MiT/TFE Family Renal Cell Carcinoma
Abstract
1. The Microphthalmia/Transcription Factor E (MiT/TFE) Family
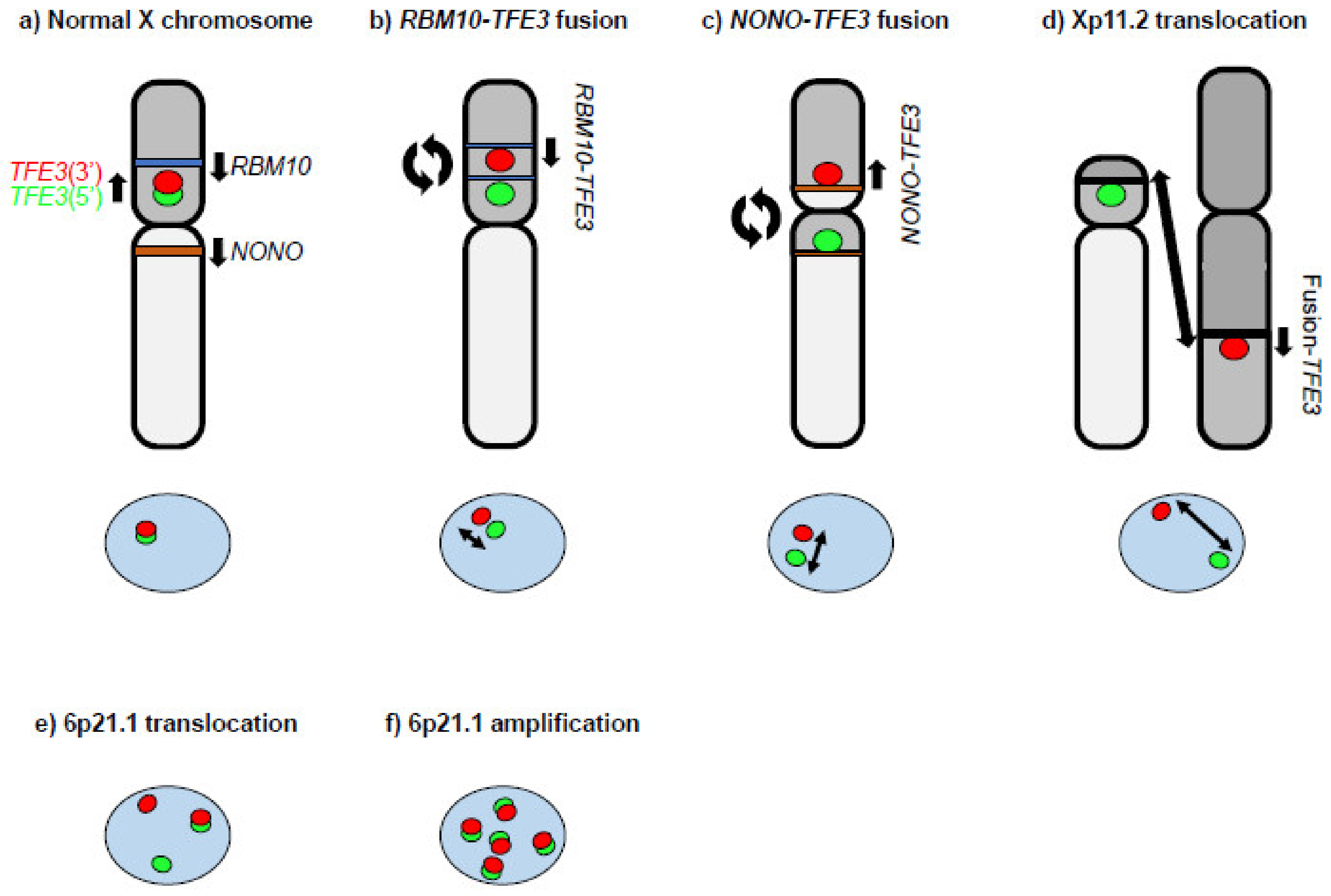
2. MiT Family Translocation Renal Cell Carcinoma (tRCC)
3. MITF p.E318K RCC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goding, C.R.; Arnheiter, H. MITF-the first 25 years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- La Spina, M.; Contreras, P.S.; Rissone, A.; Meena, N.K.; Jeong, E.; Martina, J.A. MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front. Cell Dev. Biol. 2020, 8, 609683. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, H.; Kadesch, T. The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev. 1991, 5, 1057–1066. [Google Scholar] [CrossRef]
- Hemesath, T.J.; Steingrimsson, E.; McGill, G.; Hansen, M.J.; Vaught, J.; Hodgkinson, C.A.; Arnheiter, H.; Copeland, N.G.; Jenkins, N.A.; Fisher, D.E. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994, 8, 2770–2780. [Google Scholar] [CrossRef]
- Strub, T.; Giuliano, S.; Ye, T.; Bonet, C.; Keime, C.; Kobi, D.; Le Gras, S.; Cormont, M.; Ballotti, R.; Bertolotto, C.; et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene 2011, 30, 2319–2332. [Google Scholar] [CrossRef]
- Aksan, I.; Goding, C.R. Targeting the microphthalmia basic helix-loop-helix-leucine zipper transcription factor to a subset of E-box elements in vitro and in vivo. Mol. Cell. Biol. 1998, 18, 6930–6938. [Google Scholar] [CrossRef]
- Pogenberg, V.; Ogmundsdottir, M.H.; Bergsteinsdottir, K.; Schepsky, A.; Phung, B.; Deineko, V.; Milewski, M.; Steingrimsson, E.; Wilmanns, M. Restricted leucine zipper dimerization and specificity of DNA recognition of the melanocyte master regulator MITF. Genes Dev. 2012, 26, 2647–2658. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Argani, P. Translocation carcinomas of the kidney. Genes Chromosomes Cancer 2022, 61, 219–227. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Amin, M.B.; Berney, D.M.; Comperat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Sukov, W.R.; Hodge, J.C.; Lohse, C.M.; Leibovich, B.C.; Thompson, R.H.; Pearce, K.E.; Wiktor, A.E.; Cheville, J.C. TFE3 rearrangements in adult renal cell carcinoma: Clinical and pathologic features with outcome in a large series of consecutively treated patients. Am. J. Surg. Pathol. 2012, 36, 663–670. [Google Scholar] [CrossRef]
- Komai, Y.; Fujiwara, M.; Fujii, Y.; Mukai, H.; Yonese, J.; Kawakami, S.; Yamamoto, S.; Migita, T.; Ishikawa, Y.; Kurata, M.; et al. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry. Clin. Cancer Res. 2009, 15, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; De Angelo, P.; Osborne, L.; Paniz-Mondolfi, A.E.; Geller, M.; Yang, Y.; Linehan, W.M.; Merino, M.J.; Cordon-Cardo, C.; Cai, D. Translocation renal cell carcinomas in adults: A single-institution experience. Am. J. Surg. Pathol. 2012, 36, 654–662. [Google Scholar] [CrossRef]
- Bakouny, Z.; Sadagopan, A.; Ravi, P.; Metaferia, N.Y.; Li, J.; AbuHammad, S.; Tang, S.; Denize, T.; Garner, E.R.; Gao, X.; et al. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep. 2022, 38, 110190. [Google Scholar] [CrossRef]
