Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome
Abstract
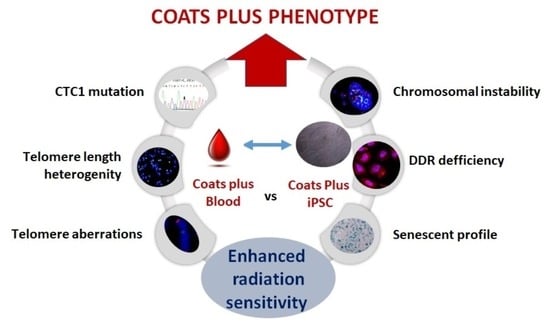
:1. Introduction
2. Materials and Methods
2.1. iPSC Line and Derivative Cells
2.2. Preparation of Metaphase Spreads
2.3. Telomere–Centromere Staining Followed by M-FISH Technique (TC + M-FISH)
2.4. Telomere Quantification
2.5. Scoring of Telomeres and Chromosomal Aberrations
2.6. Micronucleus Assay
2.7. In Vitro Irradiation
2.8. Immunofluorescence and Immunofluorecent FISH (IF-FISH)
2.9. Senescence β-Galactosidase Cell Staining
2.10. Statistical Analysis
3. Results
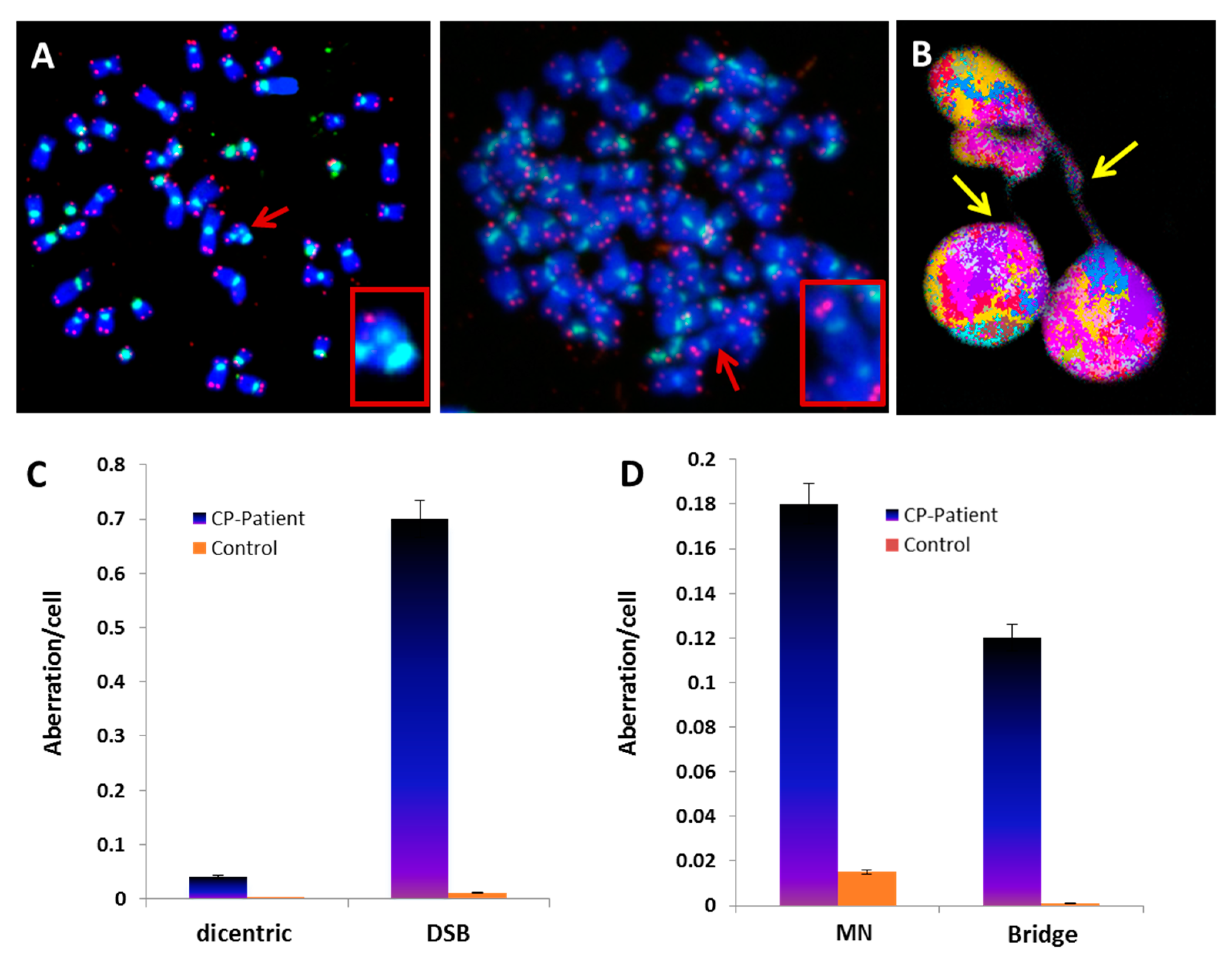
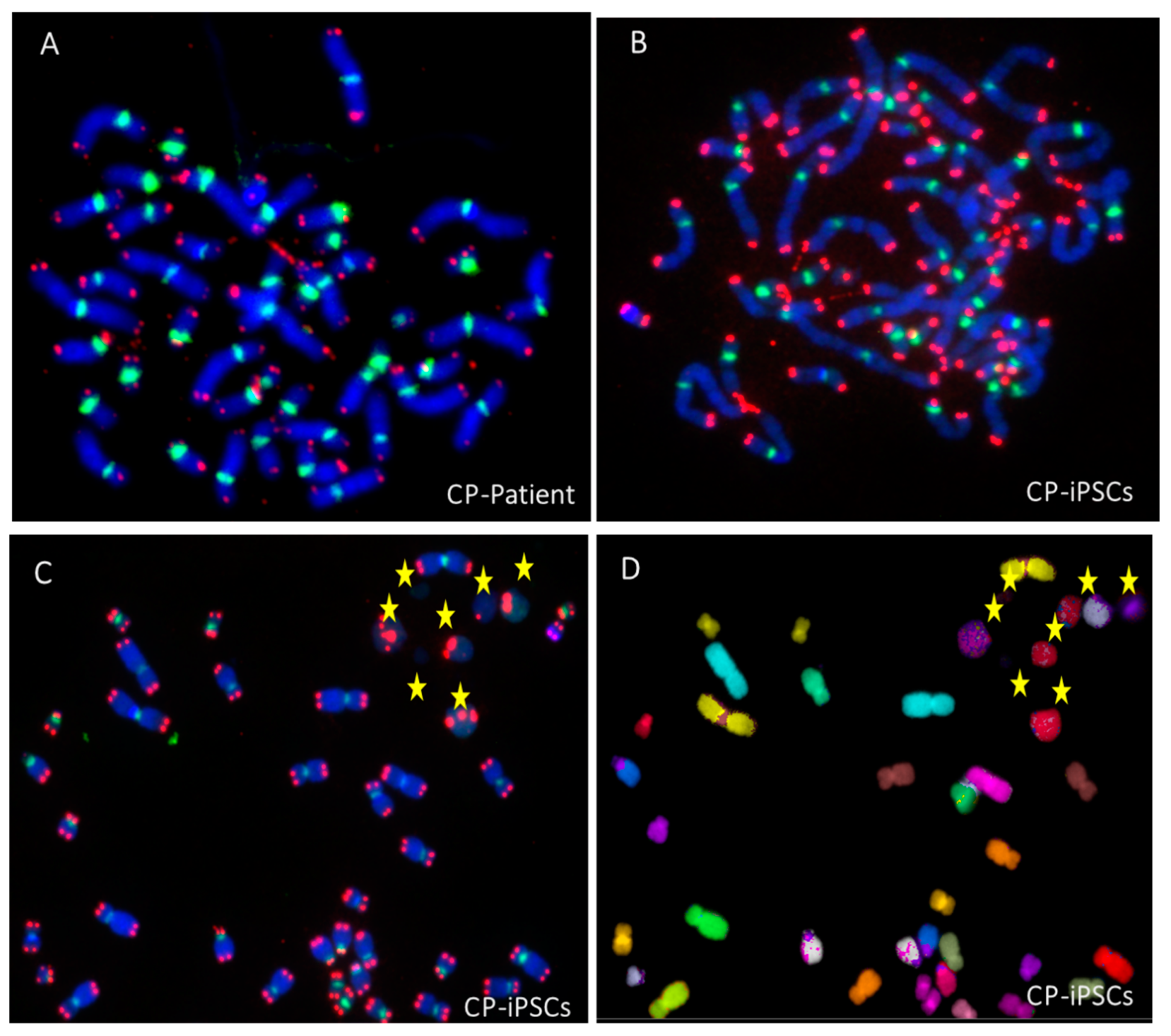
3.1. Telomere Heterogeneity and Aberrations and Chromosomal Instability in Peripheral Blood Lymphocytes of a CP Patient
3.2. Establishment of Coats Plus Induced Pluripotent Stem Cell Line
3.3. Evaluation of the Involvement of Telomerase and/or ALT Pathway for Telomere Maintenance in CP Cells
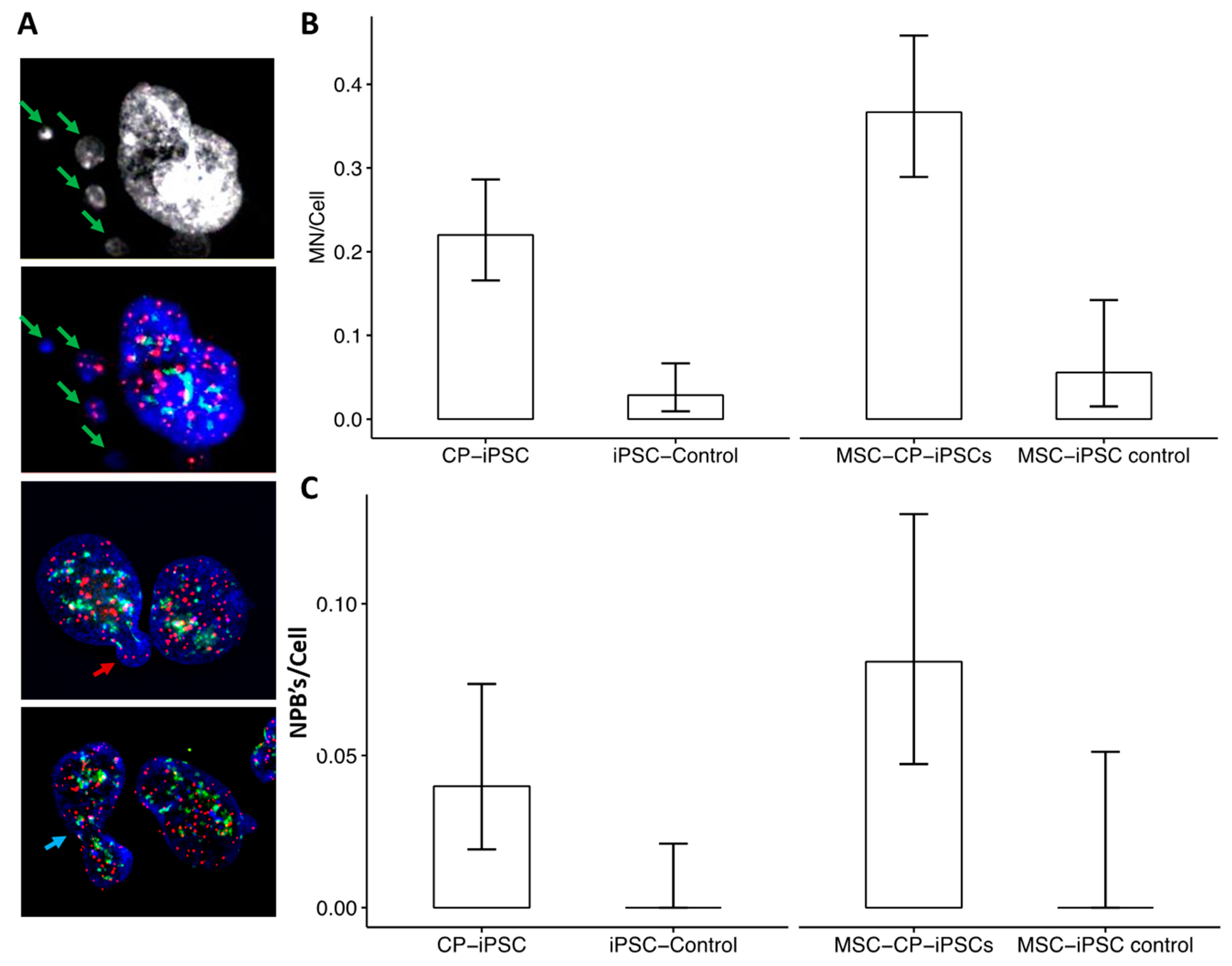
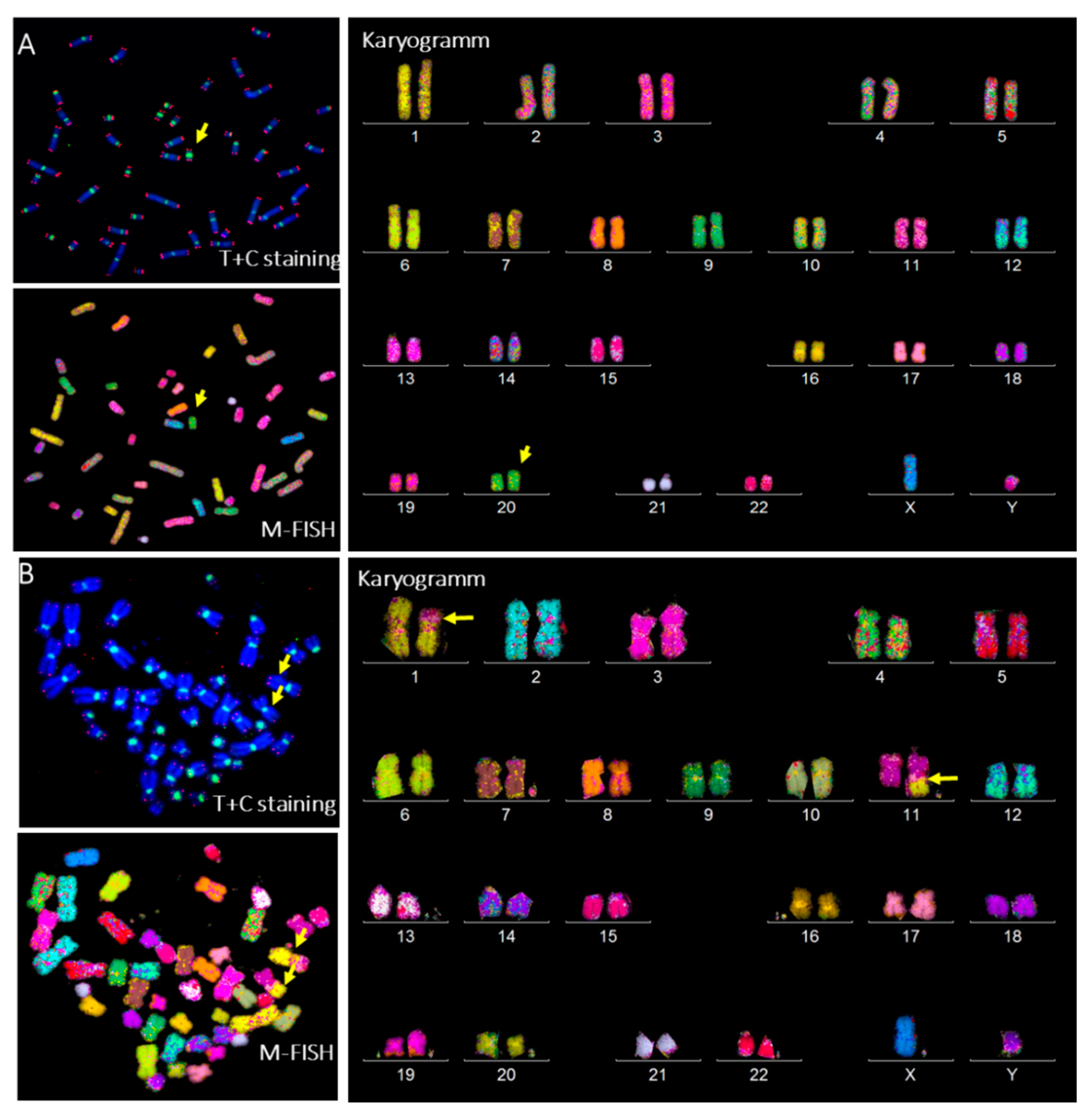
3.4. Aberrant Telomere Elongation and Chromosomal Instability in CP-iPSCs and Derivative Cells
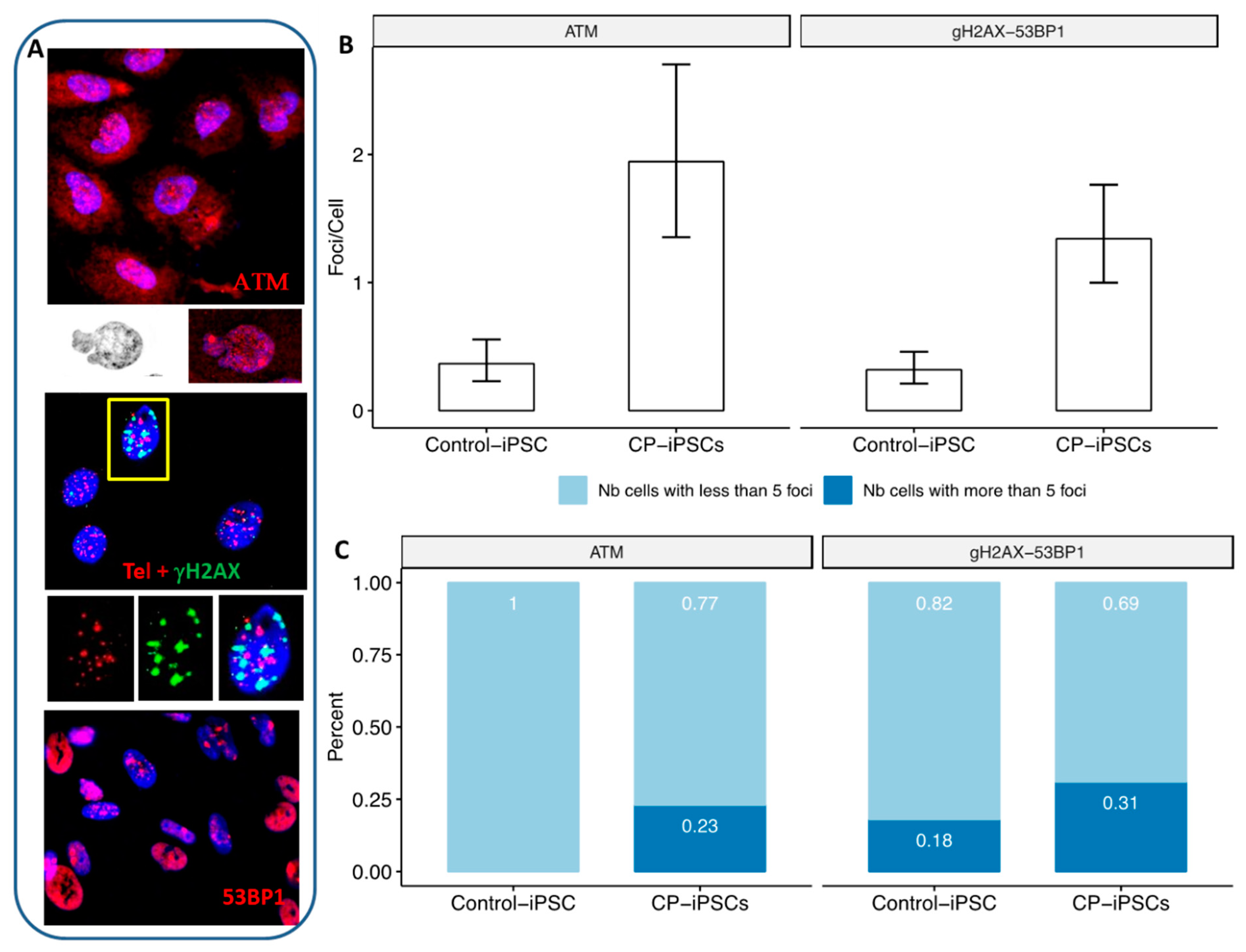
3.5. CP-iPSCs Exhibit Features of Cellular Senescence and Increased Radiosensitivity
3.6. Radiation-Induced Chromosome Instability and Formation of Clonal Dicentric Chromosomes in CP-iPSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Savage, S.A. Beginning at the ends: Telomeres and human disease. F1000Research 2018, 7, 524. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings-what telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Herate, C.; Sabatier, L. Telomere instability initiates and then boosts carcinogenesis by the butterfly effect. Curr. Opin. Genet. Dev. 2020, 60, 92–98. [Google Scholar] [CrossRef]
