What Causes Cancer in Women with a gBRCA Pathogenic Variant? Counselees’ Causal Attributions and Associations with Perceived Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedures and Participants
2.2. Measurement
2.3. Data Analysis
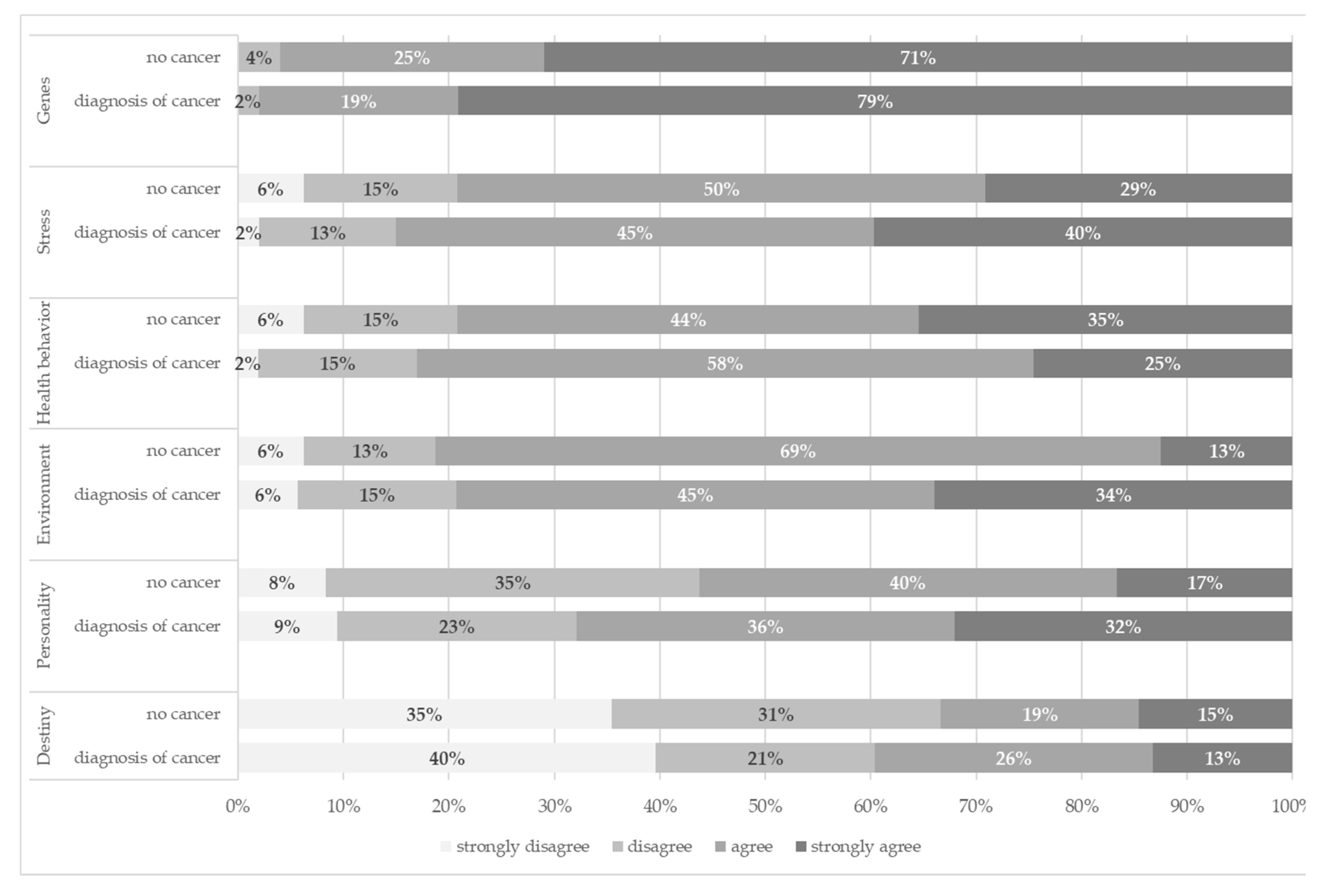
3. Results
3.1. Sample Characteristics
3.2. Causal Attributions and Control
3.3. The Association of Causal Attributions and Personal Control
4. Discussion
4.1. Stress and Personality as Causal Factors
4.2. Fate as a Causal Factor
4.3. Personal Control
4.4. Study Limitations
4.5. Practical Implications for Counseling Women with gBRCA1/2-PV
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meindl, A.; Ditsch, N.; Kast, K.; Rhiem, K.; Schmutzler, R.K. Hereditary breast and ovarian cancer: New genes, new treatments, new concepts of the article. Dtsch. Arztebl. Int. 2011, 108, 323–330. [Google Scholar] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies of the article. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers of the article. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, K.; Ditsch, N.; Kiechle, M. Lifestyle und erblicher Brustkrebs. Welche nicht-genetischen Faktoren beeinflussen das Erkrankungsrisiko? of the article. Med. Genet. 2015, 27, 237–243. [Google Scholar]
- van Erkelens, A.; Derks, L.; Sie, A.S.; Egbers, L.; Woldringh, G.; Prins, J.B.; Manders, P.; Hoogerbrugge, N. Lifestyle Risk Factors for Breast Cancer in BRCA1/2-Mutation Carriers Around Childbearing Age of the article. J. Genet. Couns. 2017, 26, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) of the article. Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer of the article. Breast Cancer (Dove Med. Press) 2021, 13, 241–257. [Google Scholar]
- Lammert, J.; Grill, S.; Kiechle, M. Modifiable Lifestyle Factors: Opportunities for (Hereditary) Breast Cancer Prevention—A Narrative Review of the article. Breast Care 2018, 13, 109–114. [Google Scholar] [CrossRef]
- Dumalaon-Canaria, J.A.; Hutchinson, A.D.; Prichard, I.; Wilson, C. What causes breast cancer? A systematic review of causal attributions among breast cancer survivors and how these compare to expert-endorsed risk factors of the article. Cancer Causes Control 2014, 25, 771–785. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Gyure, M.E.; Corona, R.; Bodurtha, J.N.; Bowen, D.J.; Quillin, J.M. What women think: Cancer causal attributions in a diverse sample of women of the article. J. Psychosoc. Oncol. 2015, 33, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Hagger, M.S.; Koch, S.; Chatzisarantis, N.L.D.; Orbell, S. The common sense model of self-regulation: Meta-analysis and test of a process model of the article. Psychol. Bull. 2017, 143, 1117–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, E.M.; Schuz, N.; Sanderson, K.; Scott, J.L.; Schuz, B. Illness representations, coping, and illness outcomes in people with cancer: A systematic review and meta-analysis of the article. Psychooncology 2017, 26, 724–737. [Google Scholar] [CrossRef]
