Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review
Abstract
:1. Introduction
- X-linked hypophosphatemia rickets (XLH) is a severe and rare disease
- The authors describe a familial case of XLH
- The clinical picture is different for the two siblings, especially in terms of bone changes
- The Burosumab treatment has favorable effects, improving growth curve and bone pain, and normalizing phosphorus levels
2. Materials and Methods
2.1. Case Report
2.2. Laboratory Investigations
2.3. Molecular Investigations
3. Results
3.1. Morphological Evaluation of the Patients
3.2. Laboratory Investigations
3.3. Radiological Investigations
3.4. Psychological Aspects: Case#1 Displays Low Self-Esteem Due to Motor Difficulties (Increased Emotional Sensitivity, Shyness in Unfamiliar Environments). Frequent Episodes of Sadness. Case#2 Periods of Anxiety and Anger
3.5. Molecular Investigations
4. Discussion
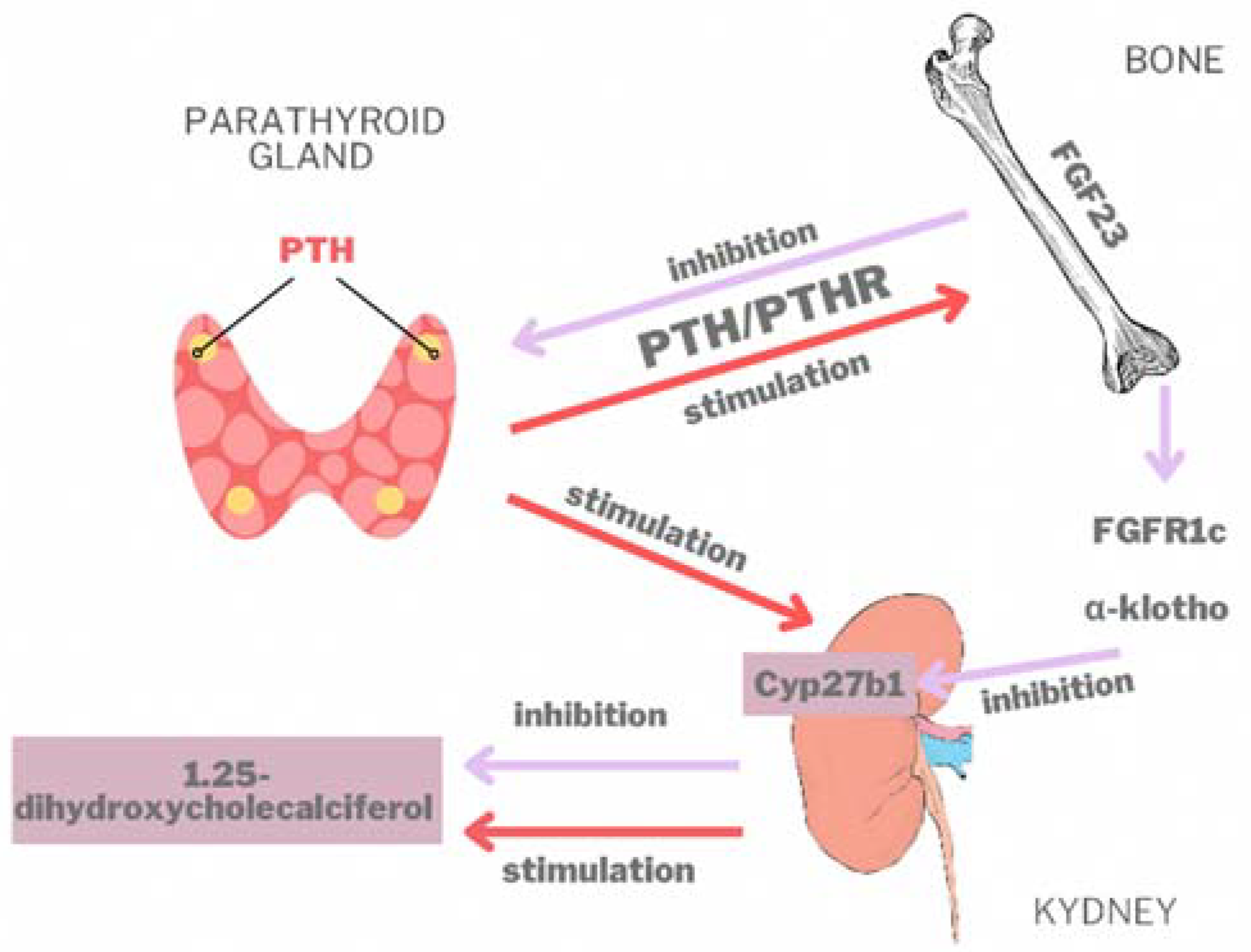
4.1. The Gene
4.2. Clinical Aspects
4.3. Paraclinical Aspects
4.4. Radiological Aspects
4.5. Genotype-Phenotype Correlation
4.6. Medical Treatment
4.7. Surgical and Orthopedic Treatment
4.8. Diagnosis and Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and Prevalence of Nutritional and Hereditary Rickets in Southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, I.A.; Huang, X.; Kunkel, L.M. Mutational Analysis of the PEX Gene in Patients with X-Linked Hypophosphatemic Rickets. Am. J. Hum. Genet. 1997, 60, 790–797. [Google Scholar] [PubMed]
- Fuente, R.; García-Bengoa, M.; Fernández-Iglesias, Á.; Gil-Peñ, H.; Santos, F.; López, J.M. Cellular and Molecular Alterations Underlying Abnormal Bone Growth in X-Linked Hypophosphatemia. Int. J. Mol. Sci. 2022, 23, 934. [Google Scholar] [CrossRef]
- Fuente, R.; Gil-Peña, H.; Claramunt-Taberner, D.; Hernández-Frías, O.; Fernández-Iglesias, Á.; Hermida-Prado, F.; Anes-González, G.; Rubio-Aliaga, I.; Lopez, J.M.; Santos, F. Marked Alterations in the Structure, Dynamics and Maturation of Growth Plate Likely Explain Growth Retardation and Bone Deformities of Young Hyp Mice. Bone 2018, 116, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Hardy, D.C.; Murphy, W.A.; Teitelbaum, S.L.; Bergfeld, M.A.; Whyte, M.P. X-Linked Hypophosphatemia: A Clinical, Biochemical, and Histopathologic Assessment of Morbidity in Adults. Medicine 1989, 68, 336–352. [Google Scholar] [CrossRef]
- Liu, S.; Quarles, L.D. How Fibroblast Growth Factor 23 Works. J. Am. Soc. Nephrol. 2007, 18, 1637–1647. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Kusano, K.; Kinosaki, M.; Ito, H.; Hirata, M.; Segawa, H.; Miyamoto, K.-I.; Fukushima, N. Human Fibroblast Growth Factor-23 Mutants Suppress Na+-Dependent Phosphate Co-Transport Activity and 1alpha,25-Dihydroxyvitamin D3 Production. J. Biol. Chem. 2003, 278, 2206–2211. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Mizutani, S.; Muto, T.; Yoneya, T.; Hino, R.; Takeda, S.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Cloning and Characterization of FGF23 as a Causative Factor of Tumor-Induced Osteomalacia. Proc. Natl. Acad. Sci. USA 2001, 98, 6500–6505. [Google Scholar] [CrossRef] [Green Version]
- Ruchon, A.F.; Marcinkiewicz, M.; Siegfried, G.; Tenenhouse, H.S.; DesGroseillers, L.; Crine, P.; Boileau, G. Pex MRNA Is Localized in Developing Mouse Osteoblasts and Odontoblasts. J. Histochem. Cytochem. 1998, 46, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, Y.; Ohata, Y.; Takeyari, S.; Kitaoka, T.; Fujiwara, M.; Nakano, Y.; Yamamoto, K.; Yamada, C.; Yamamoto, K.; Michigami, T.; et al. Genotype-Phenotype Analysis, and Assessment of the Importance of the Zinc-Binding Site in PHEX in Japanese Patients with X-Linked Hypophosphatemic Rickets Using 3D Structure Modeling. Bone 2021, 153, 116135. [Google Scholar] [CrossRef]