- Timashev, L.A.; Babcock, H.; Zhuang, X.; de Lange, T. The DDR at telomeres lacking intact shelterin does not require substantial chromatin decompaction. Genes Dev. 2017, 31, 578–589. [Google Scholar] [CrossRef]
- Anderson, B.H.; Kasher, P.R.; Mayer, J.; Szynkiewicz, M.; Jenkinson, E.M.; Bhaskar, S.S.; Urquhart, J.E.; Daly, S.B.; Dickerson, J.E.; O’Sullivan, J.; et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat. Genet. 2012, 44, 338–342. [Google Scholar] [CrossRef]
- Bisserbe, A.; Tertian, G.; Buffet, C.; Turhan, A.; Lambotte, O.; Nasser, G.; Alvin, P.; Tardieu, M.; Riant, F.; Bergametti, F.; et al. Cerebro-retinal microangiopathy with calcifications and cysts due to recessive mutations in the CTC1 gene. Rev. Neurol. 2015, 171, 445–449. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Udyaver, S.; Lim, L.S.; Mazloumi, M.; Atalay, H.T.; Khoo, C.T.L.; Shields, C.L. Coats Disease: Clinical Features and Outcomes by Age Category in 351 Cases. J. Pediatr. Ophthalmol. Strabismus 2019, 56, 288–296. [Google Scholar] [CrossRef]
- Chen, L.Y.; Majerská, J.; Lingner, J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev. 2013, 27, 2099–2108. [Google Scholar] [CrossRef]
- Gu, P.; Chang, S. Functional characterization of human CTC1 mutations reveals novel mechanisms responsible for the pathogenesis of the telomere disease Coats plus. Aging Cell 2013, 12, 1100–1109. [Google Scholar] [CrossRef]
