Emerging Roles and Potential Applications of Non-Coding RNAs in Cervical Cancer
Abstract
1. Introduction
2. Classification and Biogenesis of ncRNAs
2.1. Classification
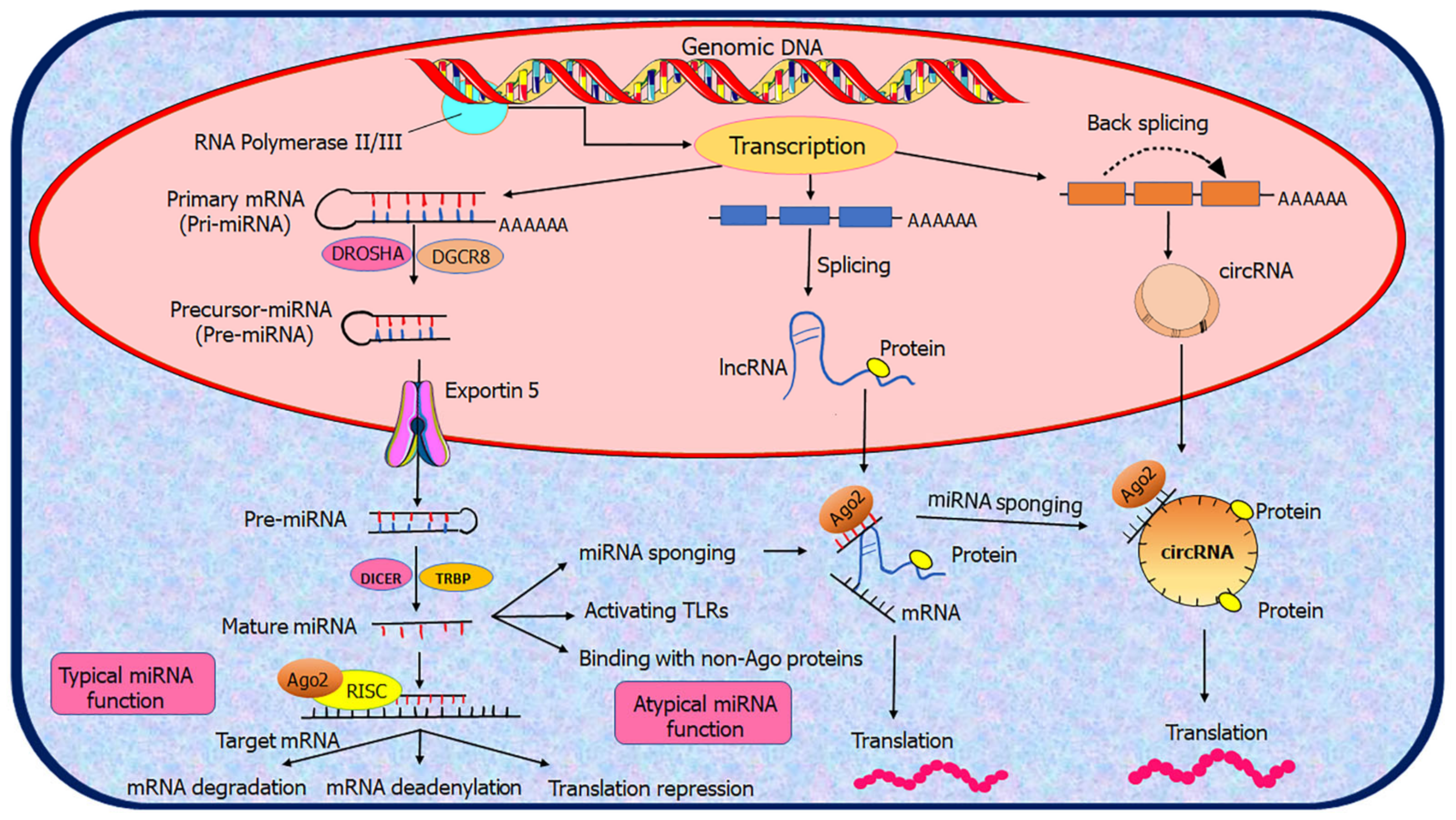
2.2. Biogenesis of ncRNAs
3. Functional Roles and Mechanisms of Action of ncRNAs
3.1. Biological Function of ncRNAs
3.2. Mechanisms of Action
4. Expression and Function of ncRNAs in CC
4.1. Dysregulated miRNAs in CC Onset/Progression
4.1.1. Oncogenic miRNAs
4.1.2. Tumor Suppressor miRNAs
4.2. Dysregulated lncRNAs in CC Onset/Progression
4.2.1. Oncogenic lncRNAs
4.2.2. Tumor Suppressor lncRNAs
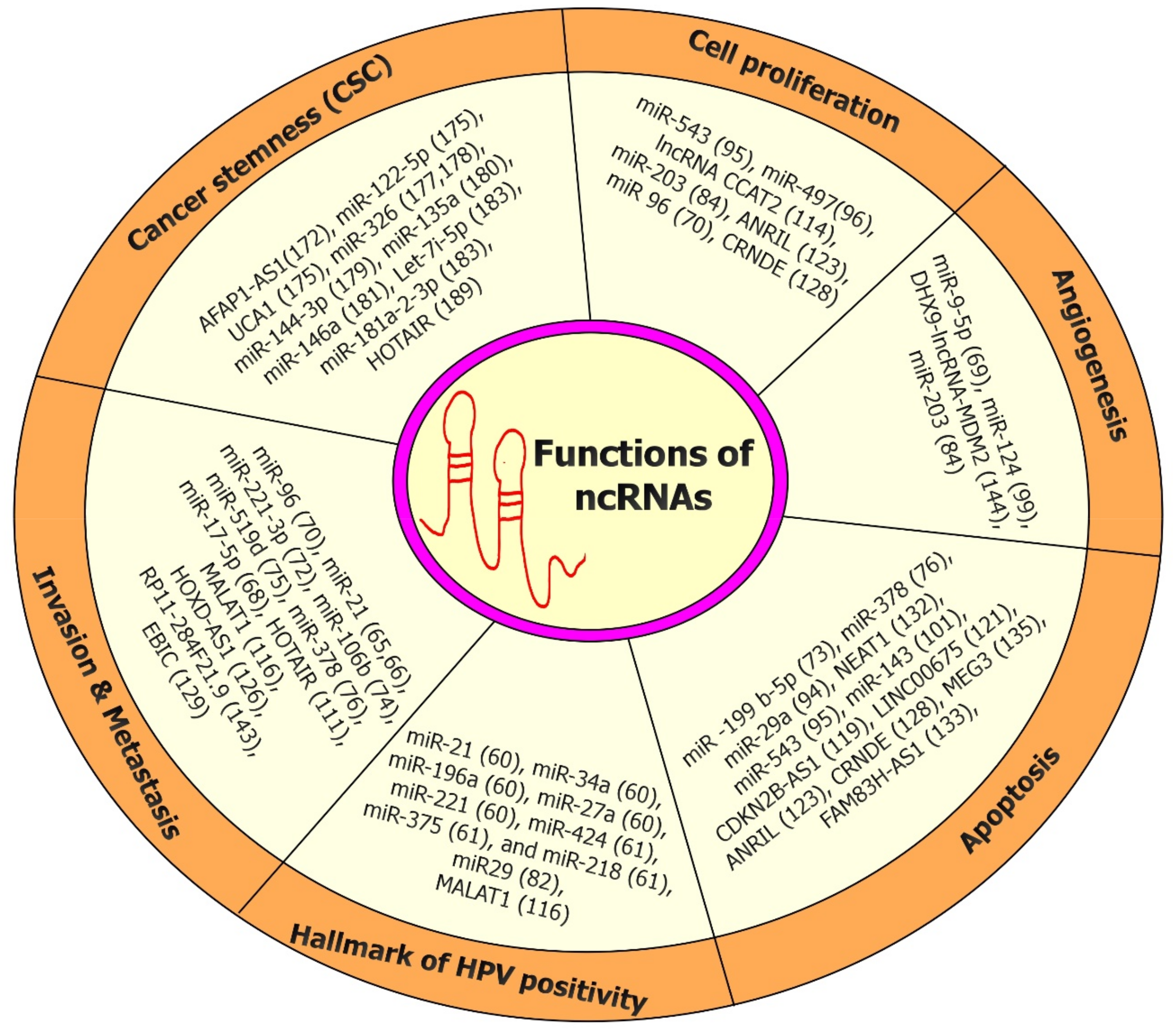
5. Role of ncRNAs (miRNAs and lncRNAs) in the Tumor Microenvironment (TME) of CC Onset/Progression
6. Role of ncRNAs (miRNAs and lncRNAs) in the Tumor Immunology of Onset/Progression
7. Role of ncRNAs (miRNAs and lncRNAs) in Cancer Stem Cells (CSCs) of CC
8. Therapeutic Approaches for Targeting ncRNAs in CC
9. Approaches for Systemic Delivery of Therapeutics ncRNAs in CC
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Párraga, S.; Alemany, L.; de Sanjosé, S.; Bosch, F.X.; Bravo, I.G. Differential HPV16 variant distribution in squamous cell carcinoma, adenocarcinoma and adenosquamous cell carcinoma. Int. J. Cancer 2017, 140, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.K.; Dan, N.; Chauhan, N.; Wang, Q.; Setua, S.; Nagesh, P.K.B.; Malik, S.; Batra, V.; Yallapu, M.M.; Miller, D.D.; et al. VERU-111 suppresses tumor growth and metastatic phenotypes of cervical cancer cells through the activation of p53 signaling pathway. Cancer Lett. 2020, 470, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Faraonio, R.; Buonaguro, L.; Annunziata, C.; Starita, N.; Cerasuolo, A.; Pezzuto, F.; Tornesello, A.L.; Buonaguro, F.M. The Role of microRNAs, Long Non-coding RNAs, and Circular RNAs in Cervical Cancer. Front. Oncol. 2020, 10, 150. [Google Scholar] [CrossRef]
- Karuri, A.R.; Kashyap, V.K.; Yallapu, M.M.; Zafar, N.; Kedia, S.K.; Jaggi, M.; Chauhan, S.C. Disparity in rates of HPV infection and cervical cancer in underserved US populations. Front. Biosci. 2017, 9, 254–269. [Google Scholar] [CrossRef][Green Version]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef]
- Ajenifuja, K.O.; Gage, J.C.; Adepiti, A.C.; Wentzensen, N.; Eklund, C.; Reilly, M.; Hutchinson, M.; Burk, R.D.; Schiffman, M. A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria. Int. J. Gynecol. Cancer 2013, 23, 507–512. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef]
- Agarwal, S.; Saini, S.; Parashar, D.; Verma, A.; Jagadish, N.; Batra, A.; Suri, S.; Bhatnagar, A.; Gupta, A.; Ansari, A.S.; et al. Expression and humoral respo nse of A-kinase anchor protein 4 in cervical cancer. Int. J. Gynecol. Cancer 2013, 23, 650–658. [Google Scholar] [CrossRef]