- Sun, G.; Chen, J.; Liang, J.; Yin, X.; Zhang, M.; Yao, J.; He, N.; Armstrong, C.M.; Zheng, L.; Zhang, X.; et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat. Commun. 2021, 12, 5262. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wu, X.; Anwaier, A.; Feng, J.; Xu, W.; Pei, X.; Zhu, Y.; Liu, Y.; Bai, L.; Yang, G.; et al. Proteogenomic characterization of MiT family translocation renal cell carcinoma. Nat. Commun. 2022, 13, 7494. [Google Scholar] [CrossRef]
- Van der Beek, J.N.; Hol, J.A.; Coulomb-l’Hermine, A.; Graf, N.; van Tinteren, H.; Pritchard-Jones, K.; Houwing, M.E.; de Krijger, R.R.; Vujanic, G.M.; Dzhuma, K.; et al. Characteristics and outcome of pediatric renal cell carcinoma patients registered in the International Society of Pediatric Oncology (SIOP) 93-01, 2001 and UK-IMPORT database: A report of the SIOP-Renal Tumor Study Group. Int. J. Cancer 2021, 148, 2724–2735. [Google Scholar] [CrossRef]
- Van der Beek, J.N.; Geller, J.I.; de Krijger, R.R.; Graf, N.; Pritchard-Jones, K.; Drost, J.; Verschuur, A.C.; Murphy, D.; Ray, S.; Spreafico, F.; et al. Characteristics and Outcome of Children with Renal Cell Carcinoma: A Narrative Review. Cancers 2020, 12, 1776. [Google Scholar] [CrossRef]
- Argani, P. MiT family translocation renal cell carcinoma. Semin. Diagn. Pathol. 2015, 32, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.C.; Ricketts, C.J.; Rais-Bahrami, S.; Yang, Y.; Merino, M.J.; Bottaro, D.P.; Srinivasan, R.; Linehan, W.M. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat. Rev. Urol. 2014, 11, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Mikami, S.; Pan, C.C.; Cohen, R.J.; Hes, O.; Michal, M.; Nagashima, Y.; Tanaka, Y.; Inoue, K.; Shuin, T.; et al. Review of renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions with focus on pathobiological aspect. Histol. Histopathol. 2012, 27, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Caliò, A.; Brunelli, M.; Segala, D.; Pedron, S.; Remo, A.; Ammendola, S.; Munari, E.; Pierconti, F.; Mosca, A.; Bollito, E.; et al. Comprehensive analysis of 34 MiT family translocation renal cell carcinomas and review of the literature: Investigating prognostic markers and therapy targets. Pathology 2020, 52, 297–309. [Google Scholar] [CrossRef]
- Zhong, M.; De Angelo, P.; Osborne, L.; Keane-Tarchichi, M.; Goldfischer, M.; Edelmann, L.; Yang, Y.; Linehan, W.M.; Merino, M.J.; Aisner, S.; et al. Dual-color, break-apart FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of Xp11 translocation renal cell carcinoma and alveolar soft part sarcoma. Am. J. Surg. Pathol. 2010, 34, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Yonescu, R.; Morsberger, L.; Morris, K.; Netto, G.J.; Smith, N.; Gonzalez, N.; Illei, P.B.; Ladanyi, M.; Griffin, C.A. Molecular confirmation of t(6;11)(p21;q12) renal cell carcinoma in archival paraffin-embedded material using a break-apart TFEB FISH assay expands its clinicopathologic spectrum. Am. J. Surg. Pathol. 2012, 36, 1516–1526. [Google Scholar] [CrossRef]