- Rabin, C.; Pinto, B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives of the article. Psychooncology 2006, 15, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, E.S.; Lutgendorf, S.K.; Roeder, S.L. Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer of the article. Psychooncology 2011, 20, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Weinman, J.; Petrie, K.J.; Sharpe, N.; Walker, S. Causal attributions in patients and spouses following first-time myocardial infarction and subsequent lifestyle changes of the article. Br. J. Health Psychol. 2000, 5, 263–273. [Google Scholar] [CrossRef]
- Speiser, D.; Rebitschek, F.G.; Feufel, M.A.; Brand, H.; Besch, L.; Kendel, F. Accuracy in risk understanding among BRCA1/2-mutation carriers of the article. Patient Educ. Couns. 2019, 102, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Brand, H.; Speiser, D.; Besch, L.; Roseman, J.; Kendel, F. Making Sense of a Health Threat: Illness Representations, Coping, and Psychological Distress among BRCA1/2 Mutation Carriers of the article. Genes 2021, 12, 741. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire of the article. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef]
- Glattacker, M.; Bengel, J.; Jäckel, W. Die deutschsprachige Version des Illness Perception Questionnaire-Revised. Psychometrische Evaluation an Patienten mit chronisch somatischen Erkrankungen of the article. Z. Für Gesundheitspsychol. 2009, 17, 158–169. [Google Scholar] [CrossRef]
- Vos, J.; Stiggelbout, A.M.; Oosterwijk, J.; Gomez-Garcia, E.; Menko, F.; Collee, J.M.; van Asperen, C.J.; Tibben, A. A counselee-oriented perspective on risk communication in genetic counseling: Explaining the inaccuracy of the counselees’ risk perception shortly after BRCA1/2 test result disclosure of the article. Genet. Med. 2011, 13, 800–811. [Google Scholar] [CrossRef]
- Anderson, G. Storytelling: A holistic foundation for genetic nursing of the article. Holist. Nurs. Pract. 1998, 12, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, M.; Engel, C.; Berling, A.; Hebestreit, K.; Bischoff, S.C.; Dukatz, R.; Siniatchkin, M.; Pfeifer, K.; Grill, S.; Yahiaoui-Doktor, M.; et al. Effects of lifestyle intervention in BRCA1/2 mutation carriers on nutrition, BMI, and physical fitness (LIBRE study): Study protocol for a randomized controlled trial of the article. Trials 2016, 17, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiechle, M.; Dukatz, R.; Yahiaoui-Doktor, M.; Berling, A.; Basrai, M.; Staiger, V.; Niederberger, U.; Marter, N.; Lammert, J.; Grill, S.; et al. Feasibility of structured endurance training and Mediterranean diet in BRCA1 and BRCA2 mutation carriers—An interventional randomized controlled multicenter trial (LIBRE-1) of the article. BMC Cancer 2017, 17, 752. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer—Review paper of the article. Ann. Agric. Environ. Med. 2019, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.J.; Myrtveit, S.M.; Partridge, A.H.; Stephens, M.; Stanton, A.L. The relationship between the belief in a genetic cause for breast cancer and bilateral mastectomy of the article. Health Psychol. 2015, 34, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Dumalaon-Canaria, J.A.; Prichard, I.; Hutchinson, A.D.; Wilson, C. Fear of cancer recurrence and psychological well-being in women with breast cancer: The role of causal cancer attributions and optimism of the article. Eur. J. Cancer Care 2018, 27, e12579. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Jeon, Y.W.; Im, E.O.; Baek, J.M. Causal Attributions and Quality of Life of Korean Breast Cancer Survivors of the article. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2021, 15, 53–59. [Google Scholar]