- St. Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef]
- Erhard, F.; Zimmer, R. Classification of ncRNAs using position and size information in deep sequencing data. Bioinformatics 2010, 26, i426–i432. [Google Scholar] [CrossRef]
- Cobb, M. 60 years ago, Francis Crick changed the logic of biology. PLoS Biol. 2017, 15, e2003243. [Google Scholar] [CrossRef]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; Oyama, R.; Ravasi, T.; Lenhard, B.; Wells, C.; et al. The transcriptional landscape of the mammalian genome. Science 2005, 309, 1559–1563. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef]
- Comfort, N. Genetics: We are the 98%. Nature 2015, 520, 615–616. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging role of non-coding RNA in health and disease. Metab. Brain Dis. 2021, 36, 1119–1134. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, J.R.; Knoll, M.; Gromatzky, A.A.; Lodish, H.F. The Super-Enhancer-Derived alncRNA-EC7/Bloodlinc Potentiates Red Blood Cell Development in trans. Cell Rep. 2017, 19, 2503–2514. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Nagano, T.; Fraser, P. No-Nonsense Functions for Long Noncoding RNAs. Cell 2011, 145, 178–181. [Google Scholar] [CrossRef]
- O’Day, E.; Lal, A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010, 12, 201. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chung, P.J.; Liu, J.; Jang, I.C.; Kean, M.J.; Xu, J.; Chua, N.H. Genome-wide identification of long noncoding natural antisense transcripts and their responses to light in Arabidopsis. Genome Res. 2014, 24, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Yu, D.; Yu, A.-M. Advances and challenges in studying noncoding RNA regulation of drug metabolism and development of RNA therapeutics. Biochem. Pharmacol. 2019, 169, 113638. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. Non-coding RNA regulation in reproduction: Their potential use as biomarkers. Noncoding RNA Res. 2019, 4, 54–62. [Google Scholar] [CrossRef]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef]
- Akerman, I.; Tu, Z.; Beucher, A.; Rolando, D.M.Y.; Sauty-Colace, C.; Benazra, M.; Nakic, N.; Yang, J.; Wang, H.; Pasquali, L.; et al. Human Pancreatic β Cell lncRNAs Control Cell-Specific Regulatory Networks. Cell Metab. 2017, 25, 400–411. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Chen, L.L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef]
- Ojha, S.; Malla, S.; Lyons, S.M. snoRNPs: Functions in Ribosome Biogenesis. Biomolecules 2020, 10, 783. [Google Scholar] [CrossRef]
- Naganuma, T.; Hirose, T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013, 10, 456–461. [Google Scholar] [CrossRef]
