Recombinant Oil-Body-Expressed Oleosin-hFGF5 in Arabidopsis thaliana Regulates Hair Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of pOTB-rhFGF5 Vector
2.2. Arabidopsis Transformation and Screening of Transgenic Lines
2.3. Recombinant Oil Body Extraction
2.4. Cell Proliferation Experiment
2.5. Establishment of a Hair Loss Mice Model and Drug Treatment
2.6. H&E Staining Analysis
2.7. Immunohistochemical Staining
2.8. Western Blot Experiment
2.9. RNA-Seq Analysis
2.10. Statistical Analysis
3. Results
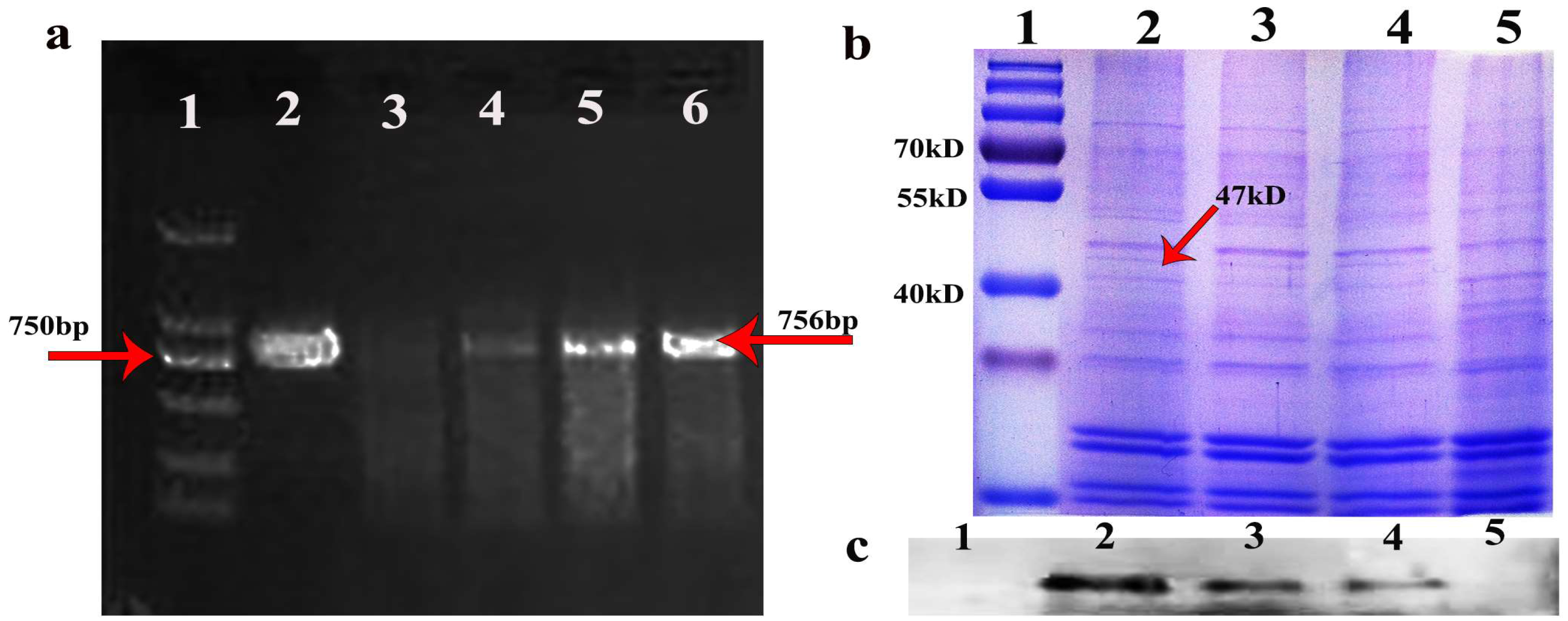
3.1. Oleosin-hFGF5 Was Successfully Expressed in Oil Body of Arabidopsis
3.2. Recombinant Oil-Body-Expressed hFGF5 Inhibits the Proliferation of Hair Follicle Epithelial Cells In Vitro
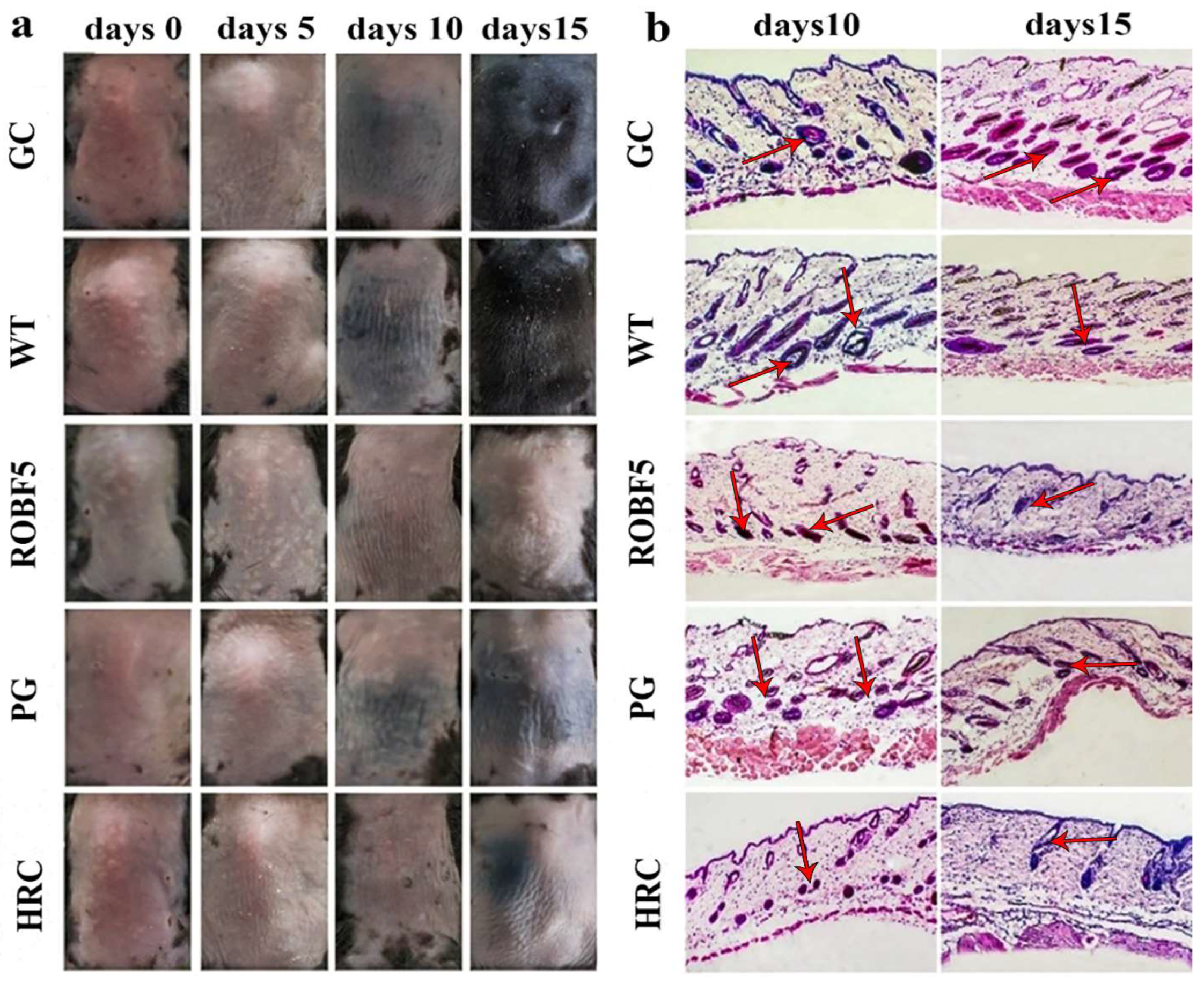
3.3. Recombinant Oil-Body-Expressed Oleosin-hFGF5 Inhibits Hair Regeneration in Mice In Vivo
3.4. Effect of Recombinant Oil Body Expressed hFGF5 on Cytokeratin 14
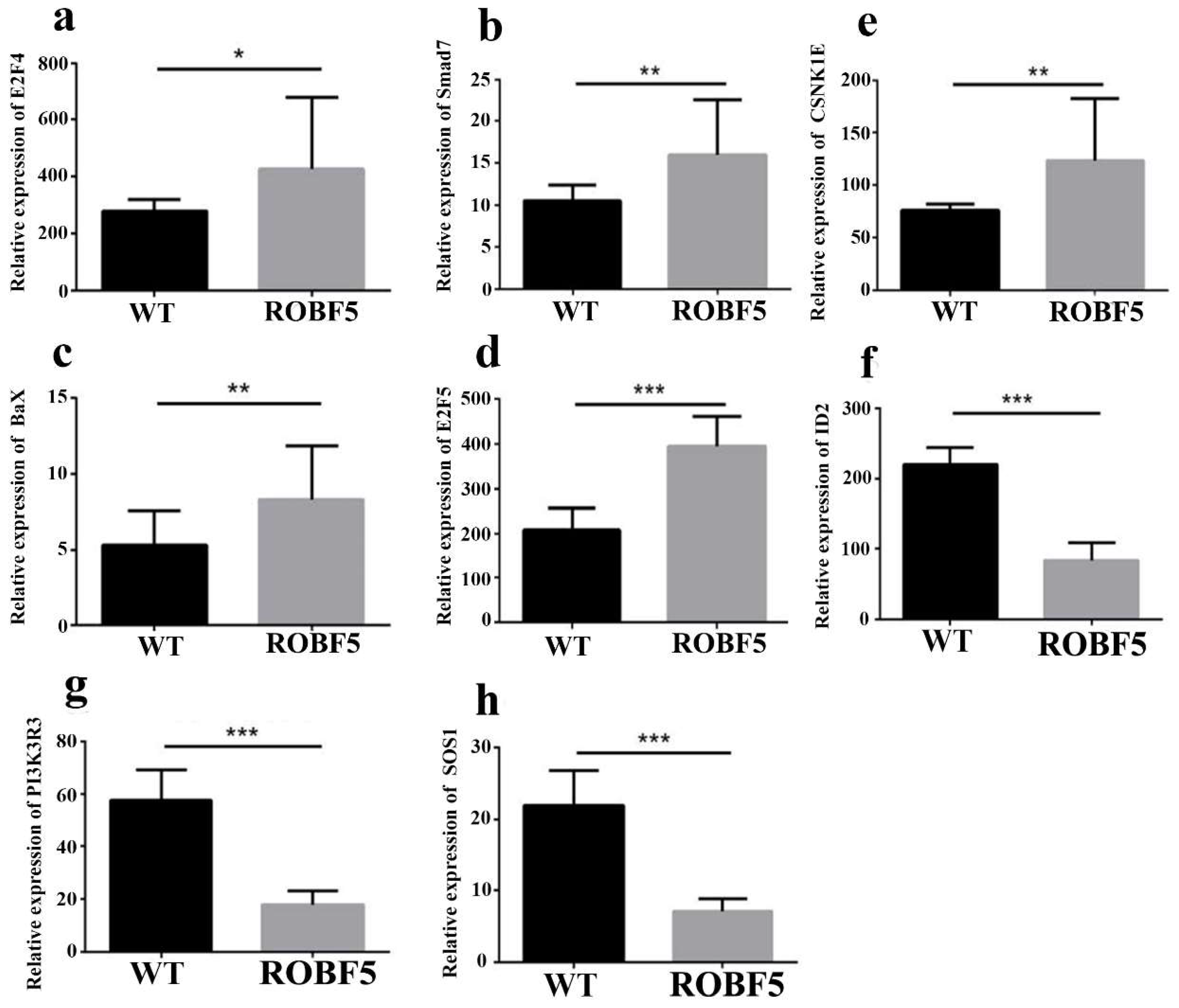
3.5. Differentially Expressed Genes by the Treatment of Recombinant Oil-Body-Expressed hFGF5
3.6. The Mechanism of Recombinant Oil-Body-Expressed hFGF5 Inhibiting Hair Growth
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huang, A.H.C. Oil bodies and oleosins in seeds. Annu. Rev. Plant Biol. 1992, 43, 177–200. [Google Scholar] [CrossRef]
