Life-Course Associations between Blood Pressure-Related Polygenic Risk Scores and Hypertension in the Bogalusa Heart Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Genotyping, PRS Derivation, and Validation
2.3. Phenotype Measurements
2.4. Statistical Analyses
2.4.1. Association between the PRSs and Incidence of Hypertension and Stage 2 Hypertension
2.4.2. Association between the PRSs and Midlife Blood Pressure
3. Results
3.1. Association between the PRSs and Incidence of Hypertension and Stage 2 Hypertension
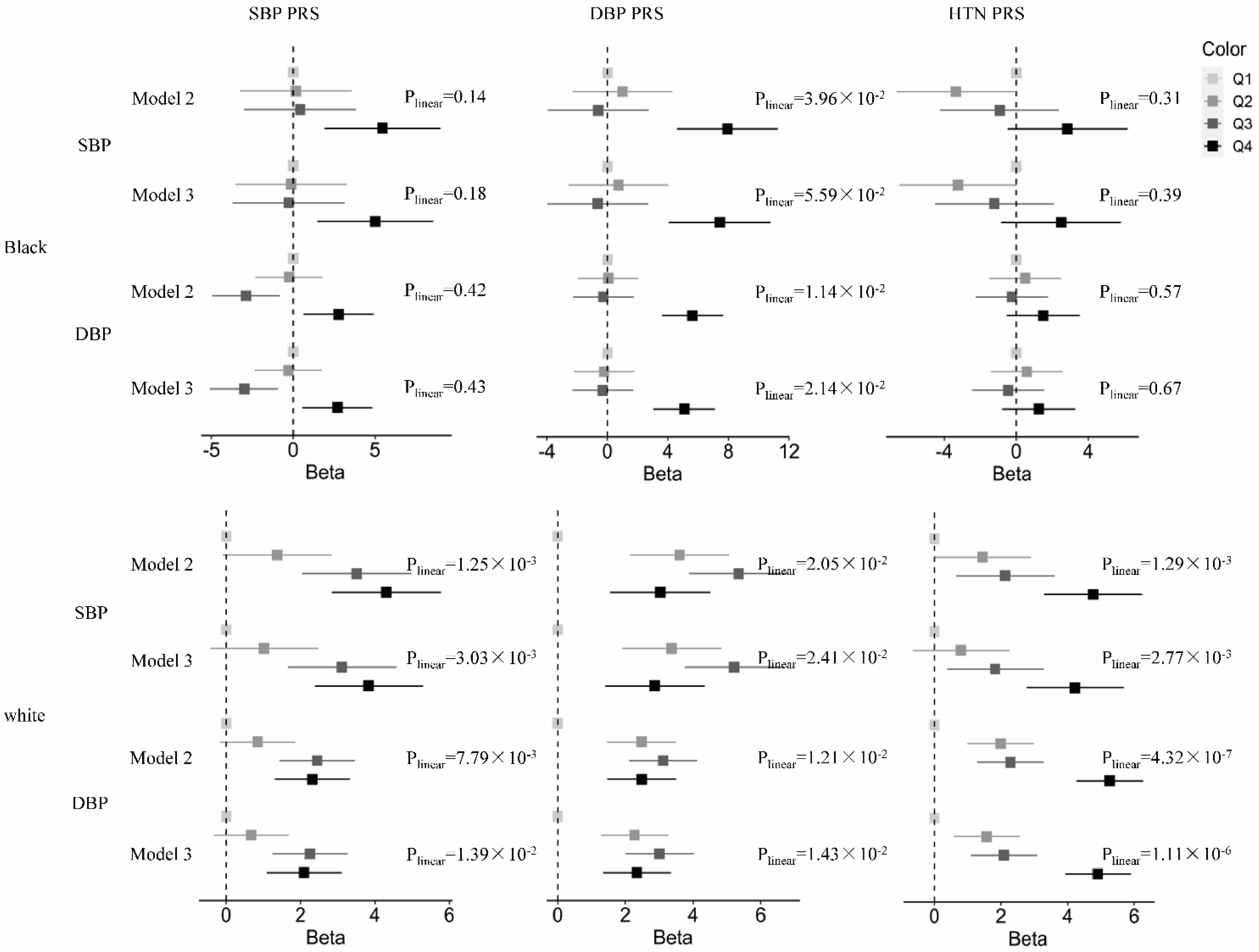
3.2. Association between the PRSs and Midlife Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Ammous, F.; Zhao, W.; Ratliff, S.M.; Mosley, T.H.; Bielak, L.F.; Zhou, X.; Peyser, P.A.; Kardia, S.L.R.; Smith, J.A. Epigenetic Age Acceleration Is Associated with Cardiometabolic Risk Factors and Clinical Cardiovascular Disease Risk Scores in African Americans. Clin. Epigenet. 2021, 13, 55. [Google Scholar] [CrossRef]
- Singh, S.; Shankar, R.; Singh, G.P. Prevalence and Associated Risk Factors of Hypertension: A Cross-Sectional Study in Urban Varanasi. Int. J. Hypertens. 2017, 2017, 5491838. [Google Scholar] [CrossRef]
- Kannel, W.B. Risk Factors in Hypertension. J. Cardiovasc. Pharmacol. 1989, 13 (Suppl. S1), S4–S10. [Google Scholar] [CrossRef]
- Wang, W.; Lee, E.T.; Fabsitz, R.R.; Devereux, R.; Best, L.; Welty, T.K.; Howard, B.V. A Longitudinal Study of Hypertension Risk Factors and Their Relation to Cardiovascular Disease: The Strong Heart Study. Hypertension 2006, 47, 403–409. [Google Scholar] [CrossRef]
- Lauer, R.M.; Clarke, W.R. Childhood Risk Factors for High Adult Blood Pressure: The Muscatine Study. Pediatrics 1989, 84, 633–641. [Google Scholar] [CrossRef]
- Theodore, R.F.; Broadbent, J.; Nagin, D.; Ambler, A.; Hogan, S.; Ramrakha, S.; Cutfield, W.; Williams, M.J.A.; Harrington, H.L.; Moffitt, T.E.; et al. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension 2015, 66, 1108–1115. [Google Scholar] [CrossRef]
- Juhola, J.; Magnussen, C.G.; Viikari, J.S.A.; Kähönen, M.; Hutri-Kähönen, N.; Jula, A.; Lehtimäki, T.; Kerblom, H.K.; Pietikäinen, M.; Laitinen, T.; et al. Tracking of Serum Lipid Levels, Blood Pressure, and Body Mass Index from Childhood to Adulthood: The Cardiovascular Risk in Young Finns Study. J. Pediatr. 2011, 159, 584–590. [Google Scholar] [CrossRef]
- Luft, F.C. Twins in Cardiovascular Genetic Research. Hypertension 2001, 37, 350–356. [Google Scholar] [CrossRef]
- Fagard, R.; Brguljan, J.; Staessen, J.; Thijs, L.; Derom, C.; Thomis, M.; Vlietinck, R. Heritability of Conventional and Ambulatory Blood Pressures. A Study in Twins. Hypertension 1995, 26, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Larson, M.G.; Vasan, R.S.; Levy, D.; Bloch, K.D.; Surti, A.; Guiducci, C.; Kathiresan, S.; Benjamin, E.J.; Struck, J.; et al. Association of Common Variants in NPPA and NPPB with Circulating Natriuretic Peptides and Blood Pressure. Nat. Genet. 