- Salehi, S.; Taheri, M.N.; Azarpira, N.; Zare, A.; Behzad-Behbahani, A. State of the art technologies to explore long non-coding RNAs in cancer. J. Cell Mol. Med. 2017, 21, 3120–3140. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Grillone, K.; Riillo, C.; Scionti, F.; Rocca, R.; Tradigo, G.; Guzzi, P.H.; Alcaro, S.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. Non-coding RNAs in cancer: Platforms and strategies for investigating the genomic “dark matter”. J. Exp. Clin. Cancer Res. 2020, 39, 117. [Google Scholar] [CrossRef]
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann. Transl. Med. 2018, 6, 3. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Santoleri, D.; Lim, H.W.; Emmett, M.J.; Stoute, J.; Gavin, M.J.; Sostre-Colón, J.; Uehara, K.; Welles, J.E.; Liu, K.F.; Lazar, M.A.; et al. Global-run on sequencing identifies Gm11967 as an Akt-dependent long noncoding RNA involved in insulin sensitivity. iScience 2022, 25, 104410. [Google Scholar] [CrossRef]
- Liu, G.X.; Tan, Y.Z.; He, G.C.; Zhang, Q.L.; Liu, P. EMX2OS plays a prognosis-associated enhancer RNA role in gastric cancer. Medicine 2021, 100, e27535. [Google Scholar] [CrossRef]
- Lee, Y.E.; Lee, J.; Lee, Y.S.; Jang, J.J.; Woo, H.; Choi, H.I.; Chai, Y.G.; Kim, T.K.; Kim, T.; Kim, L.K.; et al. Identification and Functional Characterization of Two Noncoding RNAs Transcribed from Putative Active Enhancers in Hepatocellular Carcinoma. Mol. Cells 2021, 44, 658–669. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Huang, S.; Wu, S.; Ding, J.; Lin, J.; Wei, L.; Gu, J.; He, X. MicroRNA-181a modulates gene expression of zinc finger family members by directly targeting their coding regions. Nucleic Acids Res. 2010, 38, 7211–7218. [Google Scholar] [CrossRef]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Parashar, D.; Geethadevi, A.; Aure, M.R.; Mishra, J.; George, J.; Chen, C.; Mishra, M.K.; Tahiri, A.; Zhao, W.; Nair, B.; et al. miRNA551b-3p Activates an Oncostatin Signaling Module for the Progression of Triple-Negative Breast Cancer. Cell Rep. 2019, 29, 4389–4406. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Zhu, L.; Li, T.; Yan, Z.; Guo, J. Transfer RNA-derived fragments and tRNA halves: Biogenesis, biological functions and their roles in diseases. J. Mol. Med. 2018, 96, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Jiang, Z.; Li, T.; Hu, Y.; Guo, J. Circular RNAs in hepatocellular carcinoma: Functions and implications. Cancer Med. 2018, 7, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- McHugh, C.A.; Chen, C.K.; Chow, A.; Surka, C.F.; Tran, C.; McDonel, P.; Pandya-Jones, A.; Blanco, M.; Burghard, C.; Moradian, A.; et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 2015, 521, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ahmad, A.; Sarkar, F.H. The role of microRNAs in breast cancer migration, invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 13414–13437. [Google Scholar] [CrossRef] [PubMed]