- Siloto, R.M.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The accumulation of oleosins determines the size of seed oil bodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, F.; Wilkinson, B.M.; Stirling, C.J.; Napier, J.A. In vivo targeting of a sunflower oil body protein in yeast secretory (sec) mutants. Plant J. 2000, 23, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ling, H. Oleosin fusion expression systems for the production of recombinant proteins. Biologia 2007, 62, 119–123. [Google Scholar] [CrossRef]
- Qiang, W.; Zhou, T.; Lan, X.; Zhang, X.; Guo, Y.; Noman, M.; Du, L.; Zheng, J.; Li, W.; Li, H.; et al. A new nanoscale transdermal drug delivery system: Oil body-linked oleosin-hEGF improves skin regeneration to accelerate wound healing. J. Nanobiotechnology 2018, 16, 62–80. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Cai, J.; Wang, H.; Tian, H.; Huang, J.; Qiang, W.; Zhang, L.; Li, H.; Li, X.; et al. Oil Body-Bound Oleosin-rhFGF-10: A Novel Drug Delivery System that Improves Skin Penetration to Accelerate Wound Healing and Hair Growth in Mice. Int. J. Mol. Sci. 2017, 18, 2177. [Google Scholar] [CrossRef]
- Yang, J.; Guan, L.; Guo, Y.; Du, L.; Wang, F.; Wang, Y.; Zhen, L.; Wang, Q.; Zou, D.; Chen, W.; et al. Expression of biologically recombinant human acidic fibroblast growth factor in Arabidopsis thaliana seeds via oleosin fusion technology. Gene 2015, 566, 89–94. [Google Scholar] [CrossRef]
