Abstract
Glaucoma is the leading cause of irreversible blindness worldwide. Primary open-angle glaucoma (POAG), the most common glaucoma subtype, is more prevalent and severe in individuals of African ancestry. Unfortunately, this ancestral group has been historically under-represented among genetic studies of POAG. Moreover, both genetic and polygenic risk scores (GRS, PRS) that are typically based on genetic data from European-descent populations are not transferable to individuals without a majority of European ancestry. Given the aspirations of leveraging genetic information for precision medicine, GRS and PRS demonstrate clinical potential but fall short, in part due to the lack of diversity in these studies. Prioritizing diversity in the discovery of risk variants will improve the performance and utility of GRS and PRS-derived risk estimation for disease stratification, which could bring about earlier POAG intervention and treatment for a disease that often goes undetected until significant damage has occurred.
1. Introduction
Glaucoma is the leading cause of irreversible blindness worldwide [1]; primary open-angle glaucoma (POAG) is the most common subtype. POAG is a degenerative optic nerve disease characterized by progressive peripheral vision loss that results in complete and irreversible blindness without early diagnosis and treatment. POAG is a complex, multifactorial disease, for which genetic factors are one of the most prominent culprits, given the high heritability (ranging from 0.17–0.81 [2,3]). Others have extensively reviewed the current state of the glaucoma genetic literature [4,5]. We focus herein on genetic and polygenic risk scores as well as the importance of diversity in polygenic risk of POAG.
2. Genetic and Polygenic Risk Scores
Genome-wide association studies (GWAS) have identified associations between hundreds of thousands of potential loci and thousands of phenotypes, with many loci conferring very small effect sizes [6], consequently limiting clinical applications of individual variants. Therefore, a need has emerged to aggregate the effects of individual risk loci into a cumulative risk estimate. In recent years, genetic risk scores (GRS) and polygenic risk scores (PRS) have become an area of focus [7]. GRS and PRS are similar in that they combine effects at associated loci into a single measure. GRS commonly include variants that show a statistically significant association at the genome-wide level. PRS aggregate genome-wide variants and additional risk variants spanning the genome to capture the additive effects of genome-wide variation. PRS cast a wider net and have shown promise for predicting the genetic risk of complex diseases [8] including breast cancer [9], cardiovascular [10], and Alzheimer’s [11] diseases. As clinical translational tools, GRS and PRS could expedite the attainment of precision medicine [12]. With further refinement, clinicians may be able to better estimate patient risk for disease and identify appropriate personalized prevention or disease management measures [13].
Glaucoma is a disease for which early screening is beneficial; due to the high heritability of glaucoma, a goal in the field is to expand early detection methods to include screening via genetics. In glaucoma, variation in MYOC can cause early-onset glaucoma. Indeed, the clinical utility of screening for POAG-causing MYOC variants has been shown [14] though in a study design not necessarily reflective of a typical clinical setting. Furthermore, MYOC-driven glaucoma is one of the few that occurs early in life and where variation in a single gene can have a significant effect. By contrast, most genetic variants that influence glaucoma arise from combined factors or interactions that would have a negligible effect if presented singularly. Growing evidence indicates the likely future utility of GRS and PRS in POAG prediction and classification [15,16]. Despite crucial yet incremental steps toward clinical utility, the broad application of GRS/PRS remains limited by many factors, including the fact that POAG risk variants identified through GWAS are not all causative, functional variants. Therefore, the future implementation of GRS and PRS in POAG hinges on the discovery of more genetic loci. Furthermore, while individual genetic screening can be performed via DNA sequencing, the diagnostic yield remains low (20%) [17]. Generalized genetic screening for POAG may be useful for those over the age of 50, as recent studies have shown an area under the receiver operating characteristic curve score of 0.76 [17]. However, barriers to this type of genetic testing include the following: (i) individual variants often have a small effect compared to sociodemographic or environmental factors, (ii) the sensitivity and specificity of these tests do not align with cutoffs for cost-effectiveness, and (iii) each proposed test needs additional validation in a clinical setting. When considering screening for populations younger than 50 years of age, more genetic risk factors will need to be discovered to propose and implement a clinically useful model. Importantly, and as the main focus of this overview, the genetic diversity in these studies is sparse, limiting the generalizability to other ancestral groups. While expanding diversity is a key priority in the field, an exact timeline for a predictive tool cannot be established at this time.