2009, 41, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Gaunt, T.R.; Newhouse, S.J.; Padmanabhan, S.; Tomaszewski, M.; Kumari, M.; Morris, R.W.; Tzoulaki, I.; O’Brien, E.T.; Poulter, N.R.; et al. Blood Pressure Loci Identified with a Gene-Centric Array. Am. J. Hum. Genet. 2011, 89, 688–700. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Tragante, V.; Guo, W.; Guo, Y.; Lanktree, M.; Smith, E.N.; Johnson, T.; Castillo, B.A.; Barnard, J.; Baumert, J.; et al. Loci Influencing Blood Pressure Identified Using a Cardiovascular Gene-Centric Array. Hum. Mol. Genet. 2013, 22, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; Kanoni, S.; et al. The Genetics of Blood Pressure Regulation and Its Target Organs from Association Studies in 342,415 Individuals. Nat. Genet. 2016, 48, 1171. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Ehret, G.B.; Nandakumar, P.; Ranatunga, D.; Schaefer, C.; Kwok, P.-Y.; Iribarren, C.; Chakravarti, A.; Risch, N. Genome-Wide Association Analyses Using Electronic Health Records Identify New Loci Influencing Blood Pressure Variation. Nat. Genet. 2017, 49, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic Analysis of over 1 Million People Identifies 535 New Loci Associated with Blood Pressure Traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Giri, A.; Hellwege, J.N.; Keaton, J.M.; Park, J.; Qiu, C.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-Ethnic Association Study of Blood Pressure Determinants in over 750,000 Individuals. Nat. Genet. 2019, 51, 51–62. [Google Scholar] [CrossRef]
- Kato, N.; Loh, M.; Takeuchi, F.; Verweij, N.; Wang, X.; Zhang, W.; NKelly, T.; Saleheen, D.; Lehne, B.; Leach, I.M.; et al. Trans-Ancestry Genome-Wide Association Study Identifies 12 Genetic Loci Influencing Blood Pressure and Implicates a Role for DNA Methylation. Nat. Genet. 2015, 47, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- GB, E.; PB, M.; KM, R.; M, B.; AD, J.; DI, C.; AV, S.; MD, T.; GC, V.; SJ, H.; et al. Genetic Variants in Novel Pathways Influence Blood Pressure and Cardiovascular Disease Risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.; et al. Genome-Wide Association Study Identifies Eight Loci Associated with Blood Pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Kettunen, J.; Ukkola, O.; Osmond, C.; Eriksson, J.G.; Kesäniemi, Y.A.; Jula, A.; Peltonen, L.; Kontula, K.; Salomaa, V.; et al. A Blood Pressure Genetic Risk Score Is a Significant Predictor of Incident Cardiovascular Events in 32,669 Individuals. Hypertension 2013, 61, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Fava, C.; Sjögren, M.; Montagnana, M.; Danese, E.; Almgren, P.; Engström, G.; Nilsson, P.; Hedblad, B.; Guidi, G.C.; Minuz, P.; et al. Prediction of Blood Pressure Changes over Time and Incidence of Hypertension by a Genetic Risk Score in Swedes. Hypertension 2013, 61, 319–326. [Google Scholar] [CrossRef]
- Smith, C.J.; Saftlas, A.F.; Spracklen, C.N.; Triche, E.W.; Bjonnes, A.; Keating, B.; Saxena, R.; Breheny, P.J.; Dewan, A.T.; Robinson, J.G.; et al. Genetic Risk Score for Essential Hypertension and Risk of Preeclampsia. Am. J. Hypertens. 2016, 29, 17–24. [Google Scholar] [CrossRef][Green Version]