- Santos, F.; Fuente, R.; Mejia, N.; Mantecon, L.; Gil-Peña, H.; Ordoñez, F.A. Hypophosphatemia and Growth. Pediatr. Nephrol. 2013, 28, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Forero-Delgadillo, J.M.; Cleves, D.; Ochoa, V.; Londoño-Correa, H.; Restrepo, J.M.; Nastasi-Catanese, J.A.; Pachajoa, H. PHEX Gene Mutation in a Patient with X-Linked Hypophosphatemic Rickets in a Developing Country. Appl. Clin. Genet. 2020, 13, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razali, N.N.; Hwu, T.T.; Thilakavathy, K. Phosphate Homeostasis and Genetic Mutations of Familial Hypophosphatemic Rickets. J. Pediatr. Endocrinol. Metab. 2015, 28, 1009–1017. [Google Scholar] [CrossRef]
- Francis, F.; Hennig, S.; Korn, B.; Reinhardt, R.; de Jong, P.; Poustka, A.; Lehrach, H.; Rowe, P.S.N.; Goulding, J.N.; Summerfield, T.; et al. A Gene (PEX) with Homologies to Endopeptidases Is Mutated in Patients with X-Linked Hypophosphatemic Rickets. The HYP Consortium. Nat. Genet. 1995, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Pavone, V.; Testa, G.; Gioitta Iachino, S.; Evola, F.R.; Avondo, S.; Sessa, G. Hypophosphatemic Rickets: Etiology, Clinical Features and Treatment. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 221–226. [Google Scholar] [CrossRef]
- Filisetti, D.; Ostermann, G.; von Bredow, M.; Strom, T.; Filler, G.; Ehrich, J.; Pannetier, S.; Garnier, J.M.; Rowe, P.; Francis, F.; et al. Non-Random Distribution of Mutations in the PHEX Gene, and under-Detected Missense Mutations at Non-Conserved Residues. Eur. J. Hum. Genet. 1999, 7, 615–619. [Google Scholar] [CrossRef]
- Barros, N.M.T.; Hoac, B.; Neves, R.L.; Addison, W.N.; Assis, D.M.; Murshed, M.; Carmona, A.K.; McKee, M.D. Proteolytic Processing of Osteopontin by PHEX and Accumulation of Osteopontin Fragments in Hyp Mouse Bone, the Murine Model of X-Linked Hypophosphatemia. J. Bone Miner. Res. 2013, 28, 688–699. [Google Scholar] [CrossRef]
- Qin, C.; Baba, O.; Butler, W.T. Post-Translational Modifications of Sibling Proteins and Their Roles in Osteogenesis and Dentinogenesis. Crit. Rev. Oral Biol. Med. 2004, 15, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Yoshiko, Y.; Wang, H.; Minamizaki, T.; Ijuin, C.; Yamamoto, R.; Suemune, S.; Kozai, K.; Tanne, K.; Aubin, J.E.; Maeda, N. Mineralized Tissue Cells Are a Principal Source of FGF23. Bone 2007, 40, 1565–1573. [Google Scholar] [CrossRef]
- Yu, X.; Xia, Y.; Jia, J.; Yuan, G. The Role of Fibroblast Growth Factor 19 Subfamily in Different Populations Suffering from Osteoporosis. Front. Endocrinol. (Lausanne) 2022, 13, 830022. [Google Scholar] [CrossRef]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yoshioka, M.; Itoh, N. Identification of a Novel Fibroblast Growth Factor, FGF-23, Preferentially Expressed in the Ventrolateral Thalamic Nucleus of the Brain. Biochem. Biophys. Res. Commun. 2000, 277, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D. FGF23, PHEX, and MEPE Regulation of Phosphate Homeostasis and Skeletal Mineralization. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1–E9. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Carpenter, T.O.; Demay, M.B. Hypophosphatemia Leads to Rickets by Impairing Caspase-Mediated Apoptosis of Hypertrophic Chondrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9637–9642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkitie, O.; Doria, A.; Kooh, S.W.; Cole, W.G.; Daneman, A.; Sochett, E. Early Treatment Improves Growth and Biochemical and Radiographic Outcome in X-Linked Hypophosphatemic Rickets. J. Clin. Endocrinol. Metab. 2003, 88, 3591–3597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, S.K.; Andrukhova, O.; Clinkenbeard, E.L.; White, K.E.; Erben, R.G. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol. 2016, 14, e1002427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degirolamo, C.; Sabbà, C.; Moschetta, A. Therapeutic Potential of the Endocrine Fibroblast Growth Factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 2016, 15, 51–69. [Google Scholar] [CrossRef]