3. Limited Cross-Ancestry Application of Genetic and Polygenic Risk Scores
Upwards of 80% of GWAS were performed in populations of predominantly European ancestry [12,18,19]. In the construction of GRS and PRS, the use of base data (GWAS results) from European-descent populations typically leads to worse predictive ability in diverse (non-majority European ancestry) populations. This likely reflects the different effects that variants may have and the different allele frequencies at which those variants are present in geographically separated and/or isolated ancestral groups [12,19,20,21]. The predictive value of European-derived PRS for complex phenotypes such as lipid traits, cardiometabolic diseases (coronary artery disease and type 2 diabetes), and cancer is significantly diminished in diverse populations (e.g., [20,21,22,23,24]). PRS performance improves when risk variants discovered in diverse ancestral groups are included [19]. Therefore, the next logical step to improve PRS performance in diverse groups is to expand the diversity of samples in the underlying GWAS analyses.
In an early attempt to emphasize the need for diverse representation in POAG genetic studies, Liu and colleagues evaluated POAG-associated variants discovered in European-descent individuals in a sample of majority African ancestry individuals [25]. Unsurprisingly, the 57 single nucleotide polymorphisms (SNPs) including well-established SNPs in CDKN2B-AS1, TMCO1, CAV1/CAV2, chromosome 8q22 intergenic region, and SIX1/SIX6 were not as significantly associated with POAG in a majority African-descent samples. It logically follows that applying a GRS based on the majority of existing literature would yield a risk-screening tool that is not universally applicable across diverse ancestral groups.
4. Representation of Populations of African Descent in Primary Open-Angle Glaucoma Studies
Historically, POAG GWAS have predominantly been performed in samples with an overwhelming majority of European ancestry [26]. Unsurprisingly, most POAG loci identified in this way demonstrate a stronger association with POAG risk in European-descent than African-descent individuals (e.g., [27,28]). Given the variability in allele frequency, linkage disequilibrium (LD), and underlying genetic architecture between ancestral populations, limited cross-ancestry variation is to be expected [29]. Others have consistently shown that the failure of known POAG loci to replicate at genome-wide significance in POAG GWAS of African ancestry populations [27,28,30,31,32,33] results, in part because of reduced statistical power due to admixture. Population diversity in Africa raises similar challenges to genetic analysis [34]. These findings likely reflect real differences in the genetic architecture of POAG among populations with global African genetic ancestry.
In the first glaucoma GWAS and admixture analysis in African Americans, Hoffman and colleagues detected an association between African ancestry and glaucoma incidence in African Americans in the Women’s Health Initiative (WHI) [30]. Furthermore, known POAG variants failed to replicate. A GRS of 10 POAG lead variants identified in European-descent GWAS was only nominally significant in African American prevalent cases.
The first POAG GWAS performed in continental African participants, the Genetics in Glaucoma patients of African descent (GIGA) study, validated TXNRD2, CDKN2B-AS1, and TMCO1 loci previously reported to confer POAG risk in Europeans [31]. Additionally, they identified a novel association between POAG and EXOC4, a ubiquitously expressed gene involved in exocytosis. Replication of this effect was not achieved due to the rarity of this variant in other continental African populations, for which small sample sizes were available. To investigate GRS performance across different ancestries, they tested how POAG risk variants identified from European and Asian populations fared in estimating risk in an African cohort. The GRS, comprised of variants for which allele frequencies varied greatly between the groups, was significantly associated with POAG but only explained four per cent of the variance after adjustment.
The African Descent and Glaucoma Evaluation Study (ADAGES) III GWAS detected a putative novel association with advanced glaucoma at EN04 [35]. Association or trending association was observed at known European ancestry-associated loci, including: 9p21 (CDKN2BAS), FNDC3B, 8q22, AFAP1, and TMCO1. However, LD and conditional analyses indicated potential independence from previously reported SNPs. A 400-SNP PRS based on these results had an area under the receiver operating characteristic curve of 0.94 and performed significantly better than GRS based on European-descent POAG loci. This is not surprising given that base and test data overlapped, a practice that is generally avoided in contemporary GRS and PRS analyses.