- Skala, S.L.; Xiao, H.; Udager, A.M.; Dhanasekaran, S.M.; Shukla, S.; Zhang, Y.; Landau, C.; Shao, L.; Roulston, D.; Wang, L.; et al. Detection of 6 TFEB-amplified renal cell carcinomas and 25 renal cell carcinomas with MITF translocations: Systematic morphologic analysis of 85 cases evaluated by clinical TFE3 and TFEB FISH assays. Mod. Pathol. 2018, 31, 179–197. [Google Scholar] [CrossRef]
- Baba, M.; Furuya, M.; Motoshima, T.; Lang, M.; Funasaki, S.; Ma, W.; Sun, H.W.; Hasumi, H.; Huang, Y.; Kato, I.; et al. TFE3 Xp11.2 Translocation Renal Cell Carcinoma Mouse Model Reveals Novel Therapeutic Targets and Identifies GPNMB as a Diagnostic Marker for Human Disease. Mol. Cancer Res. 2019, 17, 1613–1626. [Google Scholar] [CrossRef]
- Kato, I.; Furuya, M.; Baba, M.; Kameda, Y.; Yasuda, M.; Nishimoto, K.; Oyama, M.; Yamasaki, T.; Ogawa, O.; Niino, H.; et al. RBM10-TFE3 renal cell carcinoma characterised by paracentric inversion with consistent closely split signals in break-apart fluorescence in-situ hybridisation: Study of 10 cases and a literature review. Histopathology 2019, 75, 254–265. [Google Scholar] [CrossRef]
- Liu, N.; Guo, W.; Shi, Q.; Zhuang, W.; Pu, X.; Chen, S.; Qu, F.; Xu, L.; Zhao, X.; Li, X.; et al. The suitability of NONO-TFE3 dual-fusion FISH assay as a diagnostic tool for NONO-TFE3 renal cell carcinoma. Sci. Rep. 2020, 10, 16361. [Google Scholar] [CrossRef]
- Gupta, S.; Argani, P.; Jungbluth, A.A.; Chen, Y.B.; Tickoo, S.K.; Fine, S.W.; Gopalan, A.; Al-Ahmadie, H.A.; Sirintrapun, S.J.; Sanchez, A.; et al. TFEB Expression Profiling in Renal Cell Carcinomas: Clinicopathologic Correlations. Am. J. Surg. Pathol. 2019, 43, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.Y.; Wang, X.T.; Fang, R.; Wang, Z.; Zhao, M.; Chen, H.; Chen, N.; Teng, X.D.; Wang, X.; Wei, X.; et al. Clinicopathologic and Molecular Analysis of the TFEB Fusion Variant Reveals New Members of TFEB Translocation Renal Cell Carcinomas (RCCs): Expanding the Genomic Spectrum. Am. J. Surg. Pathol. 2020, 44, 477–489. [Google Scholar] [CrossRef]
- Antic, T.; Taxy, J.B.; Alikhan, M.; Segal, J. Melanotic Translocation Renal Cell Carcinoma With a Novel ARID1B-TFE3 Gene Fusion. Am. J. Surg. Pathol. 2017, 41, 1576–1580. [Google Scholar] [CrossRef]
- Huang, W.; Goldfischer, M.; Babyeva, S.; Mao, Y.; Volyanskyy, K.; Dimitrova, N.; Fallon, J.T.; Zhong, M. Identification of a novel PARP14-TFE3 gene fusion from 10-year-old FFPE tissue by RNA-seq. Genes Chromosomes Cancer 2015, 54, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Malouf, G.G.; Su, X.; Yao, H.; Gao, J.; Xiong, L.; He, Q.; Comperat, E.; Couturier, J.; Molinie, V.; Escudier, B.; et al. Next-generation sequencing of translocation renal cell carcinoma reveals novel RNA splicing partners and frequent mutations of chromatin-remodeling genes. Clin. Cancer Res. 2014, 20, 4129–4140. [Google Scholar] [CrossRef]
- Wei, S.; Testa, J.R.; Argani, P. A review of neoplasms with MITF/MiT family translocations. Histol. Histopathol. 2022, 37, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef]