- Mace, M.L.; Olgaard, K.; Lewin, E. New Aspects of the Kidney in the Regulation of Fibroblast Growth Factor 23 (FGF23) and Mineral Homeostasis. Int. J. Mol. Sci. 2020, 21, 8810. [Google Scholar] [CrossRef]
- Ewendt, F.; Feger, M.; Föller, M. Role of Fibroblast Growth Factor 23 (FGF23) and AKlotho in Cancer. Front. Cell Dev. Biol. 2020, 8, 601006. [Google Scholar] [CrossRef]
- Saki, F.; Kassaee, S.R.; Salehifar, A.; Omrani, G.H.R. Interaction between Serum FGF-23 and PTH in Renal Phosphate Excretion, a Case-Control Study in Hypoparathyroid Patients. BMC Nephrol. 2020, 21, 176. [Google Scholar] [CrossRef]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, C.A.; Föller, M. Phosphate Homeostasis, Inflammation and the Regulation of FGF-23. Kidney Blood Press. Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Sarafrazi, S.; Daugherty, S.C.; Miller, N.; Boada, P.; Carpenter, T.O.; Chunn, L.; Dill, K.; Econs, M.J.; Eisenbeis, S.; Imel, E.A.; et al. Novel PHEX Gene Locus-Specific Database: Comprehensive Characterization of Vast Number of Variants Associated with X-Linked Hypophosphatemia (XLH). Hum. Mutat. 2022, 43, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.T.; Johnson, B.; Aradhya, S.; Beltran, D.; Bristow, S.L.; Eisenbeis, S.; Guerra, N.E.; Krolczyk, S.; Miller, N.; Morales, A.; et al. Molecular Diagnoses of X-Linked and Other Genetic Hypophosphatemias: Results from a Sponsored Genetic Testing Program. J. Bone Miner. Res. 2022, 37, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Okazaki, R.; Shibata, M.; Hasegawa, Y.; Satoh, K.; Tajima, T.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Yamashita, T.; et al. Increased Circulatory Level of Biologically Active Full-Length FGF-23 in Patients with Hypophosphatemic Rickets/Osteomalacia. J. Clin. Endocrinol. Metab. 2002, 87, 4957–4960. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Sun, Y.; Xu, L.; JiaJue, R.; Cui, L.; Pang, Q.; Jiang, Y.; Li, M.; Wang, O.; et al. Clinical and Genetic Analysis in a Large Chinese Cohort of Patients with X-Linked Hypophosphatemia. Bone 2019, 121, 212–220. [Google Scholar] [CrossRef]
- Morey, M.; Castro-Feijóo, L.; Barreiro, J.; Cabanas, P.; Pombo, M.; Gil, M.; Bernabeu, I.; Díaz-Grande, J.M.; Rey-Cordo, L.; Ariceta, G.; et al. Genetic Diagnosis of X-Linked Dominant Hypophosphatemic Rickets in a Cohort Study: Tubular Reabsorption of Phosphate and 1,25(OH)2D Serum Levels Are Associated with PHEX Mutation Type. BMC Med. Genet. 2011, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Vila-Pérez, D.; Marín-del-Barrio, S.; Vila-Cots, J.; Camacho-Díaz, J.A.; Morey, M.; Loidi, L. Four Cases of X-Linked Hypophosphatemic Rickets, Clinical Description and Genetic Testing. Open J. Genet. 2014, 4, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rubio, E.; Gil-Peña, H.; Chocron, S.; Madariaga, L.; de la Cerda-Ojeda, F.; Fernández-Fernández, M.; de Lucas-Collantes, C.; Gil, M.; Luis-Yanes, M.I.; Vergara, I.; et al. Phenotypic Characterization of X-Linked Hypophosphatemia in Pediatric Spanish Population. Orphanet J. Rare Dis. 2021, 16, 104. [Google Scholar] [CrossRef]