The unique genetic architecture of POAG in African-descent populations is also supported by the discovery of a POAG locus unique to individuals of African ancestry in the Genetics of Glaucoma in People of African Descent (GGLAD) Consortium [32]. GGLAD identified a novel POAG risk locus at amyloid-β A4 precursor protein-binding family B member 2 (APBB2), an association that was replicated in multiple independent datasets. Importantly, while the rs59892895*C risk allele was present at an appreciable minor allele frequency in African ancestry populations, it was less than 0.1% in individuals of European or Asian ancestry. It would follow, then, that ancestry is a major key to understanding genetic risk for POAG among African and African-descent samples, and there are African genetic signals that have yet to be detected. Furthermore, a subset of known POAG loci (26 variants identified in individuals of European and Asian ancestry) was evaluated in the GGLAD study via inverse-variance, fixed-effects meta-analysis. Odd ratios (OR) for 23 of 26 variants were smaller in African than European ancestry individuals, demonstrating both the utility of African-descent discovery analyses and the lack of transferability between populations for a subset of POAG loci identified in studies of European and Asian ancestry groups.
The Primary Open-Angle African American Glaucoma Genetics (POAAGG) study performed GWAS and admixture mapping in their cohort. Significant differences in case-control ancestry were detected, and a 23-SNP GRS was significant, providing evidence that known POAG SNPs together with omni-genic ancestry effects influence POAG risk [27,36].
Importantly, more recent large-scale, global efforts to elucidate the genetic architecture of POAG have included samples from populations of diverse ancestry. The most contemporary, which included the GGLAD and other African-ancestry focused studies in the largest to-date trans-ancestry glaucoma genetics study by the International Glaucoma Genetics Consortium (IGGC) identified 127 POAG loci [28]. African-specific subset analyses identified a locus in IQGAP1 that failed to replicate in samples of majority European and Asian ancestry. While this work represents a major global effort, it is important to note that because more than 98% of the samples were of majority European and Asian descent, African-specific signals are likely drowned out. It is additionally important to acknowledge that this trans-ancestry study reported 127 variants, some of which are vastly different effects across different ancestries. Figure 1 shows that allele frequencies for many of the 127 most recently identified POAG loci differ between European-descent (CEU) and African (YRI) ancestral populations as represented by 1000 Genomes (Phase 3) data [37].
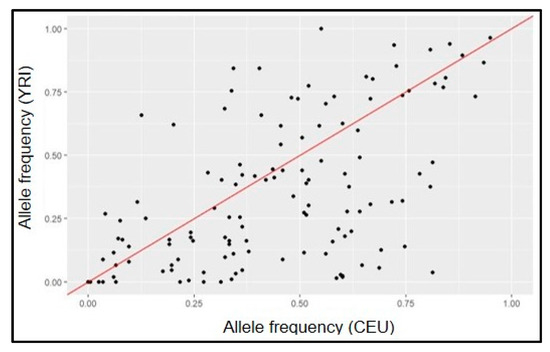
Figure 1.
Comparison of allele frequencies of 127 known POAG SNPs in ancestral populations (1000G YRI vs. CEU).
We recently showed that a GRS built on this most current set of POAG risk variants consistently underperforms in African Americans [33]. Expanding diverse representation in PRS development and testing represents an opportunity for increased understanding of POAG genetic risk and facilitate a path for precision medicine for those at risk for POAG.
5. Clinical Applicability of Genetic Data in POAG Risk Stratification and Intervention Relies on Transferability to Ensure Reduction of Health Disparities
Growing evidence indicates utility of GRS and PRS in POAG prediction and classification [4,15,16], yet most is shown with data from mostly individuals of European ancestry. GRS have not yet been established as a clinical screening tool for POAG. Neustaeter and colleagues are testing feasibility of POAG GRS for clinical screening using a prospective approach [38], but the variants included in the GRS were identified from POAG GWAS in European-descent individuals. In European-descent samples, a higher burden of genetic risk captured by PRS is associated with POAG age at diagnosis and disease-relevant clinical parameters [15,16]. However, in African-descent groups the predictive value diminishes [33]. This is not surprising, as current POAG PRS fail to incorporate genetic ancestry, significantly dampening transferability across ancestral groups [29].
