, motility indole sulfide, and urea medium. For each sample collected, a technical sheet was completed anonymously with the following variables: age, sex, breed, and history of prior antibiotic treatment.
2.2. Antibiotic Susceptibility Test
The susceptibility to antibiotics was determined via the disk diffusion method on Muller Hinton agar (HiMedia Labs; Mumbai, India) [36], and the results obtained were interpreted according to the Clinical and Laboratory Standards Institute [37]. A total of 16 antibiotic discs were used: ampicillin (AMP, 10 µg), amoxicillin–clavulanate (AMC, 20/10 µg), aztreonam (ATM, 30 μg), cefepime (FEP, 30 µg), cefotaxime (CTX, 30 µg), cefoxitin (FOX, 30 µg), ceftazidime (CAZ, 30 μg), chloramphenicol (CPL, 30 µg), ciprofloxacin (CIP, 5 μg), gentamicin (GEN, 10 μg), imipenem (IPM, 10 μg), kanamycin (KAN, 30 μg), nalidixic acid (NAL, 30 µg), streptomycin (STS, 300 μg), tetracycline (TET, 30 μg), and trimethoprim–sulfamethoxazole (SXT, 1.25/23.75 µg). The sensitivity profiles allowed classification of the isolates as resistant, intermediate, and sensitive.
2.3. Molecular Typing Analyses and Molecular Identification of blaESBL/AmpC
The molecular typing analyses were performed using Pasteur’s MLST scheme. The MLST scheme was performed using a standardized protocol specific for E. coli [38]. The internal fragments of eight housekeeping genes (dinB, icdA, pabB, polB, putP, trpA, trpB, and uidA) were amplified from the chromosomal DNA of the E. coli strain. Sequencing of the polymerase chain reaction (PCR) products was performed with the services of Macrogen Inc. (Seoul, Republic of Korea). The gene sequences were analyzed using Geneious Prime v. 2020.5 (Biomatters, Ltd.; Auckland, New Zealand), and the allelic profiling was conducted using the Pasteur Institute’s E. coli Bacterial Isolate Genome Sequence Database (BIGSDB; https://bigsdb.pasteur.fr/ecoli/ecoli.html, accessed on 24 October 2022) for the specific MLST allele profiles and STs [39].
All isolates with β-lactam and quinolone resistance phenotypes were tested for blaCTX-M, blaTEM, blaSHV, blaCMY, AmpC, qnrA, qnrB, and qnrS [40,41,42] using PCR-specific primers (Table 1). The amplified samples were sequenced and analyzed using the BLASTN program of the National Center for Biotechnology Information (NCBI; https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 24 October 2022). For the AmpC gene, E. coli K-12 was used as the reference [21].

Table 1.
β-lactamase genes analyzed.
2.4. Statistical Analyses
The data were recorded in MS Excel (The Microsoft Corporation; Redmond, WA, USA). The data analyses were performed in Stata v. 11.0 (StataCorp, LLC; College Station, TX, USA). The descriptive statistics were calculated; the goodness-of-fit chi-square test was used to compare proportions with the alpha set at 0.05 for statistical significance.
3. Results
A total of 40 strains of E. coli were isolated from domestic dogs in central Panama. The mean age was 3.9 years (SD = 3.1) and most (n = 23; 58%) of the dogs were female. Most (n = 30; 75%) of the dogs had a known breed: Schnauzer (n = 7), Cocker (n = 4), Pitbull, Maltese, and Labrador (n = 3 each); 25% of the dogs had a mixed or unidentified breed. Almost half of the dogs (n = 17; 43%) had a history of prior antibiotic use; the most commonly used antibiotics reported were doxycycline (35%), cephalexin (20%), and enrofloxacin (10%).