- Rechavi, G.; Somech, R.; Wang, Y.; Chai, W. Pathogenic CTC1 mutations cause global genome instabilities under replication stress. J. Exp. Med. 2018, 46, 3981–3992. [Google Scholar] [CrossRef]
- Simon, A.J.; Lev, A.; Zhang, Y.; Weiss, B.; Rylova, A.; Eyal, E.; Kol, N.; Barel, O. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J. Exp. Med. 2016, 213, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Sanchis, D.; Xu, Y.; Taheem, D.; Yu, M.; Tilgner, K.; Barta, T.; Gassner, K.; Anyfantis, G.; Wan, T.; Elango, R.; et al. iPSC modeling of severe aplastic anemia reveals impaired differentiation and telomere shortening in blood progenitors. Cell Death Dis. 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Griscelli, F.; Oudrhiri, N.; Feraud, O.; Divers, D.; Portier, L.; Turhan, A.G.; Bennaceur Griscelli, A. Generation of induced pluripotent stem cell (iPSC) line from a patient with triple negative breast cancer with hereditary exon 17 deletion of BRCA1 gene. Stem Cell Res. 2017, 24, 135–138. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Bennaceur-Griscelli, A.; Girinsky, T.; Koscielny, S.; Delhommeau, F.; Dossou, J.; Violot, D.; Leclercq, E.; Courtier, M.H.; Beron-Gaillard, N.; et al. Telomere shortening and associated chromosomal instability in peripheral blood lymphocytes of patients with Hodgkin’s lymphoma prior to any treatment are predictive of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 465–471. [Google Scholar] [CrossRef]
- M’kacher, R.; Maalouf, E.E.L.; Ricoul, M.; Heidingsfelder, L.; Laplagne, E.; Cuceu, C.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; Sabatier, L. New tool for biological dosimetry: Reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat. Res. Mol. Mech. Mutagen. 2014, 770, 45–53. [Google Scholar] [CrossRef]