Importantly, when evaluated in aggregate via GRS, known POAG variants effectively classify people of European descent who are at the highest genetic risk for POAG. We [39] and others [4,16,40,41,42] have shown varying degrees of applicability for GRS and/or PRS to target POAG screening and initiate timely therapy. Risk scores of 12–1200 variants associated with intraocular pressure (IOP) and/or POAG have shown an association with increased POAG risk in case-control analyses. In case-only analyses, these variants are associated with earlier age at diagnosis, more family members with POAG, need for incisional surgery, maximum recorded IOP, and more severe disease [16,39,40,41,42,43]. We previously showed that a 12-variant GRS is associated with increased POAG risk. Each higher GRS unit is associated with 0.36-year earlier POAG diagnosis [43] and mean age at diagnosis was 5.2 years earlier in the top vs. bottom 5% of the GRS distribution. The most recent report examining PRS in POAG showed that high glaucoma polygenic risk was associated with a more rapid rate of glaucomatous visual field worsening and a faster rate of nerve fiber layer thinning [4]. Siggs and colleagues showed that, despite early detection, some patients with POAG experience rapid disease onset and vision loss. They also demonstrated the benefit of informative PRS in clinical detection of severe disease risk. Individuals with high polygenic risk, defined as those in the top 5% of a normal distribution, were 50% more likely to experience vision loss compared to the bottom 95%, despite this group receiving more intense treatment. Notably, none of the studies have been replicated in people of African descent, thus representing an important gap in the field of POAG genetics.
To this end, we recently evaluated the 127 known POAG variants via GRS in the Million Veteran Program (MVP) Biobank, taking into account ancestry-specific and meta-analysis effect sizes from the IGGC [33]. While the GRS were significantly associated with POAG in European Americans and African Americans in the MVP, a higher proportion of people of European descent were consistently categorized in the top GRS decile when compared to people of African descent (21.9–23.6% and 12.9–14.5%, respectively). We further found that GRS was significantly associated with the documentation of invasive glaucoma surgery in European-descent cases; however, this association was significant in African-descent cases only if the GRS was weighted by ancestry-specific effect estimates. While unsurprising, these data further emphasize the need for understanding POAG genomic discovery in African-descent populations.
6. Beyond Genetics: A Note on Prioritizing Diversity in Ophthalmology
As efforts are being made to increase diversity of genetic data, so too are efforts needed to expand the diversity of clinical ophthalmological data. Such efforts are recently underway. Addis and colleagues call for the expansion of standard, normalized clinical databases containing clinical ocular data [44]. In evaluating the Cirrus High-Definition Optical Coherence Tomography database, they found that clinical measures differed significantly when comparing the normative database to POAAGG controls for disc area, rim area, cup-to-disc ratio, and cup volume. This study is another call to diversity in “standard” or “normalized” databases used for diagnosing diseases. Since we are aware of these racial and ethnic discrepancies in the presentation and progression of glaucoma, a one-size-fits-all diagnostic device may not be sufficient to catch all glaucoma-affected people, especially those of minoritized groups. As such efforts expand, care must be taken to clinically incorporate appropriate measures of ancestral diversity rather than apply race-based measures or modifiers.