- Palmini, G.; Marini, F.; Brandi, M.L. What Is New in the miRNA World Regarding Osteosarcoma and Chondrosarcoma? Molecules 2017, 22, 417. [Google Scholar] [CrossRef]
- Lui, W.O.; Pourmand, N.; Patterson, B.K.; Fire, A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007, 67, 6031–6043. [Google Scholar] [CrossRef]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Muralidhar, B.; Goldstein, L.D.; Ng, G.; Winder, D.M.; Palmer, R.D.; Gooding, E.L.; Barbosa-Morais, N.L.; Mukherjee, G.; Thorne, N.P.; Roberts, I.; et al. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. J. Pathol. 2007, 212, 368–377. [Google Scholar] [CrossRef]
- Gupta, S.; Panda, P.K.; Hashimoto, R.F.; Samal, S.K.; Mishra, S.; Verma, S.K.; Mishra, Y.K.; Ahuja, R. Dynamical modeling of miR-34a, miR-449a, and miR-16 reveals numerous DDR signaling pathways regulating senescence, autophagy, and apoptosis in HeLa cells. Sci. Rep. 2022, 12, 4911. [Google Scholar] [CrossRef]
- He, Y.; Lin, J.; Ding, Y.; Liu, G.; Luo, Y.; Huang, M.; Xu, C.; Kim, T.K.; Etheridge, A.; Lin, M.; et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int. J. Cancer 2016, 138, 1312–1327. [Google Scholar] [CrossRef]
- Gocze, K.; Gombos, K.; Juhasz, K.; Kovacs, K.; Kajtar, B.; Benczik, M.; Gocze, P.; Patczai, B.; Arany, I.; Ember, I. Unique microRNA expression profiles in cervical cancer. Anticancer. Res. 2013, 33, 2561–2567. [Google Scholar]
- Tian, Q.; Li, Y.; Wang, F.; Li, Y.; Xu, J.; Shen, Y.; Ye, F.; Wang, X.; Cheng, X.; Chen, Y.; et al. MicroRNA detection in cervical exfoliated cells as a triage for human papillomavirus-positive women. J. Natl. Cancer Inst. 2014, 106, dju241. [Google Scholar] [CrossRef]
- Kawai, S.; Fujii, T.; Kukimoto, I.; Yamada, H.; Yamamoto, N.; Kuroda, M.; Otani, S.; Ichikawa, R.; Nishio, E.; Torii, Y.; et al. Identification of miRNAs in cervical mucus as a novel diagnostic marker for cervical neoplasia. Sci. Rep. 2018, 8, 7070. [Google Scholar] [CrossRef]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef]
- Cheng, T.; Huang, S. Roles of Non-Coding RNAs in Cervical Cancer Metastasis. Front. Oncol. 2021, 11, 646192. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Lv, Q.; Zhu, D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol. Rep. 2015, 33, 3108–3116. [Google Scholar] [CrossRef]
- Deng, Z.M.; Chen, G.H.; Dai, F.F.; Liu, S.Y.; Yang, D.Y.; Bao, A.Y.; Cheng, Y.X. The clinical value of miRNA-21 in cervical cancer: A comprehensive investigation based on microarray datasets. PLoS ONE 2022, 17, e0267108. [Google Scholar] [CrossRef]