- Nierenberg, J.L.; Li, C.; He, J.; Gu, D.; Chen, J.; Lu, X.; Li, J.; Wu, X.; Gu, C.C.; Hixson, J.E.; et al. Blood Pressure Genetic Risk Score Predicts Blood Pressure Responses to Dietary Sodium and Potassium: The GenSalt Study (Genetic Epidemiology Network of Salt Sensitivity). Hypertension 2017, 70, 1106–1112. [Google Scholar] [CrossRef]
- Vaura, F.; Kauko, A.; Suvila, K.; Havulinna, A.S.; Mars, N.; Salomaa, V.; Finngen; Cheng, S.; Niiranen, T. Polygenic Risk Scores Predict Hypertension Onset and Cardiovascular Risk. Hypertension 2021, 77, 1119–1127. [Google Scholar] [CrossRef]
- Weng, Z.; Liu, Q.; Yan, Q.; Liang, J.; Zhang, X.; Xu, J.; Li, W.; Xu, C.; Gu, A. Associations of Genetic Risk Factors and Air Pollution with Incident Hypertension among Participants in the UK Biobank Study. Chemosphere 2022, 299, 134398. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Havulinna, A.S.; Langén, V.L.; Salomaa, V.; Jula, A.M. Prediction of Blood Pressure and Blood Pressure Change with a Genetic Risk Score. J. Clin. Hypertens. 2016, 18, 181–186. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Wang, L.; Chen, S.; Yang, X.; Li, J.; Cao, J.; Chen, J.; Li, Y.; Zhao, L.; et al. Genetic Predisposition to Higher Blood Pressure Increases Risk of Incident Hypertension and Cardiovascular Diseases in Chinese. Hypertension 2015, 66, 786–792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giontella, A.; Sjögren, M.; Lotta, L.A.; Overton, J.D.; Baras, A.; Minuz, P.; Fava, C.; Melander, O. Clinical Evaluation of the Polygenetic Background of Blood Pressure in the Population-Based Setting. Hypertension 2020, 77, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Oikonen, M.; Tikkanen, E.; Juhola, J.; Tuovinen, T.; Seppälä, I.; Juonala, M.; Taittonen, L.; Mikkilä, V.; Kähönen, M.; Ripatti, S.; et al. Genetic Variants and Blood Pressure in a Population-Based Cohort: The Cardiovascular Risk in Young Finns Study. Hypertension 2011, 58, 1079. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.K.; Lee, J.Y.; Lee, J.Y.; Park, H.Y.; Cho, M.C. The Role of Genetic Risk Score in Predicting the Risk of Hypertension in the Korean Population: Korean Genome and Epidemiology Study. PLoS ONE 2015, 10, e0131603. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587. [Google Scholar] [CrossRef]
- Shi, M.; Chen, W.; Sun, X.; Bazzano, L.A.; He, J.; Razavi, A.C.; Li, C.; Qi, L.; Khera, A.V.; Kelly, T.N. Association of Genome-Wide Polygenic Risk Score for Body Mass Index With Cardiometabolic Health From Childhood through Midlife. Circ. Genomic Precis. Med. 2021, 15, 101161CIRCGEN121003375. [Google Scholar] [CrossRef]
- Kurniansyah, N.; Goodman, M.O.; Kelly, T.N.; Elfassy, T.; Wiggins, K.L.; Bis, J.C.; Guo, X.; Palmas, W.; Taylor, K.D.; Lin, H.J.; et al. A Multi-Ethnic Polygenic Risk Score Is Associated with Hypertension Prevalence and Progression throughout Adulthood. Nat. Commun. 2022, 13, 3549. [Google Scholar] [CrossRef]