- M’Kacher, R.; Cuceu, C.; Al Jawhari, M.; Morat, L.; Frenzel, M.; Shim, G.; Lenain, A.; Hempel, W.M.; Junker, S.; Girinsky, T.; et al. The Transition between Telomerase and ALT Mechanisms in Hodgkin Lymphoma and Its Predictive Value in Clinical Outcomes. Cancers 2018, 10, 169. [Google Scholar] [CrossRef]
- Kaddour, A.; Colicchio, B.; Buron, D.; El Maalouf, E.; Laplagne, E.; Borie, C.; Ricoul, M.; Lenain, A.; Hempel, W.M.; Morat, L.; et al. Transmission of Induced Chromosomal Aberrations through Successive Mitotic Divisions in Human Lymphocytes after In Vitro and In Vivo Radiation. Sci. Rep. 2017, 7, 3291. [Google Scholar] [CrossRef]
- Murnane, J.P. Telomere dysfunction and chromosome instability. Mutat. Res. 2012, 730, 28–36. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef]
- Mitchell, T.R.; Glenfield, K.; Jeyanthan, K.; Zhu, X.D. Arginine methylation regulates telomere length and stability. Mol. Cell. Biol. 2009, 29, 4918–4934. [Google Scholar] [CrossRef] [PubMed]
- Finot, F.; Kaddour, A.; Morat, L.; Mouche, I.; Zaguia, N.; Cuceu, C.; Souverville, D.; Négrault, S.; Cariou, O.; Essahli, A.; et al. Genotoxic risk of ethyl-paraben could be related to telomere shortening. J. Appl. Toxicol. 2017, 37, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Zaguia, N.; Laplagne, E.; Colicchio, B.; Cariou, O.; Al Jawhari, M.; Heidingsfelder, L.; Hempel, W.M.; Jrad, B.B.H.; Jeandidier, E.; Dieterlen, A.; et al. A new tool for genotoxic risk assessment: Reevaluation of the cytokinesis-block micronucleus assay using semi-automated scoring following telomere and centromere staining. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 850-851, 503143. [Google Scholar] [CrossRef] [PubMed]