- Makras, P.; Hamdy, N.A.T.; Kant, S.G.; Papapoulos, S.E. Normal Growth and Muscle Dysfunction in X-Linked Hypophosphatemic Rickets Associated with a Novel Mutation in the PHEX Gene. J. Clin. Endocrinol. Metab. 2008, 93, 1386–1389. [Google Scholar] [CrossRef]
- Cauliez, A.; Zhukouskaya, V.V.; Hilliquin, S.; Sadoine, J.; Slimani, L.; Miceli-Richard, C.; Briot, K.; Linglart, A.; Chaussain, C.; Bardet, C. Impact of Early Conventional Treatment on Adult Bone and Joints in a Murine Model of X-Linked Hypophosphatemia. Front. Cell Dev. Biol. 2020, 8, 591417. [Google Scholar] [CrossRef]
- Zivičnjak, M.; Schnabel, D.; Billing, H.; Staude, H.; Filler, G.; Querfeld, U.; Schumacher, M.; Pyper, A.; Schröder, C.; Brämswig, J.; et al. Age-Related Stature and Linear Body Segments in Children with X-Linked Hypophosphatemic Rickets. Pediatr. Nephrol. 2011, 26, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chanchlani, R.; Nemer, P.; Sinha, R.; Nemer, L.; Krishnappa, V.; Sochett, E.; Safadi, F.; Raina, R. An Overview of Rickets in Children. Kidney Int. Rep. 2020, 5, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical Practice Recommendations for the Diagnosis and Management of X-Linked Hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, M.S.; Grunseich, K.; Carpenter, T.O. Contemporary Medical and Surgical Management of X-Linked Hypophosphatemic Rickets. J. Am. Acad. Orthop. Surg. 2015, 23, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Sochett, E.; Doria, A.S.; Henriques, F.; Kooh, S.W.; Daneman, A.; Mäkitie, O. Growth and Metabolic Control during Puberty in Girls with X-Linked Hypophosphataemic Rickets. Horm. Res. 2004, 61, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Shaw, N.J.; Portale, A.A.; Ward, L.M.; Abrams, S.A.; Pettifor, J.M. Rickets. Nat. Rev. Dis. Primers 2017, 3, 17101. [Google Scholar] [CrossRef]
- Holm, I.A.; Nelson, A.E.; Robinson, B.G.; Mason, R.S.; Marsh, D.J.; Cowell, C.T.; Carpenter, T.O. Mutational Analysis and Genotype-Phenotype Correlation of the PHEX Gene in X-Linked Hypophosphatemic Rickets. J. Clin. Endocrinol. Metab. 2001, 86, 3889–3899. [Google Scholar] [CrossRef]
- Rafaelsen, S.; Johansson, S.; Ræder, H.; Bjerknes, R. Hereditary Hypophosphatemia in Norway: A Retrospective Population-Based Study of Genotypes, Phenotypes, and Treatment Complications. Eur. J. Endocrinol. 2016, 174, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Chesher, D.; Oddy, M.; Darbar, U.; Sayal, P.; Casey, A.; Ryan, A.; Sechi, A.; Simister, C.; Waters, A.; Wedatilake, Y.; et al. Outcome of Adult Patients with X-Linked Hypophosphatemia Caused by PHEX Gene Mutations. J. Inherit. Metab. Dis. 2018, 41, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Whyte, M.P.; Schranck, F.W.; Armamento-Villareal, R. X-Linked Hypophosphatemia: A Search for Gender, Race, Anticipation, or Parent of Origin Effects on Disease Expression in Children. J. Clin. Endocrinol. Metab. 1996, 81, 4075–4080. [Google Scholar] [CrossRef]
- Beck-Nielsen, S.S.; Mughal, Z.; Haffner, D.; Nilsson, O.; Levtchenko, E.; Ariceta, G.; de Lucas Collantes, C.; Schnabel, D.; Jandhyala, R.; Mäkitie, O. FGF23 and Its Role in X-Linked Hypophosphatemia-Related Morbidity. Orphanet J. Rare Dis. 2019, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Jones, A.O.; Tenenhouse, H.S. PHEXdb, a Locus-Specific Database for Mutations Causing X-Linked Hypophosphatemia. Hum. Mutat. 2000, 16, 1–6. [Google Scholar] [CrossRef]
