MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues Collected and RNA Isolation
2.2. Real-Time Quantitative PCR (RT-qPCR)
2.3. Vector Construction
2.4. Cell Culture and Differentiation
2.5. Oil Red O Staining
2.6. Cell Proliferation Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
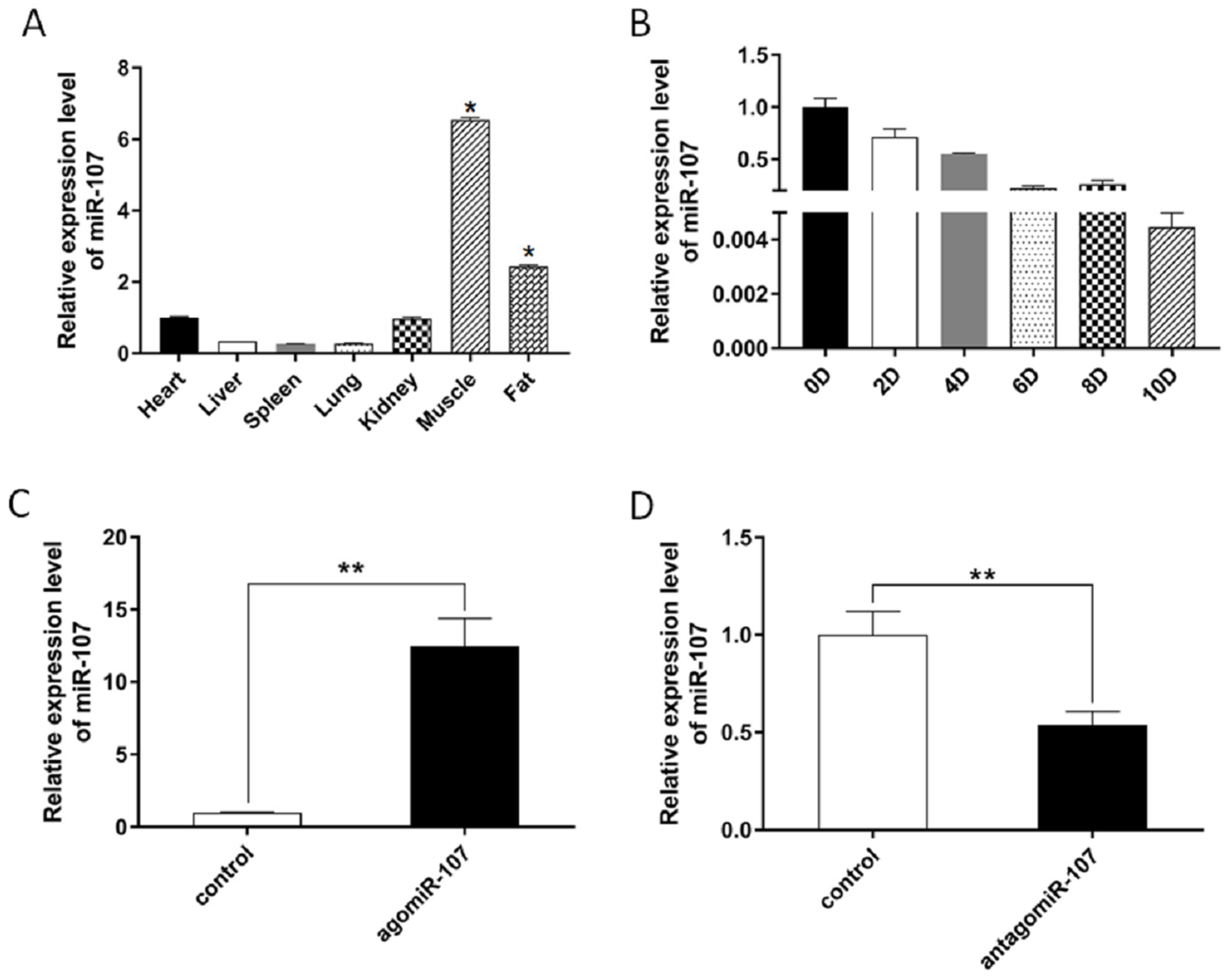
3.1. miR-107 Expression Profiles in Different Tissues
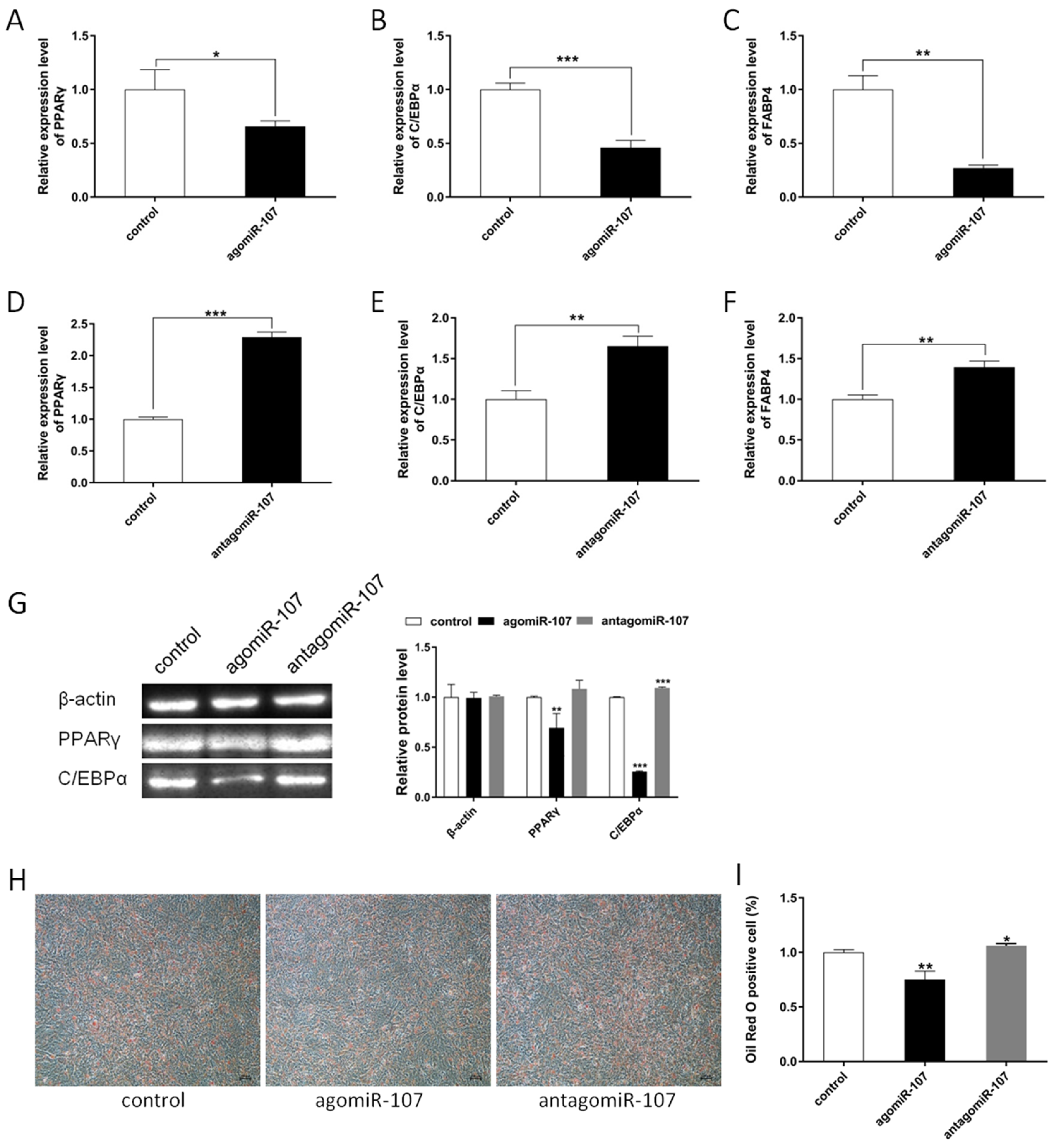
3.2. miR-107 Suppressed Adipocyte Differentiation
3.3. miR-107 Suppressed Adipocyte Proliferation
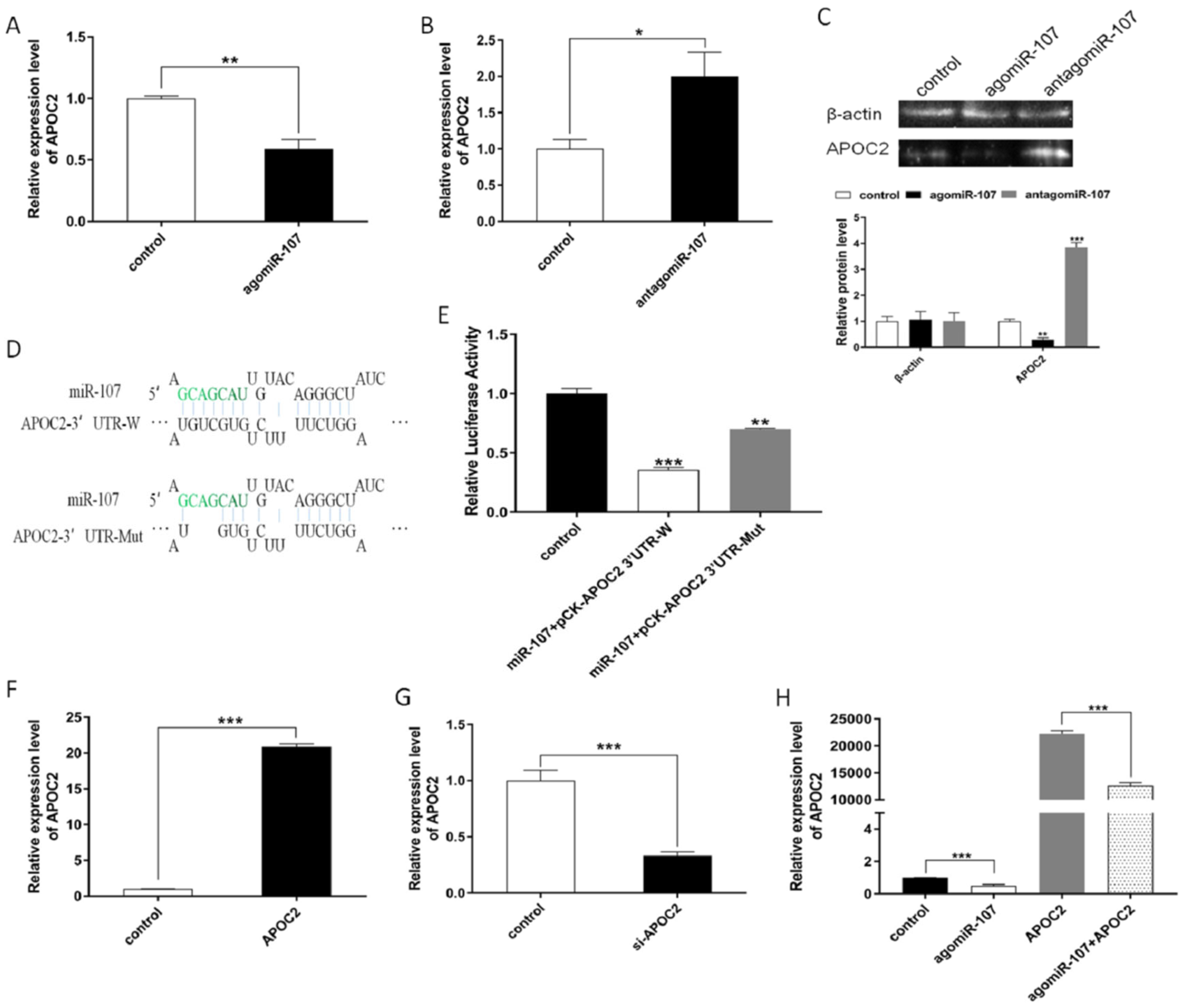
3.4. miR-107 Directly Targeted APOC2
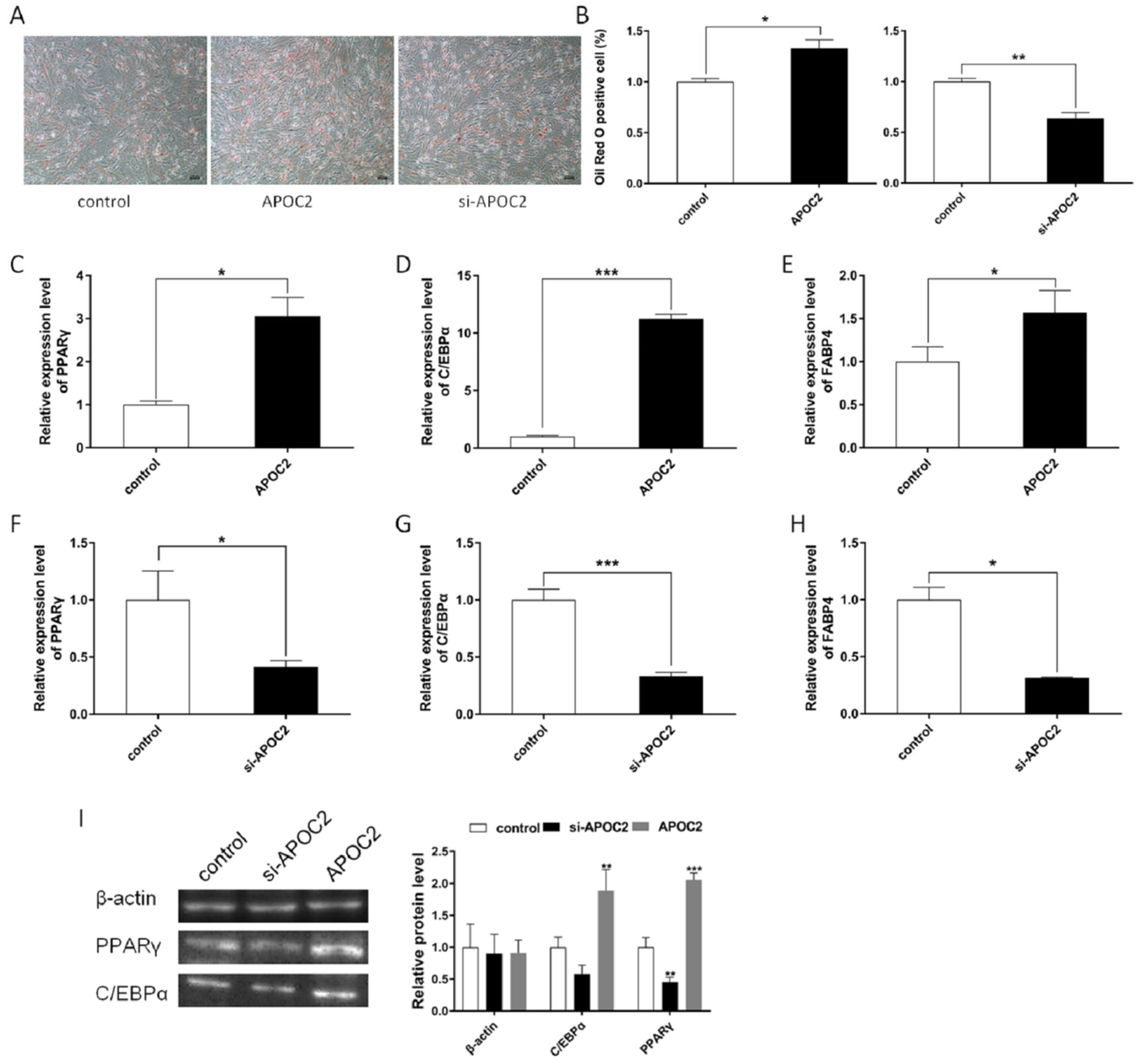
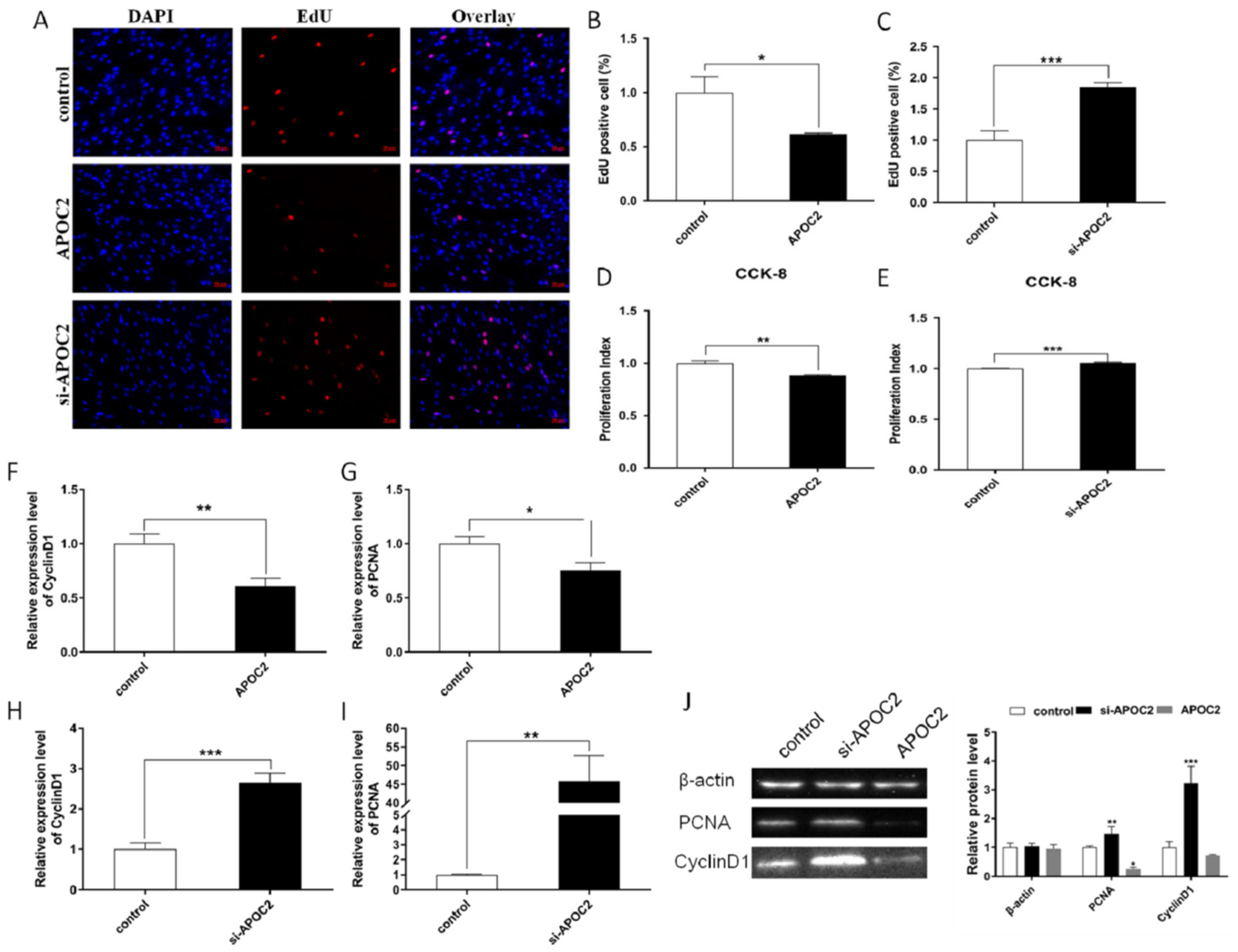
3.5. APOC2 Regulated Adipocyte Differentiation and Proliferation
4. Discussion
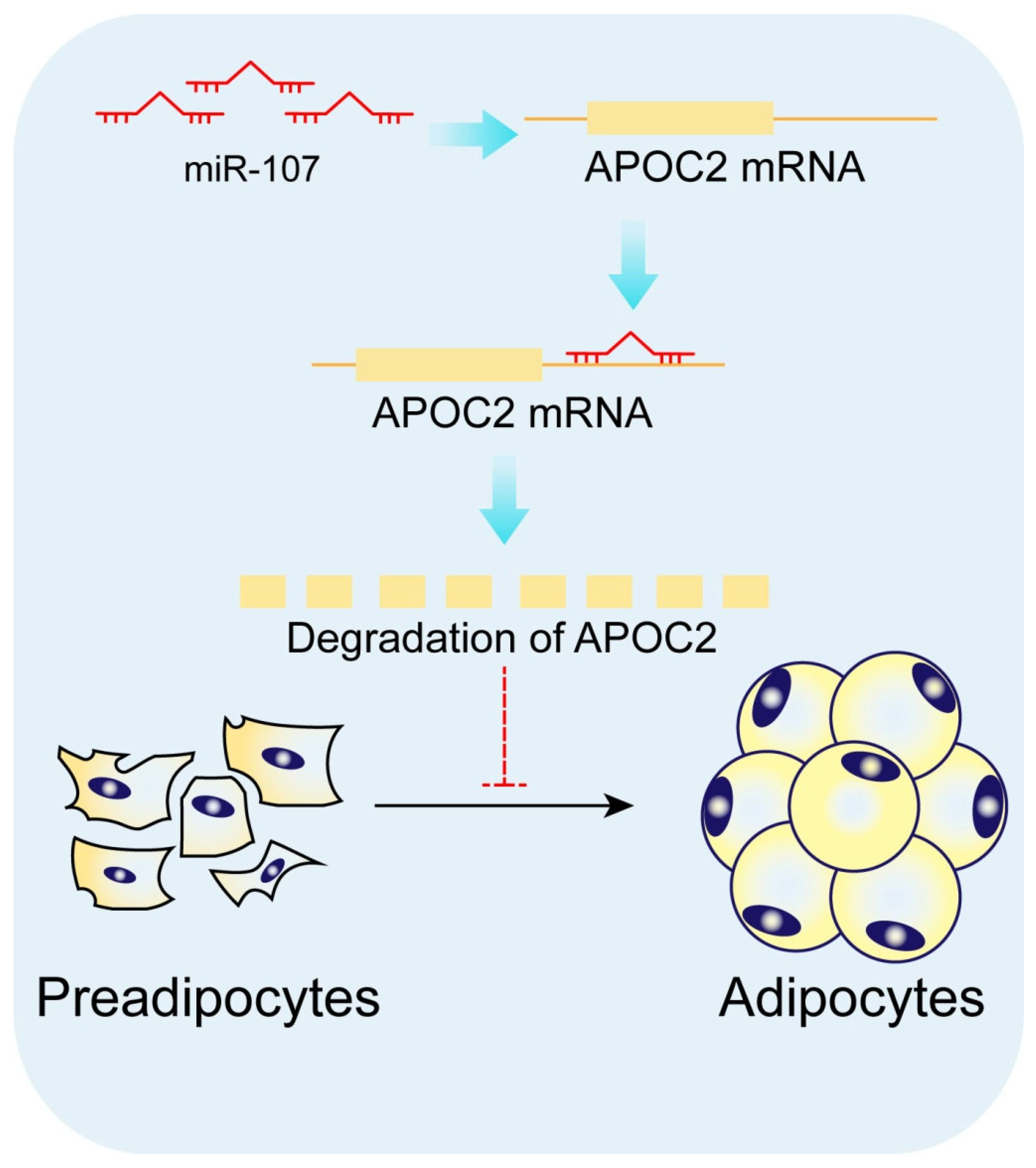
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lefterova, M.I.; Lazar, M.A. New Developments in Adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M. The Fat Controller: Adipocyte Development. PLoS Biol. 2012, 10, e1001436. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, A.Y.; Lee, H.W.; Son, Y.H.; Lee, G.Y.; Lee, J.W.; Lee, Y.S.; Kim, J.B. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem. Biophys. Res. Commun. 2010, 392, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Guan, L.; Qi, Q.; Shu, G.; Jiang, Q.; Yuan, L.; Xi, Q.; Zhang, Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-alpha (TNF-alpha) in the porcine model. PLoS ONE 2013, 8, e71568. [Google Scholar]
- Goljanek-Whysall, K.; Pais, H.; Rathjen, T.; Sweetman, D.; Dalmay, T.; Munsterberg, A. Regulation of multiple target genes by miR-1 and miR-206 is pivotal for C2C12 myoblast differentiation. J. Cell Sci. 2012, 125, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, H.; Zhang, B.; Li, C.; Dong, D.; Lan, X.; Huang, Y.; Bai, Y.; Lin, F.; Zhao, X.; et al. miR-378a-3p promotes differentiation and inhibits proliferation of myoblasts by targeting HDAC4 in skeletal muscle development. RNA Biol. 2006, 13, 1300–1309. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, F.; Song, R.; Li, Y.; Wu, P.; Chen, L.; Cheng, J.; Du, S.; Zhang, J. Proteomic and microRNA Transcriptome Analysis revealed the microRNA-SmyD1 network regulation in Skeletal Muscle Fibers performance of Chinese perch. Sci. Rep. 2007, 7, 16498. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Yang, J.; Dong, D.; Hao, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Ma, Y.; et al. circFGFR4 Promotes Differentiation of Myoblasts via Binding miR-107 to Relieve Its Inhibition of Wnt3a. Mol. Ther. Nucleic Acids 2018, 11, 272–283. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Wang, L.; Zhou, Q.; Li, Y.; Shen, C.; Zhang, X.; Wang, C. MicroRNA 107 Partly Inhibits Endothelial Progenitor Cells Differentiation via HIF-1b. PLoS ONE 2012, 7, e40323. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. miR-107-5p promotes tumor proliferation and invasion by targeting estrogen receptoralpha in endometrial carcinoma. Oncol. Rep. 2019, 41, 1575–1585. [Google Scholar] [PubMed]