- Yang, J.; Qiang, W.; Ren, S.; Yi, S.; Li, J.; Guan, L.; Du, L.; Guo, Y.; Hu, H.; Li, H.; et al. High-efficiency production of bioactive oleosin-basic fibroblast growth factor in A. thaliana and evaluation of wound healing. Gene 2018, 639, 69–76. [Google Scholar] [CrossRef]
- Qiang, W.; Gao, T.; Lan, X.; Guo, J.; Noman, M.; Li, Y.; Guo, Y.; Kong, J.; Li, H.; Du, L.; et al. Molecular Pharming of the Recombinant Protein hEGF-hEGF Concatenated with Oleosin Using Transgenic Arabidopsis. Genes 2020, 11, 959. [Google Scholar] [CrossRef]
- Allerstorfer, S.; Sonvilla, G.; Fischer, H.; Spiegl-Kreinecker, S.; Gauglhofer, C.; Setinek, U.; Czech, T.; Marosi, C.; Buchroithner, J.; Pichler, J.; et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: Autocrine and paracrine activities. Oncogene 2008, 27, 4180–4190. [Google Scholar] [CrossRef]
- Clase, K.L.; Mitchell, P.J.; Ward, P.J.; Dorman, C.M.; Johnson, S.E.; Hannon, K. FGF5 stimulates expansion of connective tissue fibroblasts and inhibits skeletal muscle development in the limb. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 219, 368–380. [Google Scholar] [CrossRef]
- Ren, Y.; Jiao, X.; Zhang, L. Expression level of fibroblast growth factor 5 (FGF5) in the peripheral blood of primary hypertension and its clinical significance. Saudi J. Biol. Sci. 2018, 25, 469–473. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chao, Y.; Zhou, G.; Chen, Y. Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hair follicle dermal papilla cells of cashmere goats. Gene Int. J. Focus. Gene Cloning Gene Struct. Funct. 