- Niebauer, E.; Fry, N.; Auster-Gussman, L.A.; Wahbeh, H. Patient perspectives on the causes of breast cancer: A qualitative study on the relationship between stress, trauma, and breast cancer development of the article. Int. J. Qual. Stud. Health Well-Being 2021, 16, 1983949. [Google Scholar] [CrossRef]
- Nakaya, N.; Bidstrup, P.E.; Saito-Nakaya, K.; Frederiksen, K.; Koskenvuo, M.; Pukkala, E.; Kaprio, J.; Floderus, B.; Uchitomi, Y.; Johansen, C. Personality traits and cancer risk and survival based on Finnish and Swedish registry data of the article. Am. J. Epidemiol. 2010, 172, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.E.; Lichtman, R.R.; Wood, J.V. Attributions, beliefs about control, and adjustment to breast cancer of the article. J. Personal. Soc. Psychol. 1984, 46, 489–502. [Google Scholar] [CrossRef]
- Weiner, B. An Attributional Theory of Motivation and Emotion; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Taylor, E.J. Whys and wherefores: Adult patient perspectives of the meaning of cancer of the article. Semin. Oncol. Nurs. 1995, 11, 32–40. [Google Scholar] [CrossRef]
- Shiloh, S.; Rashuk-Rosenthal, D.; Benyamini, Y. Illness causal attributions: An exploratory study of their structure and associations with other illness cognitions and perceptions of control of the article. J. Behav. Med. 2002, 25, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Moss-Morris, R.; Weinman, J.; Petrie, K.; Horne, R.; Cameron, L.; Buick, D. The revised Illness Perception Questionnaire (IPQ-R) of the article. Psychol. Health 2002, 17, 1–16. [Google Scholar] [CrossRef]
- Kenen, R.; Ardern-Jones, A.; Eeles, R. Living with chronic risk: Healthy women with a family history of breast/ovarian cancer of the article. Health Risk Soc. 2003, 5, 315–331. [Google Scholar] [CrossRef]
- Leventhal, H.; Leventhal, E.; Contrada, R. Self-regulation, health, and behavior: A perceptual-cognitive approach of the article. Psychol. Health 1998, 13, 717–733. [Google Scholar] [CrossRef]
- Dunkel, A.; Kendel, F.; Lehmkuhl, E.; Hetzer, R.; Regitz-Zagrosek, V. Causal attributions among patients undergoing coronary artery bypass surgery: Gender aspects and relation to depressive symptomatology of the article. J. Behav. Med. 2011, 34, 351–359. [Google Scholar] [CrossRef]
| Entire Sample (N = 101) | Diagnosis of Breast/ Ovarian Cancer (n = 53) | No Breast/ Ovarian Cancer (n = 48) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), M (SD) | 43.3 (10.9) | 46.3 (9.8) | 40.0 (11.2) |
| Partnership, n (%) | |||
| Living with a partner | 80 (79.2%) | 38 (71.7%) | 40 (83.3%) |
| Living without a partner | 21 (20.8%) | 13 (24.5%) | 8 (16.7%) |
| Presence of children, n (%) | 69 (68.4%) | 37 (69.8%) | 32 (66.7%) |
| Level of education, n (%) | |||
| High school degree | 60 (59.4%) | 27 (50.9%) | 33 (68.7%) |
| Secondary school | 31 (30.7%) | 17 (32.1%) | 14 (29.2%) |