- Cuceu, C.; Hempel, W.M.; Sabatier, L.; Bosq, J.; Carde, P.; M’Kacher, R. Chromosomal Instability in Hodgkin Lymphoma: An In-Depth Review and Perspectives. Cancers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Colicchio, B.; Borie, C.; Junker, S.; Marquet, V.; Heidingsfelder, L.; Soehnlen, K.; Najar, W.; Hempel, W.M.; Oudrhiri, N.; et al. Telomere and Centromere Staining Followed by M-FISH Improves Diagnosis of Chromosomal Instability and Its Clinical Utility. Genes 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription Regulation of the Human Telomerase Reverse Transcriptase (hTERT) Gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089. [Google Scholar] [CrossRef]
- Draskovic, I.; Arnoult, N.; Steiner, V.; Bacchetti, S.; Lomonte, P.; Londoño-Vallejo, A. Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 15726–15731. [Google Scholar] [CrossRef]
- Fasching, C.L.; Neumann, A.A.; Muntoni, A.; Yeager, T.R.; Reddel, R.R. DNA damage induces alternative lengthening of telomeres (ALT) associated promyelocytic leukemia bodies that preferentially associate with linear telomeric DNA. Cancer Res. 2007, 67, 7072–7077. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef] [PubMed]
- Armando, R.G.; Mengual Gomez, D.L. Telomeropathies: Etiology, diagnosis, treatment and follow-up. Ethical and legal considerations. Clin. Genet. 2019, 96, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Telomeres and aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Polvi, A.; Linnankivi, T.; Kivela, T.; Herva, R.; Keating, J.P.; Makitie, O.; Pareyson, D.; Vainionpaa, L.; Lahtinen, J.; Hovatta, I.; et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am. J. Hum. Genet. 