7. Global Perspectives
Recently, Soh and colleagues reported that globally, more than half of all glaucoma cases were undetected [45]. Africa and Asia had higher odds of undetected glaucoma when compared to Europe. Asia is projected to have the most cases of undetected glaucoma due to population size alone, while Africa is projected to have the highest increase in undetected cases. Countries with lower human development indices (HDI) had higher proportions of undetected glaucoma when compared to countries with medium to high HDIs. However, the percentage of undetected glaucoma in high HDI groups (70%) is still concerning because glaucoma is often asymptomatic until the late stages of the disease. A large proportion of undetected glaucoma cases presented with at least a moderate visual field deficit. The blindness due to glaucoma is irreversible, and it is vital that screening and outreach efforts be proportionate to the amount of risk in each country. Tailored interventions need to be implemented in high-risk populations as well as underserved or resource-poor areas. Timely, cost-effective glaucoma screening is crucial due to the asymptomatic nature of the disease and the cost of vision care. It follows that a majority of those living with glaucoma are undiagnosed, and therefore, untreated. The accessibility of vision care needs to be more critically assessed due to the associations between poor visual function and worse quality of life [46].
Early POAG intervention preserves vision [47]; to intervene early in high-risk groups including African-descent populations, wherein POAG presents earlier [48] and progresses faster, current screening approaches must be updated. If genetics are expected to contribute to early detection, which is highly likely given the high heritability of POAG, then it is crucial to [26,49,50] increase African and African-descent representation in POAG studies that identify genetic loci influencing POAG risk. Prioritizing African-descent POAG in genetics is logical and necessary to attain precision ocular health in historically excluded and high-risk populations. Care should also be taken to acknowledge different recruitment strategies and study design efforts that go beyond Eurocentric models, including partnering with Black leaders to ensure collaboration and active participation in research [51]. Without this intentional focus on increasing diversity in POAG research, the disparity between those at highest risk and those receiving appropriate preventative care will likely widen.
8. Summary
The lack of diversity in POAG genetics research is consistent with GWAS of many complex diseases, which have historically been performed using overwhelmingly Eurocentric data [6,29]. These studies have consequently lacked sufficient data to enable extrapolation to populations that have historically been underrepresented in research. This dearth of data could limit the reach of the benefits of personalized medicine and the genomics revolution in underrepresented populations. This is especially true with diseases such as glaucoma, where both the prevalence and severity of disease is greater in African-descent populations, which have been historically underrepresented in genomics studies. When addressing diseases characterized by such dramatic health disparities, it is imperative that GRS and PRS include data from all populations, especially those at most risk for the disease. Inclusion of diverse data in genomic discovery studies increases the likelihood that novel risk variants will be identified. In the long-term, the glaucoma genetics literature suggests that improving glaucoma risk modeling may make early intervention possible, which could ensure better health outcomes. These advancements in risk stratification and intervention would help make the visions of precision medicine and health equity more realistic.