- Peralta-Zaragoza, O.; Deas, J.; Meneses-Acosta, A.; De la O-Gómez, F.; Fernández-Tilapa, G.; Gómez-Cerón, C.; Benítez-Boijseauneau, O.; Burguete-García, A.; Torres-Poveda, K.; Bermúdez-Morales, V.H.; et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells. BMC Cancer 2016, 16, 215. [Google Scholar] [CrossRef]
- Cai, N.; Hu, L.; Xie, Y.; Gao, J.H.; Zhai, W.; Wang, L.; Jin, Q.J.; Qin, C.Y.; Qiang, R. MiR-17-5p promotes cervical cancer cell proliferation and metastasis by targeting transforming growth factor-β receptor 2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1899–1906. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Jiao, X.L.; Zhang, S.Y.; Xu, Y.; Li, S.; Kong, B.H. MiR-9-5p could promote angiogenesis and radiosensitivity in cervical cancer by targeting SOCS5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7314–7326. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Xie, B.; Chen, S.; Zhuang, Y.; Zhang, S. miR-96 exerts an oncogenic role in the progression of cervical cancer by targeting CAV-1. Mol. Med. Rep. 2020, 22, 543–550. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, X.; Yan, D.; Wang, Z. Circulating MicroRNA-21 Is Involved in Lymph Node Metastasis in Cervical Cancer by Targeting RASA1. Int. J. Gynecol. Cancer 2016, 26, 810–816. [Google Scholar] [CrossRef]
- Wei, W.F.; Zhou, C.F.; Wu, X.G.; He, L.N.; Wu, L.F.; Chen, X.J.; Yan, R.M.; Zhong, M.; Yu, Y.H.; Liang, L.; et al. MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2. Cell Death Dis. 2017, 8, 3220. [Google Scholar] [CrossRef]
- Xu, L.J.; Duan, Y.; Wang, P.; Yin, H.Q. MiR-199b-5p promotes tumor growth and metastasis in cervical cancer by down-regulating KLK10. Biochem. Biophys. Res. Commun. 2018, 503, 556–563. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Y.; Zhang, Y.; You, K.; Li, Z.; Geng, L. MicroRNA-106b is involved in transforming growth factor β1-induced cell migration by targeting disabled homolog 2 in cervical carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 11. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Zheng, S.R.; Liu, J.; Shi, R.; Yu, H.L.; Wei, M. MiR-519d facilitates the progression and metastasis of cervical cancer through direct targeting Smad7. Cancer Cell Int. 2016, 16, 21. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, C.; Han, S.; Hou, X.; Kang, S.; Zhang, Y. MicroRNA-378 enhances migration and invasion in cervical cancer by directly targeting autophagy-related protein 12. Mol. Med. Rep. 2018, 17, 6319–6326. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, D.; Chen, J.; Ding, N.; Ren, F. MiR-20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS ONE 2015, 10, e0120905. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Tao, L.; Yu, C. MicroRNA-106a promotes cell migration and invasion by targeting tissue inhibitor of matrix metalloproteinase 2 in cervical cancer. Oncol. Rep. 2017, 38, 1774–1782. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Li, J.; Wang, X.; Song, W. MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4. Biomed. Pharmacother. 2018, 97, 511–517. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Zhou, J. miR-31 Functions as an Oncomir Which Promotes Epithelial-Mesenchymal Transition via Regulating BAP1 in Cervical Cancer. Biomed. Res. Int. 2017, 2017, 6361420. [Google Scholar] [CrossRef]
- Lao, G.; Liu, P.; Wu, Q.; Zhang, W.; Liu, Y.; Yang, L.; Ma, C. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour. Biol. 2014, 35, 11933–11938. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A Systematic Review of miR-29 in Cancer. Mol. Ther. Oncolytics 2019, 12, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, F.; Fu, Y.; Chen, X.; Zhang, D. MiR-520d-5p functions as a tumor-suppressor gene in cervical cancer through targeting PTK2. Life Sci. 2020, 254, 117558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Er, K.; Mao, C.; Yan, Q.; Xu, H.; Zhang, Y.; Zhu, J.; Cui, F.; Zhao, W.; Shi, H. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol. Biochem. 2013, 32, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fontemaggi, G.; Turco, C.; Esposito, G.; Di Agostino, S. New Molecular Mechanisms and Clinical Impact of circRNAs in Human Cancer. Cancers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Kulcheski, F.R.; Christoff, A.P.; Margis, R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016, 238, 42–51. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Shen, J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017, 14, 514–521. [Google Scholar] [CrossRef]
- Ma, H.-B.; Yao, Y.-N.; Yu, J.-J.; Chen, X.-X.; Li, H.-F. Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am. J. Transl. Res. 2018, 10, 592–604. [Google Scholar]
- Yang, M.; Hu, H.; Wu, S.; Ding, J.; Yin, B.; Huang, B.; Li, F.; Guo, X.; Han, L. EIF4A3-regulated circ_0087429 can reverse EMT and inhibit the progression of cervical cancer via miR-5003-3p-dependent upregulation of OGN expression. J. Exp. Clin. Cancer Res. 2022, 41, 165. [Google Scholar] [CrossRef]
- Chen, R.; Gan, Q.; Zhao, S.; Zhang, D.; Wang, S.; Yao, L.; Yuan, M.; Cheng, J. DNA methylation of miR-138 regulates cell proliferation and EMT in cervical cancer by targeting EZH2. BMC Cancer 2022, 22, 488. [Google Scholar] [CrossRef]