Most (n = 32; 80%) of the E. coli strains analyzed showed resistance to at least one of the antibiotics analyzed; 20% (8/40) were sensitive to all antibiotics (p < 0.001). Figure 1 shows the proportion of sensitivity and resistance to the antibiotics analyzed in the isolated E. coli strains. We observed that 27.5% of the E. coli strains presented resistance to a single type of antibiotic, 15% to two types, 12.5% to three, 7.5% to four, 7.5% to five, 2.5% to six, and 7.5% to seven antibiotics. Table 2 shows the antimicrobial resistance phenotype of the E. coli strains analyzed; it can be seen that 2.5% (1/40) showed an ESBL phenotype, 40% (16/40) presented resistance to a β-lactam, and 20% (8/40) presented resistance to ciprofloxacin. The antibiotics with the highest prevalence of resistance were tetracycline (45%) and ampicillin (30%). Eight percent of the strains analyzed showed an extended-spectrum phenotype: one ESBL phenotype (LS09) and two extended-spectrum cephalosporins (ESCs) with an AmpC phenotype (HE04 and HE12 strains; Table 2). The HE02, HE04, and LS07 strains recorded resistance to imipenem; this will be analyzed in subsequent studies to elucidate possible resistance mechanisms not related to enzymes. No significant statistical differences were found between AMRs according to the dog breeds (p = 0.28).
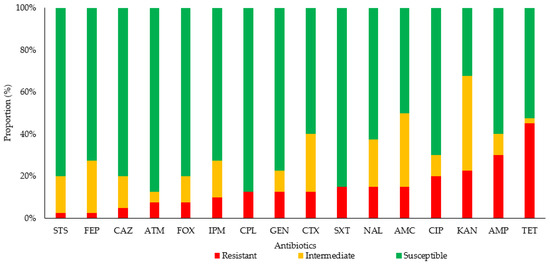
Figure 1.
Proportion of antibiotic sensitivity and resistance in E. coli strains isolated from companion dogs. Abbreviations: AMC, amoxicillin–clavulanate; AMP, ampicillin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CPL, chloramphenicol; CTX, cefotaxime; FEP, cefepime; FOX, cefoxitin; GEN, gentamicin; IPM, imipenem; KAN, kanamycin; NAL, nalidixic acid; STS, streptomycin; SXT, trimethoprim–sulfamethoxazole; TET, tetracycline.

Table 2.
Phenotypes and genotypes of E. coli strains isolated from domestic dogs.
Molecular typing using Pasteur’s MLST technique identified 14 STs in the E. coli samples analyzed (see Table 2). We most frequently observed ST399 (12%, 3/26), ST425 (12%, 3/26), ST910 (12%, 3/26), and ST960 (8%, 2/26). ST3 and ST88, which have been previously identified in human clinical isolates, also were found. We identified three new STs for the first time in this study: ST1015 (8%, 2/26), ST1016 (8%, 2/26), and ST1017 (8%, 2/26). Sequences of each of the three new STs of E. coli identified in this study can be found at https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?page=profileInfo&db=pubmlst_ecoli_seqdef&scheme_id=1&profile_id=1015, (accessed on 24 October 2022); https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?page=profileInfo&db=pubmlst_ecoli_seqdef&scheme_id=1&profile_id=1016, (accessed on 24 October 2022); and https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?page=profileInfo&db=pubmlst_ecoli_seqdef&scheme_id=1&profile_id=1017, (accessed on 24 October 2022).
Table 3 shows the genotypes and phenotypes of the strains that showed resistance to β-lactams and quinolones. The blaTEM-1 was identified in 30% (3/10) of the strains with resistance to β-lactams. In addition, 80% (8/10) had a chromosomal AmpC with a mutation in the promoter regions −35 (1/10), −28 (1/10), −18 (5/10), −1 (5/10), and +58 (7/10), thereby registering different types of combinations: type 1 (−35, −18, −1, and +58), type 2 (−18, −1, and +58), type 3 (−28), and type 4 (+58); along with changes in the following bases: −35 (A → T), −28 (G → A), −18 (G → A), −1 (T → C), and +58 (T → C).

Table 3.
Resistance genes identified in the E. coli strains that demonstrated β-lactam and quinolone resistance.
Among the strains with resistance to quinolones that were analyzed, we identified qnrS in two (20%) and qnrB in two (20%). The HE01 isolate showed the presence of both genes (qnrS and qnrB). Two of the three new STs identified in this study were carriers of antibiotic resistance genes: ST1016 (carrier of blaTEM-1) and ST1017 (carrier of blaTEM-1, qnrS, and qnrB).