- Berenson, G.S. Bogalusa Heart Study: A Long-Term Community Study of a Rural Biracial (Black/White) Population. Am. J. Med. Sci. 2001, 322, 267–274. [Google Scholar] [CrossRef]
- Smith, E.N.; Chen, W.; Kähönen, M.; Kettunen, J.; Lehtimäki, T.; Peltonen, L.; Raitakari, O.T.; Salem, R.M.; Schork, N.J.; Shaw, M.; et al. Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study. PLoS Genet. 2010, 6, e1001094. [Google Scholar] [CrossRef]
- Eberle, M.A.; Ng, P.C.; Kuhn, K.; Zhou, L.; Peiffer, D.A.; Galver, L.; Viaud-Martinez, K.A.; Lawley, C.T.; Gunderson, K.L.; Shen, R.; et al. Power to Detect Risk Alleles Using Genome-Wide Tag SNP Panels. PLoS Genet. 2007, 3, e170. [Google Scholar] [CrossRef]
- Keating, B.J.; Tischfield, S.; Murray, S.S.; Bhangale, T.; Price, T.S.; Glessner, J.T.; Galver, L.; Barrett, J.C.; Grant, S.F.A.; Farlow, D.N.; et al. Concept, Design and Implementation of a Cardiovascular Gene-Centric 50 K SNP Array for Large-Scale Genomic Association Studies. PLoS ONE 2008, 3, e3583. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.Y.; Inouye, M.; Small, K.S.; Gwilliam, R.; Deloukas, P.; Kwiatkowski, D.P.; Clark, T.G. A Genotype Calling Algorithm for the Illumina BeadArray Platform. Bioinformatics 2007, 23, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 Diverse Genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-Generation Genotype Imputation Service and Methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Fuchsberger, C.; Abecasis, G.R.; Hinds, D.A. Minimac2: Faster Genotype Imputation. Bioinformatics 2015, 31, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-Wide Complex Trait Analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score Software for Biobank-Scale Data. Gigascience 2019, 8, giz082. [Google Scholar] [CrossRef]
- Foster, T.A.; Berenson, G.S. Measurement Error and Reliability in Four Pediatric Cross-Sectional Surveys of Cardiovascular Disease Risk Factor Variables—The Bogalusa Heart Study. J. Chronic Dis. 1987, 40, 13–21. [Google Scholar] [CrossRef]
- Foster, T.A.; Webber, L.S.; Srinivasan, S.R.; Voors, A.W.; Berenson, G.S. Measurement Error of Risk Factor Variables in an Epidemiologic Study of Children-the Bogalusa Heart Study. J. Chronic Dis. 1980, 33, 661–672. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.; Bazzano, L.; He, J.; Whelton, P.; Chen, W. Trajectories of Childhood Blood Pressure and Adult Left Ventricular Hypertrophy: The Bogalusa Heart Study. Hypertension 2018, 72, 93–101. [Google Scholar] [CrossRef]
- Tobin, M.D.; Sheehan, N.A.; Scurrah, K.J.; Burton, P.R. Adjusting for Treatment Effects in Studies of Quantitative Traits: Antihypertensive Therapy and Systolic Blood Pressure. Stat. Med. 2005, 24, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, E13–E115. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.C.; Tishkoff, S.A. African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annu. Rev. Genomics Hum. Genet. 2008, 9, 403–433. [Google Scholar] [CrossRef]
- Frank, M.; Harrell, E. Package “Hmisc” Title Harrell Miscellaneous. 2022. Available online: https://hbiostat.org/R/Hmisc/ (accessed on 20 April 2022).