2012, 90, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaeiaval, F.; Zhang, J.; Schleit, J.; Lessel, D.; Kubisch, C.; Precioso, D.R.; Sillence, D.; Hisama, F.M.; Dorschner, M.; Martin, G.M.; et al. CTC1 mutations in a Brazilian family with progeroid features and recurrent bone fractures. Mol. Genet. Genom. Med. 2018, 6, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.B.; Gagne, K.E.; Usmani, G.N.; Asdourian, G.K.; Williams, D.A.; Hofmann, I.; Agarwal, S. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr. Blood Cancer 2012, 59, 311–314. [Google Scholar] [CrossRef]
- Takai, H.; Jenkinson, E.; Kabir, S.; Babul-Hirji, R.; Najm-Tehrani, N.; Chitayat, D.A.; Crow, Y.J.; de Lange, T. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev. 2016, 30, 812–826. [Google Scholar] [CrossRef]
- Walne, A.J.; Bhagat, T.; Kirwan, M.; Gitiaux, C.; Desguerre, I.; Leonard, N.; Nogales, E.; Vulliamy, T.; Dokal, I.S. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica 2013, 98, 334–338. [Google Scholar] [CrossRef]
- Maraldi, N.M. The lamin code. Biosystems 2018, 164, 68–75. [Google Scholar] [CrossRef]
- Pennarun, G.; Picotto, J.; Etourneaud, L.; Redavid, A.R. Increase in lamin B1 promotes telomere instability by disrupting the shelterin complex in human cells. Nucleic Acids Res. 2021, 49, 9886–9905. [Google Scholar] [CrossRef]