4. Discussion
Antibiotics such as β-lactams and fluoroquinolones are widely prescribed to treat infections caused by E. coli. However, resistance to these groups of antibiotics is increasing in strains isolated from humans, animals, and the environment [15]. The present study showed bacterial phenotypes in combination with genetic characteristics of E. coli strains isolated from mostly healthy domestic dogs from the central region of Panama. We observed that a high percentage of the E. coli strains showed resistance to at least one of the antibiotics analyzed. Resistance to tetracycline and to ampicillin were the most prevalent, which was in agreement with a previous study in Latin America [35]. Additionally, resistance to ciprofloxacin and to cefotaxime were also identified. We found a prevalence of 2.5% for the ESBL phenotype for central Panama, which was one of the lowest in Latin America when compared to previous studies [16,18,19]. Resistance to these antibiotics has been previously described for E. coli isolated from healthy dogs in various regions of the world [35,43] as well as from environmental strains [5]. This aspect varies by country and depends greatly on the number and enforcement of public policies that regulate the prescription of antibiotics in veterinary medicine, which has been found to be related to the emergence of multidrug-resistant (MDR) strains [44]. Other factors that contribute to MDR in veterinary medicine include the use of antibiotics in animal feed, the use of antibiotics without quantification or empirical dosage, and the preference in the use of specific antibiotics such as doxycycline and enrofloxacin without alternating them with others [42,45]. Globally, MDR E. coli poses a challenge to health systems [46]. This study evidenced that a high proportion of dogs had a history of previous use of antibiotics; the most used were doxycycline, cephalexin, and enrofloxacin (a fluroquinolone). This study confirmed the presence of E. coli strains with an extended spectrum of resistance to β-lactam antibiotics (ESBL and AmpC phenotypes) in companion animals. Few studies have examined intestinal strains of E. coli in dogs under natural conditions. One study detected ESBL-producing E. coli from a single fecal sample from a healthy dog [47], while another study reported a prevalence of 9% of ESBL-producing E. coli strains in healthy dogs [48]. These findings are concerning due to the potential for transmission of antibiotic resistance determinants from commensal bacteria to potential pathogenic bacteria, zoonotic transmission, and opportunistic infections [49].
The MLST genetic sequences analyzed in this study identified 14 STs in E. coli strains, of which ST3 and ST88 have been previously reported in clinical isolates from hospitalized patients [50,51], which suggested that there was a genetic exchange of bacterial strains between human owners and their pets [7]. ST535, ST1017, ST1016, and ST88 showed the expression of plasmid resistance genes (β-lactamases and PMQR); ST1016 and ST1017 were identified for the first time in this study, which suggested that the population genetic diversity of bacterial clones favors antibiotic resistance through horizontal plasmid-transmission mechanisms. Additionally, our study showed that the PMQR variants (qnrB and qnrS) were coexpressed with a β-lactamase. It is noteworthy that the two strains identified as ST1017 presented coexpression of plasmid resistance genes (TEM/qnrS and TEM/qnrB), as has been found elsewhere [43,52,53,54,55]. As described previously, owners of companion animals and their family members may be at risk of acquiring bacteria with antibiotic resistance genes from their pets, especially if they are carriers of conjugative plasmids. A recent study showed that ESBL and AmpC plasmid genes acquired in the community were attributed in 7.9% of the cases to companion animals, especially dogs, which constitute the most common non-human source [12].
This study reported mutations of the AmpC gene in the promoter regions −35, −28, −18, −1, and +58 in 20% of the E. coli strains. Previous studies showed that these mutations can confer resistance to antibiotic groups such as penicillins, cephalosporins, cephamycins, and aztreonam [21,22,55]. Mutations were more prevalent among the new alleles described in this study and showed Type 2 as the most frequent pattern; this represented adaptive changes of E. coli at the environmental level due to antibiotic pressure and genetic exchange, which could contribute to the emergence of resistant strains.
The WHO established quinolones, β-lactams (such as third-, fourth-, and fifth-generation cephalosporins and amino-penicillins with and without β-lactamase inhibitors), and aminoglycosides—among others also used in veterinary medicine—as critically important antibiotics [3]. Our data showed resistance to several of the critically important antibiotics tested. This finding drew attention to potential difficulties in managing infections, as well as the importance of knowing the composition and distribution of antibiotic-resistance genotypes, as important factors in limiting the impact of AMR infections with E. coli in Panama. To the best of our knowledge, this was the first study in Central America on patterns of antimicrobial sensitivity and resistance as well as the molecular epidemiology of E. coli strains isolated from fecal samples of domestic dogs. The presence of genes from plasmids and chromosomal mutations for resistance to antibiotics, the identification of isolates previously reported in humans, and the genetic diversity of STs (including three new ones identified in this study) all revealed the determinants of resistance to antibiotics within the WHO’s One Health framework.