Author Contributions
Conceptualization, J.N.C.B., K.L.F., L.A.C., A.R.W. and M.A.H.; data curation, L.A.C. and T.G.K.; writing—original draft preparation, J.N.C.B., K.L.F., L.A.C., A.R.W. and T.G.K.; writing—review and editing, J.N.C.B., A.R.W., M.A.H. and J.L.W.; visualization, T.G.K. and L.A.C.; supervision, J.N.C.B., M.A.H. and J.L.W.; funding acquisition, J.N.C.B., L.A.C., A.R.W., M.A.H. and J.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health (NIH) National Eye Institute R01 EY033829 (J.N.C.B., T.G.K., M.A.H., and J.L.W.), the CWRU Visual Sciences Training Program (T32 EY 7157-19 [A.R.W.]), the CWRU Clinical and Translational Scientist Training Program (TL1 TR 002549-04 [A.R.W.]), the National Heart, Lung, and Blood Institute (T32 HL 0075-67 [L.A.C.]), and funds from the Cleveland Institute for Computational Biology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Asefa, N.G.; Neustaeter, A.; Jansonius, N.M.; Snieder, H. Heritability of glaucoma and glaucoma-related endophenotypes: Systematic review and meta-analysis protocol. BMJ Open 2018, 8, e019049. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gaitsch, H.; Poon, H.; Cox, N.J.; Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 2017, 49, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Qassim, A.; Han, X.; Marshall, H.N.; Mullany, S.; He, W.; Souzeau, E.; Galanopoulos, A.; Agar, A.; Landers, J.; et al. Association of High Polygenic Risk with Visual Field Worsening Despite Treatment in Early Primary Open-Angle Glaucoma. JAMA Ophthalmol. 2022, 10, e224688. [Google Scholar] [CrossRef]
- Youngblood, H.; Hauser, M.A.; Liu, Y. Update on the genetics of primary open-angle glaucoma. Exp. Eye Res. 2019, 188, 107795. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Osterman, M.D.; Kinzy, T.G.; Cooke Bailey, J.N. Polygenic Risk Scores. Curr. Protoc. 2021, 1, e126. [Google Scholar] [CrossRef]
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef]
- Siu, A.L. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef]
- Damen, J.A.A.G.; Hooft, L.; Schuit, E.; Debray, T.; Collins, G.; Tzoulaki, I.; Lassale, C.; Siontis, G.C.M.; Chiocchia, V.; Roberts, C.; et al. Prediction models for cardiovascular disease risk in the general population: Systematic review. BMJ 2016, 353, i2416. [Google Scholar] [CrossRef]
- Daunt, P.; Ballard, C.G.; Creese, B.; Davidson, G.; Hardy, J.; Oshota, O.; Pither, R.J.; Gibson, A.M. Polygenic Risk Scoring is an Effective Approach to Predict Those Individuals Most Likely to Decline Cognitively Due to Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2020, 8, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Souzeau, E.; Tram, K.H.; Witney, M.; Ruddle, J.B.; Graham, S.; Healey, P.R.; Goldberg, I.; Mackey, D.A.; Hewitt, A.; Burdon, K.P.; et al. Myocilin Predictive Genetic Testing for Primary Open-Angle Glaucoma Leads to Early Identification of At-Risk Individuals. Ophthalmology 2017, 124, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.E.; Neighborhood Consortium; Han, X.; Qassim, A.; Hassall, M.; Cooke Bailey, J.N.; Kinzy, T.G.; Khawaja, A.P.; An, J.; Marshall, H.; et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet. 2020, 52, 160–166. [Google Scholar] [CrossRef]
- Qassim, A.; Souzeau, E.; Siggs, O.M.; Hassall, M.M.; Han, X.; Griffiths, H.L.; Frost, N.A.; Vallabh, N.A.; Kirwan, J.F.; Menon, G.; et al. An Intraocular Pressure Polygenic Risk Score Stratifies Multiple Primary Open-Angle Glaucoma Parameters Including Treatment Intensity. Ophthalmology 2020, 127, 901–907. [Google Scholar] [CrossRef]
- Choquet, H.; Wiggs, J.L.; Khawaja, A.P. Clinical implications of recent advances in primary open-angle glaucoma genetics. Eye 2019, 34, 29–39. [Google Scholar] [CrossRef]
- De La Vega, F.M.; Bustamante, C.D. Polygenic risk scores: A biased prediction? Genome Med. 2018, 10, 100. [Google Scholar] [CrossRef]