- Maciejowski, J.; Li, Y.; Bosco, N.; Campbell, P.J.; de Lange, T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 2015, 163, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Yehezkel, S.; Rebibo-Sabbah, A.; Segev, Y.; Tzukerman, M.; Shaked, R.; Huber, I.; Gepstein, L.; Skorecki, K.; Selig, S. Reprogramming of telomeric regions during the generation of human induced pluripotent stem cells and subsequent differentiation into fibroblast-like derivatives. Epigenetics 2011, 6, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Marion, R.M.; Strati, K.; Li, H.; Tejera, A.; Schoeftner, S.; Ortega, S.; Serrano, M.; Blasco, M.A. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 2009, 4, 141–154. [Google Scholar] [CrossRef]
- Winkler, T.; Hong, S.G.; Decker, J.E.; Morgan, M.J.; Wu, C.; Hughes, W.M.t.; Yang, Y.; Wangsa, D.; Padilla-Nash, H.M.; Ried, T.; et al. Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. J. Clin. Investig. 2013, 123, 1952–1963. [Google Scholar] [CrossRef]
- Cuceu, C.; Colicchio, B.; Jeandidier, E.; Junker, S.; Plassa, F.; Shim, G.; Mika, J.; Frenzel, M.; AL Jawhari, M.; Hempel, W.M.; et al. Independent Mechanisms Lead to Genomic Instability in Hodgkin Lymphoma: Microsatellite or Chromosomal Instability. Cancers 2018, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Laithier, V.; Valent, A.; Delhommeau, F.; Violot, D.; Deutsch, E.; Dossou, J.; Béron-Gaillard, N.; Girinsky, T.; Bourhis, J.; et al. Sensitivity to radiation and alkylating agent of peripheral lymphocytes and fibroblasts in a Hoyeraal-Hreidarsson syndrome patient. Pediatr. Hematol. Oncol. 2003, 20, 651–656. [Google Scholar] [CrossRef]
- Nelson, A.S.; Marsh, R.A.; Myers, K.C.; Davies, S.M.; Jodele, S.; O’Brien, T.A.; Mehta, P.A. A Reduced-Intensity Conditioning Regimen for Patients with Dyskeratosis Congenita Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 884–888. [Google Scholar] [CrossRef]