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the Added Predictive Ability of a New Marker: From Area under the ROC Curve to Reclassification and Beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Juhola, J.; Oikonen, M.; Magnussen, C.G.; Mikkilä, V.; Siitonen, N.; Jokinen, E.; Laitinen, T.; Würtz, P.; Gidding, S.S.; Taittonen, L.; et al. Childhood Physical, Environmental, and Genetic Predictors of Adult Hypertension: The Cardiovascular Risk in Young Finns Study. Circulation 2012, 126, 402–409. [Google Scholar] [CrossRef]
- Paynter, N.P.; Chasman, D.I.; Paré, G.; Buring, J.E.; Cook, N.R.; Miletich, J.P.; Ridker, P.M. Association Between a Literature-Based Genetic Risk Score and Cardiovascular Events in Women. JAMA 2010, 303, 631–637. [Google Scholar] [CrossRef]
- Mars, N.; Koskela, J.T.; Ripatti, P.; Kiiskinen, T.T.J.; Havulinna, A.S.; Lindbohm, J.V.; Ahola-Olli, A.; Kurki, M.; Karjalainen, J.; Palta, P.; et al. Polygenic and Clinical Risk Scores and Their Impact on Age at Onset and Prediction of Cardiometabolic Diseases and Common Cancers. Nat. Med. 2020, 26, 549–557. [Google Scholar] [CrossRef]
- Duncan, L.; Shen, H.; Gelaye, B.; Meijsen, J.; Ressler, K.; Feldman, M.; Peterson, R.; Domingue, B. Analysis of Polygenic Risk Score Usage and Performance in Diverse Human Populations. Nat. Commun. 2019, 10, 3328. [Google Scholar] [CrossRef]
- Need, A.C.; Goldstein, D.B. Next Generation Disparities in Human Genomics: Concerns and Remedies. Trends Genet. 2009, 25, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Henn, B.M.; Gutenkunst, R.N.; Indap, A.R.; Marth, G.T.; Clark, A.G.; Yu, F.; Gibbs, R.A.; Bustamante, C.D. Demographic History and Rare Allele Sharing among Human Populations. Proc. Natl. Acad. Sci. USA 2011, 108, 11983–11988. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.D.; De La Vega, F.M.; Burchard, E.G. Genomics for the World: Medical Genomics Has Focused Almost Entirely on Those of European Descent. Other Ethnic Groups Must Be Studied to Ensure That More People Benefit, Say. Nature 2011, 475, 163. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, D.L.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Collins, F.S.; De La Vega, F.M.; Donnelly, P.; Egholm, M.; et al. A Map of Human Genome Variation from Population-Scale Sequencing. Nature 2010, 467, 1061–1073. [Google Scholar] [CrossRef]
- Altshuler, D.M.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; Gabriel, S.B.; et al. An Integrated Map of Genetic Variation from 1,092 Human Genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68. [Google Scholar] [CrossRef]

| Blacks (N = 369) | Whites (N = 832) | p | |
|---|---|---|---|
| Male, n (%) | 143 (38.8) | 375 (45.1) | 0.04 |
| Time, yrs, median (IQR) | 36.2 (9.99) | 37.1 (8.88) | 0.55 |
| SBP PRS, mean (SD) | −4.90 × 10−3 (6.10 × 10−4) | −7.00 × 10−3 (4.60 × 10−4) | <0.0001 |
| DBP PRS, mean (SD) | −8.00 × 10−4 (2.20 × 10−4) | −1.30 × 10−3 (1.20 × 10−4) | <0.0001 |
| HTN PRS, mean (SD) | 1.43 × 10−5 (1.59 × 10−5) | −1.86 × 10−5 (1.45 × 10−5) | <0.0001 |
| Childhood * | |||
| Age, yrs, mean (SD) | 9.56 (2.98) | 9.94 (3.28) | 0.05 |
| BMI, kg/m2, mean (SD) | 17.6 (3.75) | 17.8 (3.47) | 0.34 |