2016, 575 Pt 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.E. The hair cycle. Sitri. It. 2006, 119, 391–393. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, K.H.; Lee, W.J.; Jung, J.W.; Song, K.Y.; Suhr, K.B.; Lee, J.H.; Park, J.K. Growth Factors Influencing the Morphology and Growth Rate of Hair Follicles in a Human Hair Organ Culture. Korean J. Dermatol. 1998, 36, 210–216. [Google Scholar]
- Lin, H.J.; Qi, J.M.; Xu, N.; Ding, M.; Chen, Y.; Li, X.N.; Hu, Z.L. Progress in the Regulation of FGF5 on Hair Follicle Cycle. Chin. J. Aesthetic Med. 2019, 11, 163–164. [Google Scholar]
- Keum, D.I.; Pi, L.Q.; Hwang, S.T.; Lee, W.S. Protective effect of Korean Red Ginseng against chemotherapeutic drug-induced premature catagen development assessed with human hair follicle organ culture model. J. Ginseng Res. 2016, 40, 169–175. [Google Scholar] [CrossRef]
- Millar, S.E. Smad7: Licensed to kill β-catenin. Dev. Cell 2006, 11, 274–276. [Google Scholar] [CrossRef]
- Sulayman, A.; Tian, K.; Huang, X.; Tian, Y.; Xu, X.; Fu, X.; Zhao, B.; Wu, W.; Wang, D.; Yasin, A.; et al. Genome-wide identification and characterization of long non-coding RNAs expressed during sheep fetal and postnatal hair follicle development. Sci. Rep. (Nat. Publ. Group) 2019, 9, 8501. [Google Scholar] [CrossRef] [PubMed]