- Gaucher, C.; Walrant-Debray, O.; Nguyen, T.-M.; Esterle, L.; Garabédian, M.; Jehan, F. PHEX Analysis in 118 Pedigrees Reveals New Genetic Clues in Hypophosphatemic Rickets. Hum. Genet. 2009, 125, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Hao, X.; Liu, Y.; Wang, Y.; Shan, C.; Ao, X.; Liu, Y.; Bao, H.; Li, P. A Novel c.2179T>C Mutation Blocked the Intracellular Transport of PHEX Protein and Caused X-Linked Hypophosphatemic Rickets in a Chinese Family. Mol. Genet. Genomic Med. 2020, 8, e1262. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Boileau, G.; Campos, M.; Carmona, A.K.; Tenenhouse, H.S. Structure and Function of Disease-Causing Missense Mutations in the PHEX Gene. J. Clin. Endocrinol. Metab. 2003, 88, 2213–2222. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, C.; Chen, Q.; Che, R.; Sha, Y.; Zhao, F.; Ding, G.; Zhou, W.; Jia, Z.; Huang, S.; et al. Functional Characterization of PHEX Gene Variants in Children with X-Linked Hypophosphatemic Rickets Shows No Evidence of Genotype-Phenotype Correlation. J. Bone Miner. Res. 2020, 35, 1718–1725. [Google Scholar] [CrossRef]
- Alenazi, B.; Molla, M.A.M.; Alshaya, A.; Saleh, M. X-Linked Hypophosphatemic Rickets (PHEX Mutation): A Case Report and Literature Review. Sudan. J. Paediatr. 2017, 17, 61–65. [Google Scholar]
- Song, H.R.; Park, J.W.; Cho, D.Y.; Yang, J.H.; Yoon, H.R.; Jung, S.C. PHEX Gene Mutations and Genotype-Phenotype Analysis of Korean Patients with Hypophosphatemic Rickets. J. Korean Med. Sci. 2007, 22, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.Y.; Lee, B.H.; Kang, J.H.; Ha, I.S.; Cheong, H.I.; Choi, Y. A Clinical and Molecular Genetic Study of Hypophosphatemic Rickets in Children. Pediatr. Res. 2005, 58, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, N.A.T.; Harvengt, P.; Usardi, A. X-Linked Hypophosphatemia: The Medical Expert’s Challenges and the Patient’s Concerns on Their Journey with the Disease. Arch. Pediatr. 2021, 28, 612–618. [Google Scholar] [CrossRef]
- Laurent, M.R.; De Schepper, J.; Trouet, D.; Godefroid, N.; Boros, E.; Heinrichs, C.; Bravenboer, B.; Velkeniers, B.; Lammens, J.; Harvengt, P.; et al. Consensus Recommendations for the Diagnosis and Management of X-Linked Hypophosphatemia in Belgium. Front. Endocrinol. 2021, 12, 641543. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.M.S.; Coates, V.; Omar, H.A. Evaluation of Stature Development during Childhood and Adolescence in Individuals with Familial Hypophosphatemic Rickets. ScientificWorldJournal 2005, 5, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Santos Rodríguez, F. X-Linked Hypophosphataemic Rickets and Growth. Adv. Ther. 2020, 37 (Suppl. 2), 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jurcă, M.C.; Bembea, M.; Kozma, K.; Şandor, M.I.; Negrean, R.A.; Dobjanschi, L.; Cuc, E.A.; Petcheşi, C.D.; Jurcă, A.D. Empty Sella Associated with Growth Hormone Deficiency and Polydactyly. Rom. J. Morphol. Embryol. 2018, 59, 381–384. [Google Scholar]
- Kaneko, I.; Segawa, H.; Ikuta, K.; Hanazaki, A.; Fujii, T.; Tatsumi, S.; Kido, S.; Hasegawa, T.; Amizuka, N.; Saito, H.; et al. Eldecalcitol Causes FGF23 Resistance for Pi Reabsorption and Improves Rachitic Bone Phenotypes in the Male Hyp Mouse. Endocrinology 2018, 159, 2741–2758. [Google Scholar] [CrossRef]
- Beck-Nielsen, S.S.; Brusgaard, K.; Rasmussen, L.M.; Brixen, K.; Brock-Jacobsen, B.; Poulsen, M.R.; Vestergaard, P.; Ralston, S.H.; Albagha, O.M.E.; Poulsen, S.; et al. Phenotype Presentation of Hypophosphatemic Rickets in Adults. Calcif. Tissue Int. 2010, 87, 108–119. [Google Scholar] [CrossRef]
- Drug Approval Package: CRYSVITA (Burosumab–Twza). Available online: https://medlineplus.gov/druginfo/meds/a618034.html (accessed on 15 July 2022).
- EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/crysvita-epar-product-information_en.p (accessed on 19 May 2022).