Author Contributions
Conceptualization, V.N.-S., G.P.-P. and I.L.; methodology, V.N.-S., G.P.-P., A.D.L.C. and I.L.; software, V.N.-S. and I.L.; validation, V.N.-S., G.P.-P., A.D.L.C. and I.L.; formal analysis, V.N.-S., G.P.-P., A.D.L.C. and I.L.; investigation, V.N.-S., G.P.-P., A.D.L.C. and I.L.; resources, V.N.-S. and I.L.; data curation, V.N.-S., G.P.-P. and I.L.; writing—original draft preparation, V.N.-S., G.P.-P. and I.L.; writing—review and editing, V.N.-S., G.P.-P. and I.L.; visualization, V.N.-S., G.P.-P. and I.L.; supervision, V.N.-S. and IL; project administration, V.N.-S. and I.L.; funding acquisition, V.N.-S. and I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was reviewed and approved by the Animal Bioethics Committee at the University of Panama: Comité de Ética de la Investigación y el Bienestar de los Animales de la Universidad de Panamá CEIBAUP (No. CEIBA-UP-027-2022, dated 11 July 2022).
Informed Consent Statement
Informed consent was obtained from all owners of dogs participating in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Humberto López Castillo and Mauricio Arcos-Holzinger for their review of the manuscript. I.L. and V.N.-S. are members of the Sistema Nacional de Investigación (SNI), which is supported by Panama’s Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pitout, J.D.D. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021. WHO: Geneva, Switzerland. Licence: CC BY-NC-SA 3.0 IGO. 2021. Available online: https://apps.who.int/iris/rest/bitstreams/1350455/retrieve (accessed on 1 February 2022).
- Critically Important Antimicrobials for Human Medicine: Categorization for the Development of Risk Management Strategies to Contain Antimicrobial Resistance Due to Non-Human Antimicrobial Use: Report of the Second WHO Expert Meeting, Copenhagen, 29–31 May 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43765/9789241595742_eng.pdf (accessed on 1 February 2022).
- Galarce, N.; Arriagada, G.; Sánchez, F.; Venegas, V.; Cornejo, J.; Lapierre, L. Antimicrobial use in companion animals: Assessing veterinarians’ prescription patterns through the first national survey in Chile. Animals 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Animal reservoirs for extended spectrum beta-lactamase producers. Clin. Microbiol. Infect. 2008, 14 (Suppl. S1), 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zogg, A.L.; Zurfluh, K.; Schmitt, S.; Nüesch-Inderbinen, M.; Stephan, R. Antimicrobial resistance, multilocus sequence types and virulence profiles of ESBL producing and non-ESBL producing uropathogenic Escherichia coli isolated from cats and dogs in Switzerland. Vet. Microbiol. 2018, 216, 79–84. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Haenni, M.; Metayer, V.; Madec, J.-Y.; Ferreira, H.M.N. Epidemic spread of IncI1/pST113 plasmid carrying the extended-spectrum beta-lactamase (ESBL) blaCTX-M-8 gene in Escherichia coli of Brazilian cattle. Vet. Microbiol. 2020, 243, 108629. [Google Scholar] [CrossRef]
- Ljungquist, O.; Ljungquist, D.; Myrenås, M.; Rydén, C.; Finn, M.; Bengtsson, B. Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs—A pilot study. Infect. Ecol. Epidemiol. 2016, 6, 31514. [Google Scholar] [CrossRef]
- Grönthal, T.; Österblad, M.; Eklund, M.; Jalava, J.; Nykäsenoja, S.; Pekkanen, K.; Rantala, M. Sharing more than friendship—Transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Euro Surveill. 2018, 23, 1700497. [Google Scholar] [CrossRef]
- The Tripartite Workplan on Antimicrobial Resistance 2019–2020: Final Draft. Available online: https://web.oie.int//downld/WG/AMR/AMR-Tripartite-Workplan-updated-08-April-2019.pdf (accessed on 1 February 2022).
- Calero Cáceres, W.R.; Núñez Arcos, E.J. Determinación de la presencia de genes de resistencia a antibióticos emergentes en aislados de Escherichia coli en caninos de la ciudad de Ambato. Bachelor’s Thesis, Universidad Técnica de Ambato, Ambato, Ecuador, 2018. Available online: https://repositorio.uta.edu.ec:8443/jspui/handle/123456789/28393 (accessed on 1 February 2022).