- Ou, R.; Lu, S.; Wang, L.; Wang, Y.; Lv, M.; Li, T.; Xu, Y.; Lu, J.; Ge, R.-s. Circular RNA circLMO1 Suppresses Cervical Cancer Growth and Metastasis by Triggering miR-4291/ACSL4-Mediated Ferroptosis. Front. Oncol. 2022, 12, 858598. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, L.; Liu, R.; Shi, Q.; Li, X.; Wang, M. MiR-125 inhibited cervical cancer progression by regulating VEGF and PI3K/AKT signaling pathway. World J. Surg. Oncol. 2020, 18, 115. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, X.J.; Yang, H.L.; Zhang, Y.B.; Li, J.C. MicroRNA-23b suppresses cervical cancer biological progression by directly targeting six1 and affecting epithelial-to-mesenchymal transition and AKT/mTOR signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4688–4697. [Google Scholar] [CrossRef]
- Gong, Y.; Wan, J.H.; Zou, W.; Lian, G.Y.; Qin, J.L.; Wang, Q.M. MiR-29a inhibits invasion and metastasis of cervical cancer via modulating methylation of tumor suppressor SOCS1. Future Oncol. 2019, 15, 1729–1744. [Google Scholar] [CrossRef]
- Liu, X.; Gan, L.; Zhang, J. miR-543 inhibites cervical cancer growth and metastasis by targeting TRPM7. Chem. Biol. Interact. 2019, 302, 83–92. [Google Scholar] [CrossRef]
- Luo, M.; Shen, D.; Zhou, X.; Chen, X.; Wang, W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery 2013, 153, 836–847. [Google Scholar] [CrossRef]
- Kogo, R.; How, C.; Chaudary, N.; Bruce, J.; Shi, W.; Hill, R.P.; Zahedi, P.; Yip, K.W.; Liu, F.F. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget 2015, 6, 1090–1100. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Chen, G.T.; Chen, C.; Zhang, Q.F.; Pan, F.; Hu, M.; Li, B.S. MicroRNA-200b inhibits epithelial-mesenchymal transition and migration of cervical cancer cells by directly targeting RhoE. Mol. Med. Rep. 2016, 13, 3139–3146. [Google Scholar] [CrossRef]
- Wan, H.Y.; Li, Q.Q.; Zhang, Y.; Tian, W.; Li, Y.N.; Liu, M.; Li, X.; Tang, H. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014, 355, 148–158. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, G.; Li, L.; Geng, P.; Song, H. microRNA-214 suppresses the growth of cervical cancer cells by targeting EZH2. Oncol. Lett. 2018, 16, 5679–5686. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Guo, X.; Tian, Z.; Su, M.; Long, Y.; Huang, C.; Zhou, F.; Liu, M.; Wu, X.; et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol. Med. Rep. 2012, 5, 753–760. [Google Scholar] [CrossRef]