- Duncan, L.; Shen, H.; Gelaye, B.; Meijsen, J.; Ressler, K.; Feldman, M.; Peterson, R.; Domingue, B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 2019, 10, 3328. [Google Scholar] [CrossRef]
- Martin, A.R.; Gignoux, C.R.; Walters, R.K.; Wojcik, G.L.; Neale, B.M.; Gravel, S.; Daly, M.J.; Bustamante, C.D.; Kenny, E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017, 100, 635–649. [Google Scholar] [CrossRef]
- Márquez-Luna, C.; Loh, P.; South Asian Type 2 Diabetes (SAT2D) Consortium; The SIGMA Type 2 Diabetes Consortium; Price, A.L. Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genet. Epidemiol. 2017, 41, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Kamiza, A.B.; Toure, S.M.; Vujkovic, M.; Machipisa, T.; Soremekun, O.S.; Kintu, C.; Corpas, M.; Pirie, F.; Young, E.; Gill, D.; et al. Transferability of genetic risk scores in African populations. Nat. Med. 2022, 28, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Weissbrod, O.; Kanai, M.; Shi, H.; Gazal, S.; Peyrot, W.J.; Khera, A.V.; Okada, Y.; Martin, A.R.; Finucane, H.; Price, A.L. Leveraging fine-mapping and non-European training data to improve trans-ethnic polygenic risk scores. medRxiv 2021. [Google Scholar] [CrossRef]
- Mars, N.; Kerminen, S.; Feng, Y.-C.A.; Kanai, M.; Läll, K.; Thomas, L.F.; Skogholt, A.H.; Parolo, P.D.B.; Neale, B.M.; Smoller, J.W.; et al. Genome-wide risk prediction of common diseases across ancestries in one million people. Cell Genom. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Liu, Y.; Hauser, M.A.; Akafo, S.K.; Qin, X.; Miura, S.; Gibson, J.R.; Wheeler, J.; Gaasterland, D.E.; Challa, P.; Herndon, L.W.; et al. Investigation of Known Genetic Risk Factors for Primary Open Angle Glaucoma in Two Populations of African Ancestry. Investig. Opthalmol. Vis. Sci. 2013, 54, 6248–6254. [Google Scholar] [CrossRef]
- Restrepo, N.A.; Cooke Bailey, J.N. Primary Open-Angle Glaucoma Genetics in African Americans. Curr. Genet. Med. Rep. 2017, 5, 167–174. [Google Scholar] [CrossRef]
- Cole, B.S.; Gudiseva, H.V.; Pistilli, M.; Salowe, R.; McHugh, C.P.; Zody, M.C.; Chavali, V.R.M.; Ying, G.S.; Moore, J.H.; O’Brien, J.M. The Role of Genetic Ancestry as a Risk Factor for Primary Open-angle Glaucoma in African Americans. Investig. Opthalmol. Vis. Sci. 2021, 62, 28. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Tang, H.; Thornton, T.A.; Caan, B.; Haan, M.; Millen, A.E.; Thomas, F.; Risch, N. Genome-wide association and admixture analysis of glaucoma in the Women’s Health Initiative. Hum. Mol. Genet. 2014, 23, 6634–6643. [Google Scholar] [CrossRef]
- Bonnemaijer, P.W.M.; GIGA Study Group; Iglesias, A.I.; Nadkarni, G.N.; Sanyiwa, A.J.; Hassan, H.G.; Cook, C.; Simcoe, M.; Taylor, K.D.; Schurmann, C.; et al. Genome-wide association study of primary open-angle glaucoma in continental and admixed African populations. Hum. Genet. 2018, 137, 847–862. [Google Scholar] [CrossRef] [PubMed]
- The Genetics of Glaucoma in People of African Descent (GGLAD) Consortium; Hauser, M.A.; Allingham, R.R.; Aung, T.; Van Der Heide, C.J.; Taylor, K.D.; Rotter, J.I.; Wang, S.-H.J.; Bonnemaijer, P.W.M.; Williams, S.E.I.; et al. Association of Genetic Variants with Primary Open-Angle Glaucoma Among Individuals with African Ancestry. JAMA 2019, 322, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Waksmunski, A.R.; Kinzy, T.G.; Cruz, L.A.; Nealon, C.L.; Halladay, C.W.; Simpson, P.; Canania, R.L.; Anthony, S.A.; Roncone, D.P.; Rogers, L.S.; et al. Glaucoma Genetic Risk Scores in the Million Veteran Program. Ophthalmology 2022, 129, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Kosoko-Lasaki, O.; Gong, G.; Haynatzki, G.; Wilson, M.R. Race, ethnicity and prevalence of primary open-angle glaucoma. J. Natl. Med. Assoc. 2006, 98, 1626–1629. [Google Scholar]