- Baradhi, K. Dramatic Transformation after Burosumab in a Young Boy with X-Linked Hypophosphatemia: A Life-Changing Saga. Cureus 2022, 14, e22340. [Google Scholar] [CrossRef]
- Kondo, S.; Takano, T.; Ono, Y.; Saito, H.; Matsumoto, T. Eldecalcitol Reduces Osteoporotic Fractures by Unique Mechanisms. J. Steroid Biochem. Mol. Biol. 2015, 148, 232–238. [Google Scholar] [CrossRef]
- Raimann, A.; Mindler, G.T.; Kocijan, R.; Bekes, K.; Zwerina, J.; Haeusler, G.; Ganger, R. Multidisciplinary Patient Care in X-Linked Hypophosphatemic Rickets: One Challenge, Many Perspectives. Wien. Med. Wochenschr. 2020, 170, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Insogna, K.L.; Rauch, F.; Kamenický, P.; Ito, N.; Kubota, T.; Nakamura, A.; Zhang, L.; Mealiffe, M.; San Martin, J.; Portale, A.A. Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J. Bone Miner. Res. 2019, 34, 2183–2191. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Högler, W.; Linglart, A.; Padidela, R.; van’t Hoff, W.; Mao, M.; Chen, C.-Y.; et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thacher, T.D.; Pettifor, J.M.; Tebben, P.J.; Creo, A.L.; Skrinar, A.; Mao, M.; Chen, C.-Y.; Chang, T.; San Martin, J.; Carpenter, T.O. Rickets Severity Predicts Clinical Outcomes in Children with X-Linked Hypophosphatemia: Utility of the Radiographic Rickets Severity Score. Bone 2019, 122, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus Conventional Therapy in Children with X-Linked Hypophosphataemia: A Randomised, Active-Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef]
- Padidela, R.; Whyte, M.P.; Glorieux, F.H.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Portale, A.A.; Simmons, J.H.; Namba, N.; Cheong, H.I.; et al. Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab versus Conventional Therapy in Children with X-Linked Hypophosphatemia. Calcif. Tissue Int. 2021, 108, 622–633. [Google Scholar] [CrossRef]
- Whyte, M.P.; Carpenter, T.O.; Gottesman, G.S.; Mao, M.; Skrinar, A.; San Martin, J.; Imel, E.A. Efficacy and Safety of Burosumab in Children Aged 1-4 Years with X-Linked Hypophosphataemia: A Multicentre, Open-Label, Phase 2 Trial. Lancet Diabetes Endocrinol. 2019, 7, 189–199. [Google Scholar] [CrossRef]
- Insogna, K.L.; Briot, K.; Imel, E.A.; Kamenický, P.; Ruppe, M.D.; Portale, A.A.; Weber, T.; Pitukcheewanont, P.; Cheong, H.I.; Jan de Beur, S.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults with X-Linked Hypophosphatemia: Week 24 Primary Analysis. J. Bone Miner. Res. 2018, 33, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Cheong, H.I.; Yoo, H.-W.; Adachi, M.; Tanaka, H.; Fujiwara, I.; Hasegawa, Y.; Harada, D.; Sugimoto, M.; Okada, Y.; Kato, M.; et al. First-in-Asian Phase I Study of the Anti-Fibroblast Growth Factor 23 Monoclonal Antibody, Burosumab: Safety and Pharmacodynamics in Adults with X-Linked Hypophosphatemia: First-in-Asian Phase i Study of Burosumab, Anti-Fgf23 Antibody. JBMR Plus 2019, 3, e10074. [Google Scholar] [CrossRef] [Green Version]