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.L.; Schimitt, H.; Hald, T.; Evers, E.; et al. ESBL Author Consortium. Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet Health 2019, 3, e357–e369. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Romero-Alvarez, D.; Valdez-Vélez, V.; Morales, R.D.; Montalvo-Hernande, A.; Gomes-Dias, C.; Calvopiña, M. Extended-spectrum beta-lactamases producing Escherichia coli in South America: A systematic review with a One Health Perspective. Infect. Drug Resist. 2022, 15, 5759. [Google Scholar] [CrossRef]
- Pan-American Health Organization. Magnitud y Tendencias de la Resistencia a los Antimicrobianos en América Latina. ReLAVRA 2014, 2015, 2016. Available online: https://www.paho.org/es/file/81258/download?token=apblM4AC (accessed on 18 December 2022).
- González-Zorn, B.; Escudero, J.A. Ecology of antimicrobial resistance: Humans, animals, food and environment. Int. Microbiol. 2012, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gracia, R.C.; Cortés-Cortés, G.; Lozano-Zarain, P.; Bello, F.; Martínez-Laguna, Y.; Torres, C. Faecal Escherichia coli isolates from healthy dogs harbour CTX-M-15 and CMY-2 β-lactamases. Vet. J. 2015, 203, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Karkaba, A.; Grinberg, A.; Benschop, J.; Pleydell, E. Characterisation of extended-spectrum β-lactamase and AmpC β-lactamase-producing Enterobacteriaceae isolated from companion animals in New Zealand. N. Z. Vet. J. 2017, 65, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Munita, J.M.; Rivas, L.; Garcia, P.; Listoni, F.J.P.; Moreno-Sitt, A.I.; Paes, A.C. Risk factors associated with faecal carriage of extended-spectrum cephalosporin-resistant Escherichia coli among dogs in Southeast Brazil. Prev. Vet. Med. 2021, 190, 105316. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.A.; Salgado-Caxito, M.; Opazo-Capurro, A.; González Muñoz, P.; Piñeiro, A.; Otto Medina, M.; Rivas, L.; Munita, J.; Millán, J. ESBL-producing Escherichia coli carrying CTX-M genes circulating among livestock, dogs, and wild mammals in small-scale farms of central Chile. Antibiotics 2021, 10, 510. [Google Scholar] [CrossRef]
- Hong, J.S.; Song, W.; Park, H.-M.; Oh, J.-Y.; Chae, J.-C.; Jeong, S.; Jeong, S.H. Molecular characterization of fecal extended-spectrum β-lactamase- and AmpC β-lactamase-producing Escherichia coli from healthy companion animals and cohabiting humans in South Korea. Front. Microbiol. 2020, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Caroff, N.; Espaze, E.; Bérard, I.; Richet, H.; Reynaud, A. Mutations in the ampC promoter of Escherichia coli isolates resistant to oxyiminocephalosporins without extended spectrum beta-lactamase production. FEMS Microbiol. Lett. 1999, 173, 459–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulvey, M.R.; Bryce, E.; Boyd, D.A.; Ofner-Agostini, M.; Land, A.M.; Simor, A.E.; Paton, S. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob. Agents Chemother. 2005, 49, 358–365. [Google Scholar] [CrossRef]
- Roscetto, E.; Varriale, C.; Galdiero, U.; Esposito, C.; Catania, M.R. Extended-spectrum beta-lactamase-producing and carbapenem-resistant Enterobacterales in companion and animal-assisted interventions dogs. Int. J. Environ. Res. Public Health 2021, 18, 12952. [Google Scholar] [CrossRef] [PubMed]