| Occupation status, n (%) | |||
| Employed | 72 (71.3%) | 34 (64.2%) | 38 (79.2%) |
| Unemployed | 13 (12.9%) | 7 (13.2%) | 6 (12.6%) |
| Retired | 14 (13.9%) | 11 (20.8%) | 3 (6.3%) |
| Clinical characteristics, n (%) | |||
| Prophylactic surgery | 46 (45.5%) | 17 (32.1%) | 29 (60.4%) |
| No Prophylactic surgery | 13 (12.9%) | 9 (17%) | 4 (8.3%) |
| Prophylactic surgery | 27 (26.7%) | 13 (24.5%) | 14 (29.2%) |
| Prophylactic salpingo-oophorectomy | 11 (10.9%) | 10 (18.9%) | 1 (2.1%) |
| Mastectomy and salpingo-oophorectomy | |||
| History of cancer, n (%) | |||
| Breast cancer | - | 44 (83.3%) | - |
| Ovarian Cancer | - | 5 (9.4%) | - |
| Months since diagnosis, M (SD) | - | 62.1 (62.5) | - |
| Pathogenic germline variant, n (%) | |||
| gBRCA1 | 62 (61.4%) | 35 (66%) | 27 (56.3%) |
| gBRCA2 | 39 (38.6%) | 18 (34%) | 21 (43.8%) |
| Months since genetic analysis, M (SD) | 14.2 (12.6) | 14.3 (11.7) | 14.1 (13.6) |
| Personal Control | Stress | Genes | Personality | Health Behavior | Destiny | Environment | |
|---|---|---|---|---|---|---|---|
| Treatment control | --- | −0.03 | 0.20 * | 0.13 | 0.21 * | −0.01 | <0.01 |
| Personal control | --- | 0.22 | −0.12 | 0.39 ** | 0.44 ** | −0.02 | 0.22 ** |
| Stress | 1 | −0.06 | 0.40 ** | 0.11 ** | 0.15 * | 0.33 ** | |
| Genes | 1 | −0.01 *** | 0.17 ** | −0.01 | −0.10 *** | ||
| Personality | 1 | 0.36 ** | 0.14 * | 0.33 ** | |||
| Health behavior | 1 | −0.02 | 0.32 ** | ||||
| Destiny | 1 | 0.22 ** | |||||
| Environment | 1 |
| Predictor | SE | 95%-CI | T | p | |
|---|---|---|---|---|---|
| Intercept | 4.026 | 1.379 | [1.287, 6.764] | 2.919 | 0.004 |
| Stress | 0.196 | 0.294 | [−0.387, 0.780] | 0.669 | 0.505 |
| Genes | −0.725 | 0.419 | [−1.556, 0.106] | −1.733 | 0.086 |
| Personality | 0.540 | 0.265 | [0.013, 1.067] | 2.034 | 0.045 * |
| Health behavior | 1.062 | 0.316 | [0.435, 1.689] | 3.364 | 0.001 ** |
| Destiny | −0.156 | 0.202 | [−0.557, 0.245] | −0.772 | 0.442 |
| Environment | 0.086 | 0.313 | [−0.536, 0.708] | 0.275 | 0.784 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kendel, F.; Klein, K.; Schüürhuis, S.; Besch, L.; Feufel, M.A.; Speiser, D. What Causes Cancer in Women with a gBRCA Pathogenic Variant? Counselees’ Causal Attributions and Associations with Perceived Control. Genes 2022, 13, 1399. https://doi.org/10.3390/genes13081399
Kendel F, Klein K, Schüürhuis S, Besch L, Feufel MA, Speiser D. What Causes Cancer in Women with a gBRCA Pathogenic Variant? Counselees’ Causal Attributions and Associations with Perceived Control. Genes. 2022; 13(8):1399. https://doi.org/10.3390/genes13081399
Chicago/Turabian StyleKendel, Friederike, Katharina Klein, Stephen Schüürhuis, Laura Besch, Markus A. Feufel, and Dorothee Speiser. 2022. "What Causes Cancer in Women with a gBRCA Pathogenic Variant? Counselees’ Causal Attributions and Associations with Perceived Control" Genes 13, no. 8: 1399. https://doi.org/10.3390/genes13081399
APA StyleKendel, F., Klein, K., Schüürhuis, S., Besch, L., Feufel, M. A., & Speiser, D. (2022). What Causes Cancer in Women with a gBRCA Pathogenic Variant? Counselees’ Causal Attributions and Associations with Perceived Control. Genes, 13(8), 1399. https://doi.org/10.3390/genes13081399