- Li, F.; Liu, B.; Gao, Y.; Liu, Y.; Xu, Y.; Tong, W.; Zhang, A. Upregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014, 588, 538–544. [Google Scholar] [CrossRef]
- Jiang, J.D.; Zheng, X.C.; Huang, F.Y.; Gao, F.; You, M.Z.; Zheng, T. MicroRNA-107 regulates anesthesia-induced neural injury in embryonic stem cell derived neurons. IUBMB Life 2019, 71, 20–27. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B.; Lan, X.; Zhang, C.; Lei, C.; Chen, H. Comparative transcriptome analysis reveals significant differences in MicroRNA expression and their target genes between adipose and muscular tissues in cattle. PLoS ONE 2014, 9, e102142. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Jong, M.C.; Hofker, M.H.; Havekes, L.M. Role of ApoCs in Lipoprotein Metabolism Functional Differences Between ApoC1, ApoC2, and ApoC3. Arter. Thromb. Vasc. Biol. 1999, 19, 472–484. [Google Scholar] [CrossRef]
- Isshiki, M.; Hirayama, S.; Ueno, T.; Ito, M.; Furuta, A.; Yano, K.; Yamatani, K.; Sugihara, M.; Idei, M.; Miida, T. Apolipoproteins C-II and C-III as nutritional markers unaffected by inflammation. Clin. Chim. Acta 2018, 481, 225–230. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Ling, Y.; Kayoumu, A.; Liu, G.; Gao, X. A novel APOC2 gene mutation identified in a Chinese patient with severe hypertriglyceridemia and recurrent pancreatitis. Lipids Health Dis. 2016, 15, 12. [Google Scholar] [CrossRef]
- Liu, C.; Gates, K.P.; Fang, L.; Amar, M.J.; Schneider, D.A.; Geng, H.; Huang, W.; Kim, J.; Pattison, J.; Zhang, J.; et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Models Mech. 2015, 8, 989–998. [Google Scholar] [CrossRef]
- Wei, X.; Han, S.; Wang, S.; Zheng, Q.; Li, X.; Du, J.; Zhao, J.; Li, F.; Ma, Y. ANGPTL8 regulates adipocytes differentiation and adipogenesis in bovine. Gene 2019, 707, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, T.; Qiao, S. miR-1 and miR-802 regulate mesenchymal-epithelial transition during kidney development by regulating Wnt-4_β-catenin signaling. Am. J. Transl. Res. 2019, 11, 7000–7008. [Google Scholar]