| SBP, mmHg, mean (SD) | 99.1 (10.69) | 100 (9.83) | 0.08 |
| DBP, mmHg, mean (SD) | 61.88 (8.72) | 62.03 (8.17) | 0.78 |
| Hypertension, n (%) | 16 (4.34) | 46 (5.55) | 0.38 |
| Stage-2 hypertension (%) | 2 (0.54) | 0 (0.00) | 0.03 |
| Antihypertension medication, n (%) | 0 (0.00) | 0 (0.00) | NA |
| Midlife † | |||
| Age, yrs, mean (SD) | 44.6 (7.38) | 45.4 (6.99) | 0.07 |
| BMI, kg/m2, mean (SD) | 32.8 (9.15) | 30.2 (6.93) | <0.0001 |
| SBP, mmHg, mean (SD) | 126 (19.7) | 118 (14.0) | <0.0001 |
| DBP, mmHg, mean (SD) | 82.6 (11.8) | 78.2 (9.7) | <0.0001 |
| Hypertension, n (%) | 265 (71.8) | 462 (55.6) | <0.0001 |
| Stage-2 hypertension (%) | 197 (53.4) | 286 (34.4) | <0.0001 |
| Antihypertension medication, n (%) | 144 (39.0) | 198 (23.8) | <0.0001 |
| Model 1 § | Model 2 † | Model 3 ‡ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP PRS | Hazard Ratio (CI) | p-Value | Harrell’s C | p-Value (M1 vs. Null *) | Hazard Ratio (CI) | p-Value | Harrell’s C | p-value (M2 vs. Null *) | Hazard Ratio (CI) | p-Value | Harrell’s C | p-Value (M3 vs. M2) | p-Value (M3 vs. M1) |
| Black | |||||||||||||
| Hypertension | |||||||||||||
| SBP PRS | |||||||||||||
| PRS | - | - | 0.629 | 1.07 × 10−3 | 1.42 (1.11, 1.82) | 4.87 × 10−3 | 0.615 | 1.99 × 10−2 | 1.38 (1.08, 1.77) | 9.62 × 10−3 | 0.631 | 2.87 × 10−3 | 0.07 |
| Measured SBP | 1.26 (1.07, 1.48) | 5.71 × 10−3 | - | - | 1.23 (1.05, 1.45) | 1.14 × 10−2 | |||||||
| DBP PRS | |||||||||||||
| PRS | - | - | 0.633 | 4.21 × 10−7 | 1.24 (1.06, 1.46) | 8.15 × 10−3 | 0.615 | 8.20 × 10−3 | 1.22 (1.04, 1.43) | 1.66 × 10−2 | 0.634 | 2.13 × 10−6 | 2.49 × 10−2 |
| Measured DBP | 1.29 (1.11, 1.49) | 6.15 × 10−4 | - | - | 1.27 (1.1, 1.46) | 1.22 × 10−3 | |||||||
| HTN PRS | |||||||||||||
| PRS | - | - | 0.638 | 1.27 × 10−7 | 1.18 (0.99, 1.40) | 0.06 | 0.620 | 0.06 | 1.14 (0.96, 1.36) | 0.13 | 0.643 | 3.29 × 10−7 | 0.15 |
| Measured MAP | 1.34 (1.14, 1.56) | 2.67 × 10−4 | - | - | 1.32 (1.13, 1.55) | 4.90 × 10−4 | |||||||
| Stage 2 Hypertension | |||||||||||||
| SBP PRS | |||||||||||||
| PRS | - | - | 0.613 | 1.55 × 10−2 | 1.75 (1.31, 2.32) | 1.22 × 10−4 | 0.618 | 5.00 × 10−4 | 1.74 (1.30, 2.31) | 1.59 × 10−4 | 0.627 | 2.21 × 10−2 | 8.03 × 10−4 |
| Measured SBP | 1.21 (1.01, 1.46) | 3.75 × 10−2 | - | - | 1.20 (1.00, 1.44) | 0.05 | |||||||
| DBP PRS | |||||||||||||
| PRS | - | - | 0.616 | 1.36 × 10−4 | 1.52 (1.25, 1.84) | 2.16 × 10−5 | 0.619 | 0 | 1.50 (1.24, 1.82) | 3.34 × 10−5 | 0.630 | 5.67 × 10−4 | 4.34 × 10−5 |
| Measured DBP | 1.22 (1.03, 1.44) | 1.99 × 10−2 | - | - | 1.20 (1.01, 1.41) | 3.28 × 10−2 | |||||||
| HTN PRS | |||||||||||||
| PRS | - | - | 0.621 | 9.64 × 10−5 | 1.19 (0.98, 1.44) | 0.08 | 0.603 | 0.10 | 1.17 (0.97, 1.42) | 0.11 | 0.622 | 1.31 × 10−4 | 0.12 |
| Measured MAP | 1.26 (1.06, 1.51) | 1.06 × 10−2 | - | - | 1.25 (1.05, 1.50) | 1.32 × 10−2 | |||||||
| White | |||||||||||||
| Hypertension | |||||||||||||
| SBP PRS | |||||||||||||
| PRS | - | - | 0.645 | 2.56 × 10−12 | 1.29 (1.05, 1.60) | 1.60 × 10−2 | 0.614 | 4.62 × 10−2 | 1.24 (1.00, 1.53) | 4.49 × 10−2 | 0.645 | 9.56 × 10−12 | 0.16 |
| Measured SBP | 1.37 (1.23, 1.52) | 6.42 × 10−9 | - | - | 1.36 (1.22, 1.51) | 1.67 × 10−8 | |||||||
| DBP PRS | |||||||||||||