- Gizard, A.; Rothenbuhler, A.; Pejin, Z.; Finidori, G.; Glorion, C.; de Billy, B.; Linglart, A.; Wicart, P. Outcomes of Orthopedic Surgery in a Cohort of 49 Patients with X-Linked Hypophosphatemic Rickets (XLHR). Endocr. Connect. 2017, 6, 566–573. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Fukumoto, S. X-Linked Hypophosphatemia and FGF23-Related Hypophosphatemic Diseases: Prospect for New Treatment. Endocr. Rev. 2018, 39, 274–291. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A Clinician’s Guide to X-Linked Hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Baroncelli, G.I.; Mora, S. X-Linked Hypophosphatemic Rickets: Multisystemic Disorder in Children Requiring Multidisciplinary Management. Front. Endocrinol. 2021, 12, 688309. [Google Scholar] [CrossRef] [PubMed]

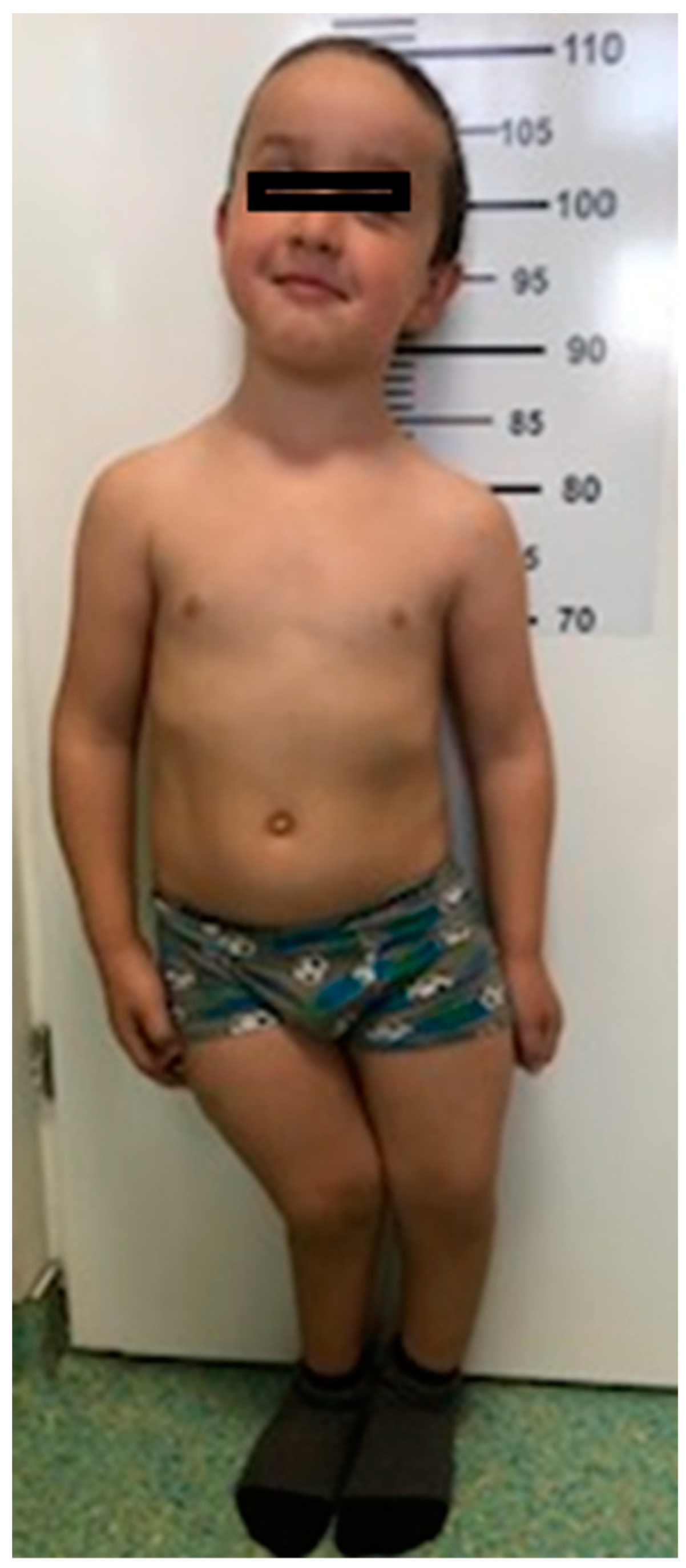

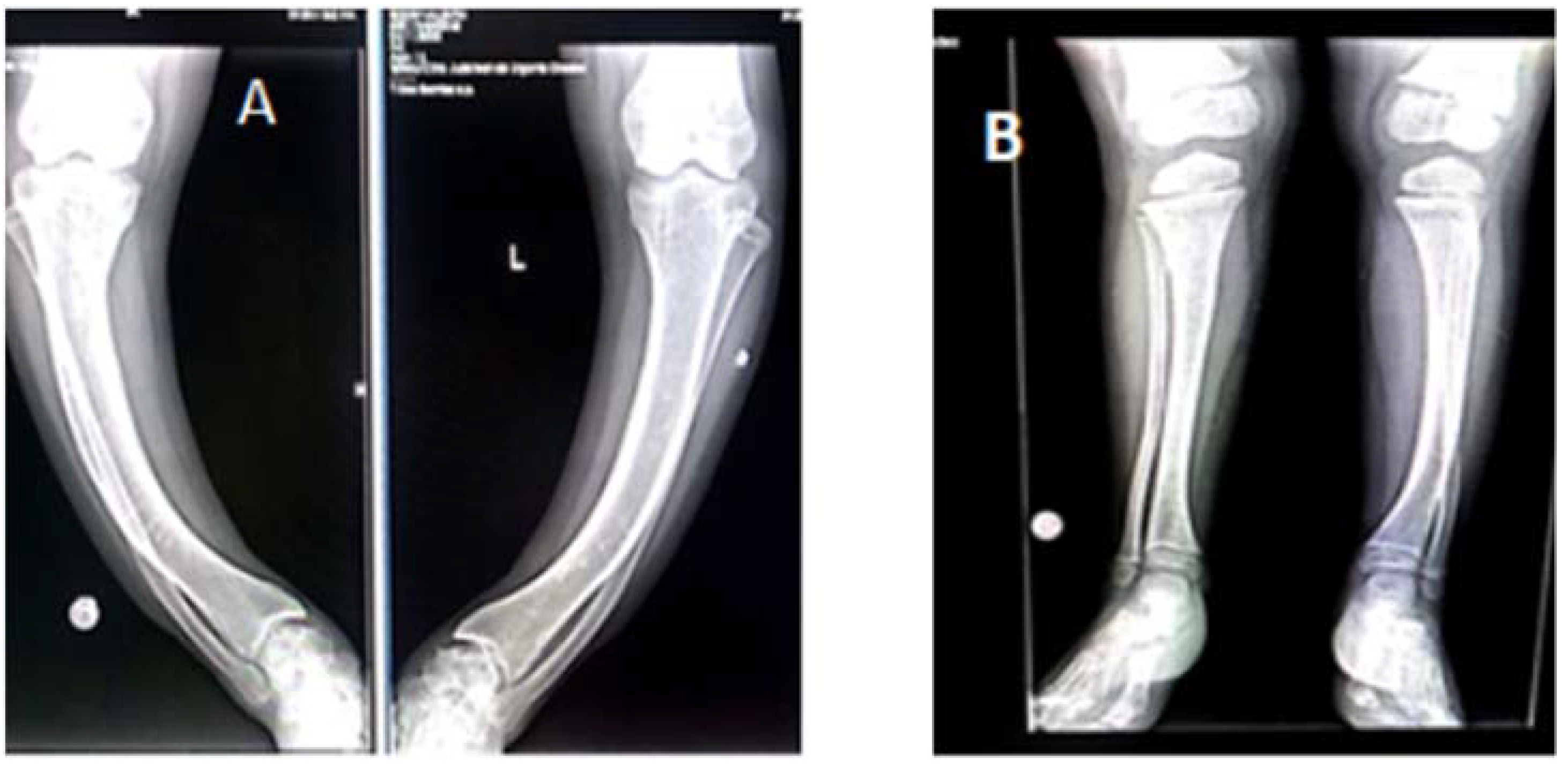
| Case #1 Sister | Case #2 Brother | |
|---|---|---|
| Age at diagnosis | 13½ years | 7 years |
| Age of onset | 3 years | 2 years |
| Weight | 39 kg | 19 kg |
| Stature | 131 cm (−3DS) | 107 cm (−3DS) |
| Craniofacial dysmorphism | macrocephaly: head circumference 53 cm frontal bossing flattened facies bilateral epicanthal folds, high arched palate | |
| Chest deformity | pectus excavatum, thoracolumbar scoliosis, Harrison’s groove and rachitic rosary | pectus excavatum, Harrison’s groove and rachitic rosary |
| Musculoskeletal abnormalities | thickened wrists and ankles, severe bilateral bowing of lower limbs (genu varum) | thickened wrists and ankles, genu valgum deformity (knees touching each other while the ankles remain spaced apart) |
| Walk | waddling gait | impaired |
| Walking fatigue | ++++++ | +++ |
| Investigations (Reference Values) | Case#1 | Case#2 | ||
|---|---|---|---|---|
| Initiation of Treatment with Burosumab | 1 Year after Starting Treatment with Burosumab | Initiation of Treatment with Burosumab | 1 Year after Starting Treatment with Burosumab | |
| Phosphorus (2.4–4.4 mg/dL) | 1.85 | 2.6 | 2.41 | 3.0 |
| Alcaline phosphatase (3–10 years: 130–260 U/L, 10–14 years 130–340 U/L) | 488 | 159 | 788 | 379 |
| Total Calcium (8.8–10.8 mg/dL) | 10.2 | 9.5 | 9.9 | 9.40 |
| FGF23 (26–110 kRU/l) | 201 | 215 | ||
| Parathormone (PTH) (12–65 pg/mL) | 24 | 63 | 63.1 | 94 |
| 1,25(HO)2 dehydrogenase (25–86 pg/mL) | 59.5 | 83.7 | 62.7 | 89.10 |
| Glomerular filtration rate (over 90 mL/min) | 122.3 | 142.7 | 134.5 | 149 |
| Initiation of Treatment with Burosumab | 1 Year after Starting Treatment with Burosumab | |
|---|---|---|
| Case# 1 | Chest: discrete dextroconvex dorsolumbar scoliosis Bilateral femur: bilateral femural scoliostosis, bilateral enlargement of the distal metaphysis and epiphysis. Bilateral knee, leg, ankle: major bilateral tibial scoliostosis; mild bilateral fibular deformation. Bilateral enlargement of the proximal and distal tibial epiphysis and metaphysis Bone demineralization of the radiographed skeleton, with fine opaque lines Bone age corresponds to chronological age | Bilateral femural scoliostosis (in varum); Marked bilateral tibial scoliostosis; mild bilateral fibular deformation. Closed growth plates. Bone age corresponds to chronological age |