- Romao, J.M.; Jin, W.; Dodson, M.V.; Hausman, G.J.; Moore, S.S.; Guan, L.L. MicroRNA regulation in mammalian adipogenesis. Exp. Biol. Med. 2011, 236, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, J.; Fan, G.G.; Zou, Z.Y.; Yin, Y.L.; Li, G.X. MiRNA-324-5p inhibits inflammatory response of diabetic vessels by targeting CPT1A. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12836–12843. [Google Scholar]
- Gu, J.; Han, T.; Sun, L.; Yan, A.H.; Jiang, X.J. miR-552 promotes laryngocarcinoma cells proliferation and metastasis by targeting p53 pathway. Cell Cycle 2020, 19, 1012–1021. [Google Scholar] [CrossRef]
- Foley, N.H.; O’Neill, L.A. miR-107: A toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetes. J. Leukoc. Biol. 2012, 92, 521–527. [Google Scholar] [CrossRef]
- Sharma, P.; Saini, N.; Sharma, R. miR-107 functions as a tumor suppressor in human esophageal squamous cell carcinoma and targets Cdc42. Oncol. Rep. 2017, 37, 3116–3127. [Google Scholar] [CrossRef][Green Version]

| Name | Forward Primer 5′→3′ | Reverse Primer 5′→3′ |
|---|---|---|
| β-actin | CTGGCATTGTCATGGACTCTG | GCTCGGCTGTGGTGGTAAA |
| PPARγ | AGCCCAAGTTCGAGTTTGCT | TCTTGTATGTCCTCAATGGGCT |
| C/EBPα | TGGACAAGAACAGCAACGAG | TTGTCACTGGTCAGCTCCAG |
| FABP4 | AAGTCAAGAGCATCGTAA | CCAGCACCATCTTATCAT |
| CyclinD1 | CGAGCACTTCCTCTCCAAGA | AATGAACTTCACGTCTGTGGCA |
| PCNA | TCCAGAACAAGAGTATAGC | TACAACAGCATCTCCAAT |
| AOPC2 | ATACAGCAAGAGCACGGCA | TCCTGACAGCACAGAAAAGACC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| miR-107 | ggAGCAGCATTGTACAGGGCTATC | GCAGGGTCCGAGGTATTC |
| miR-107 stem-loop primer | gtcgtatccagtgcagggtccgaggtattcgcactggatacgacGATAGC | |
| Name | Forward Primer 5′→3′ | Reverse Primer 5′→3′ |
|---|---|---|
| APOC2-CDS | CCAAGCTTGGAGAGTCTGCCACCTCAGTGTT | CGGAATTCCGGGCTAGAATTGGGAACAGGA |
| APOC2-3′UTR | CCGCTCGAGCAGGGATTATTACTGACCAGG | ATAAGAATGCGGCCGCGCTAGAATTGGGAACAGGATG |