| PRS | - | - | 0.649 | 1.11 × 10−15 | 1.30 (1.07, 1.59) | 8.53 × 10−3 | 0.618 | 1.03 × 10−2 | 1.29 (1.06, 1.57) | 1.23 × 10−2 | 0.651 | 1.78 × 10−15 | 1.89 × 10−2 |
| Measured DBP | 1.39 (1.26, 1.55) | 2.82 × 10−10 | - | - | 1.39 (1.25, 1.54) | 3.89 × 10−10 | |||||||
| HTN PRS | |||||||||||||
| PRS | - | - | 0.656 | 0 | 1.27 (1.13, 1.44) | 1.02 × 10−4 | 0.622 | 7.00 × 10−4 | 1.25 (1.11, 1.42) | 2.54 × 10−4 | 0.661 | 0 | 1.79 × 10−3 |
| Measured MAP | 1.46 (1.31, 1.63) | 3.64 × 10−12 | - | - | 1.45 (1.30, 1.61) | 8.57 × 10−12 | |||||||
| Stage 2 Hypertension | |||||||||||||
| SBP PRS | |||||||||||||
| PRS | - | - | 0.673 | 5.71 × 10−10 | 1.33 (1.02, 1.75) | 3.84 × 10−2 | 0.648 | 3.32 × 10−2 | 1.27 (0.97, 1.67) | 0.08 | 0.675 | 1.57 × 10−9 | 0.10 |
| Measured SBP | 1.38 (1.2, 1.58) | 3.78 × 10−6 | - | - | 1.37 (1.19, 1.57) | 7.33 × 10−6 | |||||||
| DBP PRS | |||||||||||||
| PRS | - | - | 0.668 | 2.59 × 10−7 | 1.42 (1.09, 1.84) | 8.57 × 10−3 | 0.652 | 1.11 × 10−2 | 1.39 (1.07, 1.81) | 1.31 × 10−2 | 0.671 | 4.81 × 10−7 | 2.18 × 10−2 |
| Measured DBP | 1.34 (1.17, 1.53) | 1.77 × 10−5 | - | - | 1.33 (1.16, 1.51) | 2.64 × 10−5 | |||||||
| HTN PRS | |||||||||||||
| PRS | - | - | 0.675 | 4.26 × 10−11 | 1.44 (1.22, 1.70) | 1.13 × 10−5 | 0.660 | 1.00 × 10−4 | 1.42 (1.21, 1.67) | 2.29 × 10−5 | 0.686 | 1.26 × 10−10 | 2.24 × 10−4 |
| Measured MAP | 1.43 (1.24, 1.64) | 5.14 × 10−7 | - | - | 1.41 (1.23, 1.62) | 1.02 × 10−6 | |||||||
| Model 1 § | Model 2 † | Model 3 ‡ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP PRS | Beta | SE | p-Value | R2 | p-Value (M1 vs. Null *) | Beta | SE | p-Value | R2 | p-Value (M2 vs. Null *) | Beta | SE | p-Value | R2 | p-Value (M3 vs. M2) | p-Value (M3 vs. M1) |
| Black (N = 369) | ||||||||||||||||
| Systolic blood pressure | ||||||||||||||||
| SBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.07 | 0.11 | 5.22 | 2.45 | 3.35 × 10-2 | 0.08 | 3.35 × 10-2 | 4.83 | 2.46 | 0.05 | 0.08 | 0.17 | 0.05 |
| Measured SBP | 2.29 | 1.42 | 0.11 | - | - | - | 1.96 | 1.42 | 0.17 | |||||||
| DBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.07 | 0.14 | 3.45 | 1.54 | 2.57 × 10-2 | 0.08 | 2.57 × 10-2 | 3.24 | 1.55 | 3.77 × 10-2 | 0.08 | 0.21 | 3.77 × 10-2 |
| Measured DBP | 2.00 | 1.34 | 0.14 | - | - | - | 1.68 | 1.35 | 0.21 | |||||||
| HTN PRS | ||||||||||||||||
| PRS | - | - | - | 0.07 | 0.08 | 1.11 | 1.59 | 0.48 | 0.07 | 0.48 | 0.73 | 1.60 | 0.65 | 0.07 | 0.10 | 0.65 |
| Measured MAP | 2.48 | 1.43 | 0.08 | - | - | - | 2.39 | 1.44 | 0.10 | |||||||
| Diastolic blood pressure | ||||||||||||||||
| SBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.06 | 0.82 | 2.53 | 1.49 | 0.09 | 0.06 | 0.09 | 2.53 | 1.50 | 0.09 | 0.06 | 0.98 | 0.09 |
| Measured SBP | 0.20 | 0.87 | 0.82 | - | - | - | 0.03 | 0.87 | 0.98 | |||||||
| DBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.07 | 1.81 × 10-2 | 2.51 | 0.93 | 7.45 × 10-3 | 0.07 | 7.45 × 10-3 | 2.29 | 0.94 | 1.48 × 10-2 | 0.09 | 3.66 × 10-2 | 1.48 × 10-2 |
| Measured DBP | 1.93 | 0.81 | 0.02 | - | - | - | 1.70 | 0.81 | 3.66 × 10-2 | |||||||
| HTN PRS | ||||||||||||||||
| PRS | - | - | - | 0.06 | 0.07 | 0.61 | 0.96 | 0.52 | 0.06 | 0.52 | 0.37 | 0.97 | 0.70 | 0.06 | 0.09 | 0.70 |