- Pereboeva, L.; Westin, E.; Patel, T.; Flaniken, I.; Lamb, L.; Klingelhutz, A.; Goldman, F. DNA damage responses and oxidative stress in dyskeratosis congenita. PLoS ONE 2013, 8, e76473. [Google Scholar] [CrossRef]
- Stout, G.J.; Blasco, M.A. Telomere length and telomerase activity impact the UV sensitivity syndrome xeroderma pigmentosum C. Cancer Res. 2013, 73, 1844–1854. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudrhiri, N.; M’kacher, R.; Chaker, D.; Colicchio, B.; Borie, C.; Jeandidier, E.; Dieterlen, A.; Griscelli, F.; Bennaceur-Griscelli, A.; Turhan, A.G. Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome. Genes 2022, 13, 1395. https://doi.org/10.3390/genes13081395
Oudrhiri N, M’kacher R, Chaker D, Colicchio B, Borie C, Jeandidier E, Dieterlen A, Griscelli F, Bennaceur-Griscelli A, Turhan AG. Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome. Genes. 2022; 13(8):1395. https://doi.org/10.3390/genes13081395
Chicago/Turabian StyleOudrhiri, Noufissa, Radhia M’kacher, Diana Chaker, Bruno Colicchio, Claire Borie, Eric Jeandidier, Alain Dieterlen, Frank Griscelli, Annelise Bennaceur-Griscelli, and Ali G. Turhan. 2022. "Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome" Genes 13, no. 8: 1395. https://doi.org/10.3390/genes13081395
APA StyleOudrhiri, N., M’kacher, R., Chaker, D., Colicchio, B., Borie, C., Jeandidier, E., Dieterlen, A., Griscelli, F., Bennaceur-Griscelli, A., & Turhan, A. G. (2022). Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome. Genes, 13(8), 1395. https://doi.org/10.3390/genes13081395