- Tolun, V.; Küçükbasmaci, O.; Törümküney-Akbulut, D.; Catal, C.; Anğ-Küçüker, M.; Anğ, O. Relationship between ciprofloxacin resistance and extended-spectrum beta-lactamase production in Escherichia coli and Klebsiella pneumoniae strains. Clin. Microbiol. Infect. 2004, 10, 72–75. [Google Scholar] [CrossRef]
- Corkill, J.E.; Anson, J.J.; Hart, C.A. High prevalence of the plasmid-mediated quinolone resistance determinant qnrA in multidrug-resistant Enterobacteriaceae from blood cultures in Liverpool, UK. J. Antimicrob. Chemother. 2005, 56, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats—A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef] [PubMed]
- Valat, C.; Drapeau, A.; Beurlet, S.; Bachy, V.; Boulouis, H.-J.; Pin, R.; Cazeau, G.; Madec, J.-Y.; Haenni, M. Pathogenic Escherichia coli in dogs reveals the predominance of ST372 and the human-associated st73 extra-intestinal lineages. Front. Microbiol. 2020, 11, 580. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Imirzalioglu, C.; Ghosh, H.; Gwozdzinski, K.; Schmiedel, J.; Gentil, K.; Bauefeind, R.; Kampfer, P.; Seifert, H.; Brenner Michael, G.; et al. Circulation of clonal populations of fluoroquinolone-resistant CTX-M-15-producing Escherichia coli ST410 in humans and animals in Germany. Int. J. Antimicrob. Agents 2016, 47, 457–465. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Stamm, I.; Grobbel, M.; Kopp, P.A.; Guerra, B.; Stubbe, M.; Doi, Y.; Zong, Z.; Kola, A.; et al. CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: Another pandemic clone combining multiresistance and extraintestinal virulence? J. Antimicrob. Chemother. 2014, 69, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.C.; Boisson, M.N.G.; Saras, E.; Médaille, C.; Boulouis, H.-J.; Madec, J.-Y.; Haenni, M. OXA-48-producing ST372 Escherichia coli in a French dog. J. Antimicrob. Chemother. 2017, 72, 1256–1258. [Google Scholar] [CrossRef][Green Version]
- Carvalho, A.C.; Barbosa, A.V.; Arais, L.R.; Ribeiro, P.F.; Carneiro, V.C.; Cerqueira, A.M.F. Resistance patterns, ESBL genes, and genetic relatedness of Escherichia coli from dogs and owners. Braz. J. Microbiol. 2016, 47, 150–158. [Google Scholar] [CrossRef]
- LeCuyer, T.E.; Byrne, B.A.; Daniels, J.B.; Diaz-Campos, D.V.; Hammac, G.K.; Miller, C.B.; Besser, T.E.; Davis, M.A. Population structure and antimicrobial resistance of canine uropathogenic Escherichia coli. J. Clin. Microbiol. 2018, 56, e00788-18. [Google Scholar] [CrossRef]
- Rumi, M.V.; Mas, J.; Elena, A.; Cerdeira, L.; Muñoz, M.E.; Lincopan, N.; Gentilini, E.R.; Di Conza, J.; Gutkind, G. Co-occurrence of clinically relevant β-lactamases and MCR-1 encoding genes in Escherichia coli from companion animals in Argentina. Vet. Microbiol. 2019, 230, 228–234. [Google Scholar] [CrossRef]
- Sánchez, M.D.P.; Gutiérrez, N.P.; Padilla, M.Y.; Suárez, L.L. Resistencia antimicrobiana de bacterias aisladas de clínicas veterinarias de la ciudad de Ibagué, Colombia. Univ Salud. 2015, 17, 18–31. [Google Scholar]
- Marchetti, L.; Buldain, D.; Gortari Castillo, L.; Buchamer, A.; Chirino-Trejo, M.; Mestorino, N. Pet and stray dogs as reservoirs of antimicrobial-resistant Escherichia coli. Int. J. Microbiol. 2021, 2021, 6664557. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2020; Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 1 October 2022).