| Measured MAP | 1.56 | 0.87 | 0.07 | - | - | - | 1.51 | 0.88 | 0.09 | |||||||
| White (N = 832) | ||||||||||||||||
| Systolic blood pressure | ||||||||||||||||
| SBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.17 | 1.28 × 10-9 | 4.50 | 1.31 | 6.42 × 10-4 | 0.14 | 6.42 × 10-4 | 3.93 | 1.29 | 2.42 × 10-3 | 0.18 | 4.57 × 10-9 | 2.42 × 10-3 |
| Measured SBP | 4.04 | 0.66 | 1.28 × 10-9 | - | - | - | 3.89 | 0.66 | 4.57 × 10-9 | |||||||
| DBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.15 | 3.81 × 10-6 | 3.58 | 1.24 | 3.99 × 10-3 | 0.14 | 3.99 × 10-3 | 3.41 | 1.23 | 5.61 × 10-3 | 0.16 | 5.30 × 10-6 | 5.61 × 10-3 |
| Measured DBP | 2.87 | 0.62 | 3.81 × 10-6 | - | - | - | 2.82 | 0.62 | 5.30 × 10-6 | |||||||
| HTN PRS | ||||||||||||||||
| PRS | - | - | - | 0.17 | 4.16 × 10-9 | 2.78 | 0.76 | 2.60 × 10-4 | 0.14 | 2.60 × 10-4 | 2.56 | 0.74 | 6.27 × 10-4 | 0.18 | 9.79 × 10-9 | 6.27 × 10-4 |
| Measured MAP | 3.91 | 0.66 | 4.16 × 10-9 | - | - | - | 3.79 | 0.65 | 9.79 × 10-9 | |||||||
| Diastolic blood pressure | ||||||||||||||||
| SBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.12 | 3.05 × 10-5 | 2.76 | 0.90 | 2.35 × 10-3 | 0.11 | 2.35 × 10-3 | 2.49 | 0.90 | 5.78 × 10-3 | 0.13 | 7.27 × 10-5 | 5.78 × 10-3 |
| Measured SBP | 1.92 | 0.46 | 3.05 × 10-5 | - | - | - | 1.82 | 0.46 | 7.27 × 10-5 | |||||||
| DBP PRS | ||||||||||||||||
| PRS | - | - | - | 0.14 | 3.17 × 10-8 | 2.73 | 0.85 | 1.37 × 10-3 | 0.11 | 1.37 × 10-3 | 2.59 | 0.84 | 2.01 × 10-3 | 0.15 | 4.62 × 10-8 | 2.01 × 10-3 |
| Measured DBP | 2.36 | 0.42 | 3.17 × 10-8 | - | - | - | 2.32 | 0.42 | 4.62 × 10-8 | |||||||
| HTN PRS | ||||||||||||||||
| PRS | - | - | - | 0.14 | 8.47 × 10-9 | 2.81 | 0.52 | 7.00 × 10-8 | 0.13 | 7.00 × 10-8 | 2.66 | 0.51 | 2.00 × 10-7 | 0.17 | 2.41 × 10-8 | 2.00 × 10-7 |
| Measured MAP | 2.63 | 0.45 | 8.47 × 10-9 | - | - | - | 2.51 | 0.45 | 2.41 × 10-8 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Pan, Y.; Zhang, R.; De Anda-Duran, I.; Huang, Z.; Li, C.; Shi, M.; Razavi, A.C.; Bazzano, L.A.; He, J.; et al. Life-Course Associations between Blood Pressure-Related Polygenic Risk Scores and Hypertension in the Bogalusa Heart Study. Genes 2022, 13, 1473. https://doi.org/10.3390/genes13081473
Sun X, Pan Y, Zhang R, De Anda-Duran I, Huang Z, Li C, Shi M, Razavi AC, Bazzano LA, He J, et al. Life-Course Associations between Blood Pressure-Related Polygenic Risk Scores and Hypertension in the Bogalusa Heart Study. Genes. 2022; 13(8):1473. https://doi.org/10.3390/genes13081473
Chicago/Turabian StyleSun, Xiao, Yang Pan, Ruiyuan Zhang, Ileana De Anda-Duran, Zhijie Huang, Changwei Li, Mengyao Shi, Alexander C. Razavi, Lydia A. Bazzano, Jiang He, and et al. 2022. "Life-Course Associations between Blood Pressure-Related Polygenic Risk Scores and Hypertension in the Bogalusa Heart Study" Genes 13, no. 8: 1473. https://doi.org/10.3390/genes13081473
APA StyleSun, X., Pan, Y., Zhang, R., De Anda-Duran, I., Huang, Z., Li, C., Shi, M., Razavi, A. C., Bazzano, L. A., He, J., Sofer, T., & Kelly, T. N. (2022). Life-Course Associations between Blood Pressure-Related Polygenic Risk Scores and Hypertension in the Bogalusa Heart Study. Genes, 13(8), 1473. https://doi.org/10.3390/genes13081473