| Case # 2 | Chest: Marked bilateral widening of the anterior ends of the ribs. Costal rosaries. Femur: Deformed, curved femoral diaphysis, widened distal metaphysis and irregular contour at growth plates level. Deformed, curved fibular and tibial diaphysis with widened metaphysis and irregular contours Bone age corresponds to chronological age | Bilateral femoral scoliostosis (in varum); Widened femoral metaphysis In varum scoliostosis of the left tibia, in valgum scoliostosis of the right tibia. Bone age corresponds to chronological age |
| Author | Title | References |
|---|---|---|
| Yamazaki Y et al. | Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia | [34] |
| Zhang C et al. | Clinical and genetic analysis in a large Chinese cohort of patients with X-linked hypophosphatemia. | [35] |
| Morey M et al. | Genetic diagnosis of X-linked dominant Hypophosphatemic Rickets in a cohort study: tubular reabsorption of phosphate and 1,25(OH)2D serum levels are associated with PHEX mutation type | [36] |
| Vila-Pérez D et al. | Four Cases of X-Linked Hypophosphatemic Rickets, Clinical Description and Genetic Testing | [37] |
| Study and Patients | Results | Observations | References |
|---|---|---|---|
| Open-label phase 2 trial No. of patients: 52 Age: 1–12 Tracking period: 64 weeks |
| Positive effects of Burosumab treatment. | [73] |
| Open-label phase 3 trial at 16 clinical sites No. of patients: 61: 29 received Burosumab, 32 conventional therapies Age: 1–12 Tracking period: 64 weeks |
| Burosumab offers a promising new treatment approach for children with XLH in comparison with conventional therapy | [75] |
| Open-label phase 2 trial at three hospitals in the US No. of patients: 13 Age: 1–4 Tracking period: 64 weeks |
| Burosumab had a favorable safety profile | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurca, C.M.; Iuhas, O.; Kozma, K.; Petchesi, C.D.; Zaha, D.C.; Bembea, M.; Jurca, S.; Paul, C.; Jurca, A.D. Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review. Genes 2022, 13, 1392. https://doi.org/10.3390/genes13081392
Jurca CM, Iuhas O, Kozma K, Petchesi CD, Zaha DC, Bembea M, Jurca S, Paul C, Jurca AD. Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review. Genes. 2022; 13(8):1392. https://doi.org/10.3390/genes13081392
Chicago/Turabian StyleJurca, Claudia Maria, Oana Iuhas, Kinga Kozma, Codruta Diana Petchesi, Dana Carmen Zaha, Marius Bembea, Sanziana Jurca, Corina Paul, and Alexandru Daniel Jurca. 2022. "Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review" Genes 13, no. 8: 1392. https://doi.org/10.3390/genes13081392
APA StyleJurca, C. M., Iuhas, O., Kozma, K., Petchesi, C. D., Zaha, D. C., Bembea, M., Jurca, S., Paul, C., & Jurca, A. D. (2022). Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review. Genes, 13(8), 1392. https://doi.org/10.3390/genes13081392