- Jaureguy, F.; Landraud, L.; Passet, V.; Diancourt, L.; Frapy, E.; Guigon, G.; Carbonnelle, E.; Lortholary, O.; Clermont, O.; Denamur, E.; et al. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genom. 2008, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Maiden, M.C.J. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.R.; Soule, G.; Boyd, D.; Demczuk, W.; Ahmed, R.; Multi-provincial Salmonella Typhimurium Case Control Study Group. Characterization of the first extended-spectrum beta-lactamase-producing Salmonella isolate identified in Canada. J. Clin. Microbiol. 2003, 41, 460–462. [Google Scholar] [CrossRef]
- Olesen, I.; Hasman, H.; Møller Aarestrup, F. Prevalence of β-lactamases among ampicillin-resistant Escherichia coli and Salmonella isolated from food animals in Denmark. Microb. Drug. Resist. 2004, 10, 334–340. [Google Scholar] [CrossRef]
- Dierikx, C.M.; van Duijkeren, E.; Schoormans, A.H.W.; van Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Huijsdens, X.W.; van der Zwaluw, K.; Wagenaar, J.A.; Mevius, D.J. Occurrence and characteristics of extended-spectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2012, 67, 1368–1374. [Google Scholar] [CrossRef]
- Haenni, M.; Saras, E.; Métayer, V.; Médaille, C.; Madec, J.-Y. High prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in France. Antimicrob. Agents Chemother. 2014, 58, 5358–5362. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.; Gupta, S.K.; Sharma, P.; Ahmed, M.; Hiott, L.M.; Barrett, J.B.; Woodley, T.A.; Frye, J.G.; Jackson, C.R. Circulation of emerging NDM-5-producing Escherichia coli among humans and dogs in Egypt. Zoonoses Public Health 2020, 67, 324–329. [Google Scholar] [CrossRef]
- Dolejska, M.; Duskova, E.; Rybarikova, J.; Janoszowska, D.; Roubalova, E.; Dibdakova, K.; Maceckova, G.; Kohoutova, L.; Literak, I.; Smola, J.; et al. Plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. J. Antimicrob. Chemother. 2011, 66, 757–764. [Google Scholar] [CrossRef]
- Villegas, M.V.; Blanco, M.G.; Sifuentes-Osornio, J.; Rossi, F. Increasing prevalence of extended-spectrum-beta-lactamase among Gram-negative bacilli in Latin America—2008 update from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Braz. J. Infect. Dis. 2011, 15, 34–39. [Google Scholar] [PubMed]
- Chen, J.-W.; Huang, H.H.; Chang, S.-M.; Scaria, J.; Chiu, Y.-L.; Chen, C.-M.; Ko, W.-C.; Wang, J.-L. Antibiotic-resistant Escherichia coli and sequence type 131 in fecal colonization in dogs in Taiwan. Microorganisms 2020, 8, 1439. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.M.; Pinchbeck, G.L.; Nuttall, T.; McEwan, N.; Dawson, S.; Williams, N.J. Antimicrobial resistance risk factors and characterisation of faecal E. coli isolated from healthy Labrador retrievers in the United Kingdom. Prev. Vet. Med. 2015, 119, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Agabou, A.; Pantel, A.; Ouchenane, Z.; Lezzar, N.; Khemissi, S.; Satta, D.; Sotto, A.; Lavigne, J.-P. First description of OXA-48-producing Escherichia coli and the pandemic clone ST131 from patients hospitalised at a military hospital in Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Samudio, V.; Pecchio, M.; Pimentel-Peralta, G.; Quintero, Y.; Herrera, M.; Landires, I. Molecular epidemiology of Escherichia coli clinical isolates from Central Panama. Antibiotics 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Hase, A.; Fukuda, A.; Matsuo, M.; Horimoto, T.; Ogasawara, J. Prevalence and mechanisms of fluoroquinolone-resistant Escherichia coli among sheltered companion animals. Access Microbiol. 2020, 2, acmi000077. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Muggeo, A.; El Garch, F.; Moyaert, H.; de Champs, C.; Guillard, T. Characterization of quinolone resistance mechanisms in Enterobacteriaceae isolated from companion animals in Europe (ComPath II study). Vet. Microbiol. 2018, 216, 159–167. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Li, Y.; Hao, C. High Prevalence of β-lactamase and plasmid-mediated quinolone resistance genes in extended-spectrum cephalosporin-resistant Escherichia coli from dogs in Shaanxi, China. Front. Microbiol. 2016, 7, 1843. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Younas, S.; Abosalif, K.O.A.; Junaid, K.; Alzahrani, B.; Alsrhani, A.; Abdalla, A.E.; Ullah, M.I.; Qamar, M.U.; Hamam, S.S.M. Molecular analysis of blaSHV, blaTEM, and blaCTX-M in extended-spectrum β-lactamase producing Enterobacteriaceae recovered from fecal specimens of animals. PLoS ONE 2021, 16, e0245126. [Google Scholar] [CrossRef]
- Harada, K.; Nakai, Y.; Kataoka, Y. Mechanisms of resistance to cephalosporin and emergence of O25b-ST131 clone harboring CTX-M-27 β-lactamase in extraintestinal pathogenic Escherichia coli from dogs and cats in Japan. Microbiol. Immunol. 2012, 56, 480–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).