- Hammond NJahoda, L.C. Id2, Id3, and Id4 proteins show dynamic changes in expression during vibrissae follicle development. Dev. Dyn. 2010, 237, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.-H.; Chen, J.; Gao, W.; Zhang, J.-Y.; Quan, F.-S.; Hu, J.-P.; Yuan, B.; Ren, W.-Z. Cutaneous transcriptome analysis in NIH hairless mice. PLoS ONE 2017, 12, e0182463–e0182471. [Google Scholar] [CrossRef] [PubMed]
- Amberg, N.; Sotiropoulou, P.A.; Heller, G.; Lichtenberger, B.M.; Holcmann, M.; Camurdanoglu, B.; Baykuscheva-Gentscheva, T.; Blanpain, C.; Sibilia, M. EGFR Controls Hair Shaft Differentiation in a p53-Independent Manner. iScience 2019, 15, 243–256. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lan, X.; Noman, M.; Wang, Z.; Zhang, J. Recombinant Oil-Body-Expressed Oleosin-hFGF5 in Arabidopsis thaliana Regulates Hair Growth. Genes 2023, 14, 21. https://doi.org/10.3390/genes14010021
Wang H, Lan X, Noman M, Wang Z, Zhang J. Recombinant Oil-Body-Expressed Oleosin-hFGF5 in Arabidopsis thaliana Regulates Hair Growth. Genes. 2023; 14(1):21. https://doi.org/10.3390/genes14010021
Chicago/Turabian StyleWang, Hongyu, Xinxin Lan, Muhammad Noman, Ze Wang, and Jing Zhang. 2023. "Recombinant Oil-Body-Expressed Oleosin-hFGF5 in Arabidopsis thaliana Regulates Hair Growth" Genes 14, no. 1: 21. https://doi.org/10.3390/genes14010021
APA StyleWang, H., Lan, X., Noman, M., Wang, Z., & Zhang, J. (2023). Recombinant Oil-Body-Expressed Oleosin-hFGF5 in Arabidopsis thaliana Regulates Hair Growth. Genes, 14(1), 21. https://doi.org/10.3390/genes14010021