- Cronin, J.C.; Wunderlich, J.; Loftus, S.K.; Prickett, T.D.; Wei, X.; Ridd, K.; Vemula, S.; Burrell, A.S.; Agrawal, N.S.; Lin, J.C.; et al. Frequent mutations in the MITF pathway in melanoma. Pigment Cell Melanoma Res. 2009, 22, 435–444. [Google Scholar] [CrossRef]
- Yokoyama, S.; Woods, S.L.; Boyle, G.M.; Aoude, L.G.; MacGregor, S.; Zismann, V.; Gartside, M.; Cust, A.E.; Haq, R.; Harland, M.; et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 2011, 480, 99–103. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Syed, J.S.; Espenschied, C.R.; LaDuca, H.; Bhagat, A.M.; Suarez-Sarmiento, A.; O’Rourke, T.K., Jr.; Brierley, K.L.; Hofstatter, E.W.; Shuch, B. Advances in the diagnosis of hereditary kidney cancer: Initial results of a multigene panel test. Cancer 2017, 123, 4363–4371. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Vocke, C.D.; Ricketts, C.J.; Metwalli, A.R.; Ball, M.W.; Schmidt, L.S.; Linehan, W.M. Clinical and Molecular Characterization of Microphthalmia-associated Transcription Factor (MITF)-related Renal Cell Carcinoma. Urology 2021, 149, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Levy, C.; Davis, I.J.; Razin, E.; Fisher, D.E. Sumoylation of MITF and its related family members TFE3 and TFEB. J. Biol. Chem. 2005, 280, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Arnheiter, H. Sumoylation modulates transcriptional activity of MITF in a promoter-specific manner. Pigment. Cell Res. 2005, 18, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef]
- Rosonina, E. A conserved role for transcription factor sumoylation in binding-site selection. Curr. Genet. 2019, 65, 1307–1312. [Google Scholar] [CrossRef]
- Bonet, C.; Luciani, F.; Ottavi, J.F.; Leclerc, J.; Jouenne, F.M.; Boncompagni, M.; Bille, K.; Hofman, V.; Bossis, G.; Marco de Donatis, G.; et al. Deciphering the Role of Oncogenic MITFE318K in Senescence Delay and Melanoma Progression. J. Natl. Cancer Inst. 2017, 109, djw340. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Genetic predisposition to kidney cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef]
- Hong, S.B.; Oh, H.; Valera, V.A.; Baba, M.; Schmidt, L.S.; Linehan, W.M. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS ONE 2010, 5, e15793. [Google Scholar] [CrossRef]
- Alesi, N.; Akl, E.W.; Khabibullin, D.; Liu, H.J.; Nidhiry, A.S.; Garner, E.R.; Filippakis, H.; Lam, H.C.; Shi, W.; Viswanathan, S.R.; et al. TSC2 regulates lysosome biogenesis via a non-canonical RAGC and TFEB-dependent mechanism. Nat. Commun. 2021, 12, 4245. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Baba, M. MiT/TFE Family Renal Cell Carcinoma. Genes 2023, 14, 151. https://doi.org/10.3390/genes14010151
Tang J, Baba M. MiT/TFE Family Renal Cell Carcinoma. Genes. 2023; 14(1):151. https://doi.org/10.3390/genes14010151
Chicago/Turabian StyleTang, Jinglong, and Masaya Baba. 2023. "MiT/TFE Family Renal Cell Carcinoma" Genes 14, no. 1: 151. https://doi.org/10.3390/genes14010151
APA StyleTang, J., & Baba, M. (2023). MiT/TFE Family Renal Cell Carcinoma. Genes, 14(1), 151. https://doi.org/10.3390/genes14010151