| APOC2-3′UTR-Mut-1 | GCTGAGGACAGCCGCC | CACAGAAAAGACCTGGTCAGTA |
| APOC2-3′UTR-Mut-2 | CTGACCAGGTCTTTTCTGTGTCAGGAAAAGATTAA | GATAAGTCAGGCTAGAATTGGGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Zhao, X.; Shan, X.; Zhu, Y.; Wang, S.; Chen, H.; Li, H.; Ma, Y. MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine. Genes 2022, 13, 1467. https://doi.org/10.3390/genes13081467
Wei X, Zhao X, Shan X, Zhu Y, Wang S, Chen H, Li H, Ma Y. MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine. Genes. 2022; 13(8):1467. https://doi.org/10.3390/genes13081467
Chicago/Turabian StyleWei, Xuefeng, Xue Zhao, Xinyue Shan, Yunchang Zhu, Shuzhe Wang, Hong Chen, Hui Li, and Yun Ma. 2022. "MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine" Genes 13, no. 8: 1467. https://doi.org/10.3390/genes13081467
APA StyleWei, X., Zhao, X., Shan, X., Zhu, Y., Wang, S., Chen, H., Li, H., & Ma, Y. (2022). MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine. Genes, 13(8), 1467. https://doi.org/10.3390/genes13081467