- Taylor, K.D.; Guo, X.; Zangwill, L.M.; Liebmann, J.M.; Girkin, C.A.; Feldman, R.M.; Dubiner, H.; Hai, Y.; Samuels, B.C.; Panarelli, J.F.; et al. Genetic Architecture of Primary Open-Angle Glaucoma in Individuals of African Descent. Ophthalmology 2018, 126, 38–48. [Google Scholar] [CrossRef]
- Gudiseva, H.V.; Verma, S.S.; Chavali, V.R.; Salowe, R.J.; Lucas, A.; Collins, D.W.; Rathi, S.; He, J.; Lee, R.; Merriam, S.; et al. Genome wide-association study identifies novel loci in the Primary Open-Angle African American Glaucoma Genetics (POAAGG) study. bioRxiv 2020. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Neustaeter, A.; Nolte, I.; Snieder, H.; Jansonius, N.M. Genetic pre-screening for glaucoma in population-based epidemiology: Protocol for a double-blind prospective screening study within Lifelines (EyeLife). BMC Ophthalmol. 2021, 21, 18. [Google Scholar] [CrossRef]
- Khawaja, A.P.; UK Biobank Eye and Vision Consortium; Cooke Bailey, J.N.; Wareham, N.J.; Scott, R.A.; Simcoe, M.; Igo, R.P.; Song, Y.E.; Wojciechowski, R.; Cheng, C.-Y.; et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018, 50, 778–782. [Google Scholar] [CrossRef]
- MacGregor, S.; Ong, J.-S.; An, J.; Han, X.; Zhou, T.; Siggs, O.M.; Law, M.H.; Souzeau, E.; Sharma, S.; Lynn, D.; et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet. 2018, 50, 1067–1071. [Google Scholar] [CrossRef]
- Gao, X.R.; Huang, H.; Kim, H. Polygenic Risk Score Is Associated with Intraocular Pressure and Improves Glaucoma Prediction in the UK Biobank Cohort. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Zebardast, N.; Sekimitsu, S.; Wang, J.; Elze, T.; Gharahkhani, P.; Cole, B.S.; Lin, M.M.; Segrè, A.V.; Wiggs, J.L.; Aung, T.; et al. Characteristics of p.Gln368Ter Myocilin Variant and Influence of Polygenic Risk on Glaucoma Penetrance in the UK Biobank. Ophthalmology 2021, 128, 1300–1311. [Google Scholar] [CrossRef]
- Fan, B.J.; Cooke Bailey, J.; Igo, R.P.; Kang, J.H.; Boumenna, T.; Brilliant, M.H.; Budenz, D.L.; Fingert, J.H.; Gaasterland, T.; Gaasterland, D.; et al. Association of a Primary Open-Angle Glaucoma Genetic Risk Score with Earlier Age at Diagnosis. JAMA Ophthalmol 2019, 137, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Addis, V.; Chan, L.; Chen, J.; Goodyear, K.; Pistilli, M.; Salowe, R.; Lee, R.; Sankar, P.; Miller-Ellis, E.; Cui, Q.N.; et al. Evaluation of the Cirrus High-Definition OCT Normative Database Probability Codes in a Black American Population. Ophthalmol. Glaucoma 2022, 5, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Soh, Z.; Yu, M.; Betzler, B.K.; Majithia, S.; Thakur, S.; Tham, Y.C.; Wong, T.Y.; Aung, T.; Friedman, D.S.; Cheng, C.-Y. The global extent of undetected glaucoma in adults: A systematic review and meta-analysis. Ophthalmology 2021, 128, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Caprioli, J. Glaucoma: A Disease of Early Cellular Senescence. Investig. Opthalmol. Vis. Sci. 2013, 54, ORSF60–ORSF67. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Sommer, A.; Katz, J.; Royall, R.M.; Quigley, H.A.; Javitt, J. Racial Variations in the Prevalence of Primary Open-angle Glaucoma: The Baltimore Eye Survey. JAMA 1991, 266, 369–374. [Google Scholar] [CrossRef]
- Grant, W.M.; Burke, J.F. Why Do Some People Go Blind from Glaucoma? Ophthalmology 1982, 89, 991–998. [Google Scholar] [CrossRef]
- Martin, M.J.; Summer, A.; Gold, E.B.; Diamond, E.L. Race and Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 1985, 99, 383–387. [Google Scholar] [CrossRef]
- Kikut, A.; Sanyal, M.; Vaughn, M.; Ridley-Merriweather, K.E.; Head, K.; Salowe, R.; Lomax-Reese, S.; Lewis, M.; Ross, A.G.; Cui, Q.N.; et al. Learning from Black/African American Participants: Applying the Integrated Behavioral Model to Assess Recruitment Strategies for a Glaucoma Genetic Study. Health Commun. 2020, 37, 515–524. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).