- Shen, W.; Xie, X.Y.; Liu, M.R.; Wang, L.L. MicroRNA-101-5p inhibits the growth and metastasis of cervical cancer cell by inhibiting CXCL6. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1957–1968. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhang, L.; Guo, X.; Wang, J.H.; Zhou, W.; Liu, M.; Li, X.; Tang, H. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life 2015, 67, 380–394. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, H.Y.; Shi, X.Q.; Wang, Y. Upregulation of microRNA-129-5p inhibits cell invasion, migration and tumor angiogenesis by inhibiting ZIC2 via downregulation of the Hedgehog signaling pathway in cervical cancer. Cancer Biol. Ther. 2018, 19, 1162–1173. [Google Scholar] [CrossRef]
- Ou, L.; Wang, D.; Zhang, H.; Yu, Q.; Hua, F. Decreased Expression of miR-138-5p by lncRNA H19 in Cervical Cancer Promotes Tumor Proliferation. Oncol. Res. 2018, 26, 401–410. [Google Scholar] [CrossRef]
- Cao, X.C.; Yu, Y.; Hou, L.K.; Sun, X.H.; Ge, J.; Zhang, B.; Wang, X. miR-142-3p inhibits cancer cell proliferation by targeting CDC25C. Cell Prolif. 2016, 49, 58–68. [Google Scholar] [CrossRef]
- Mou, Z.; Xu, X.; Dong, M.; Xu, J. MicroRNA-148b Acts as a Tumor Suppressor in Cervical Cancer by Inducing G1/S-Phase Cell Cycle Arrest and Apoptosis in a Caspase-3-Dependent Manner. Med. Sci. Monit. 2016, 22, 2809–2815. [Google Scholar] [CrossRef]
- Sun, J.; Ji, J.; Huo, G.; Song, Q.; Zhang, X. miR-182 induces cervical cancer cell apoptosis through inhibiting the expression of DNMT3a. Int. J. Clin. Exp. Pathol. 2015, 8, 4755–4763. [Google Scholar] [PubMed]
- Zhou, Q.; Han, L.R.; Zhou, Y.X.; Li, Y. MiR-195 Suppresses Cervical Cancer Migration and Invasion Through Targeting Smad3. Int. J. Gynecol. Cancer 2016, 26, 817–824. [Google Scholar] [CrossRef] [PubMed]
- How, C.; Hui, A.B.; Alajez, N.M.; Shi, W.; Boutros, P.C.; Clarke, B.A.; Yan, R.; Pintilie, M.; Fyles, A.; Hedley, D.W.; et al. MicroRNA-196b regulates the homeobox B7-vascular endothelial growth factor axis in cervical cancer. PLoS ONE 2013, 8, e67846. [Google Scholar] [CrossRef] [PubMed]
- Aalijahan, H.; Ghorbian, S. Long non-coding RNAs and cervical cancer. Exp. Mol. Pathol. 2019, 106, 7–16. [Google Scholar] [CrossRef]
- Huo, H.; Tian, J.; Wang, R.; Li, Y.; Qu, C.; Wang, N. Long non-coding RNA NORAD upregulate SIP1 expression to promote cell proliferation and invasion in cervical cancer. Biomed. Pharmacother. 2018, 106, 1454–1460. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.X.; Sun, X.X.; Wang, C.; Liu, T.F.; Wang, D.J. Long non-coding RNA CCHE1 overexpression predicts a poor prognosis for cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 479–483. [Google Scholar]
- Wu, L.; Jin, L.; Zhang, W.; Zhang, L. Roles of Long Non-Coding RNA CCAT2 in Cervical Cancer Cell Growth and Apoptosis. Med. Sci. Monit. 2016, 22, 875–879. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, L. New Insights into Long Non-Coding RNA MALAT1 in Cancer and Metastasis. Cancers 2019, 11, 216. [Google Scholar] [CrossRef]
- Lu, H.; He, Y.; Lin, L.; Qi, Z.; Ma, L.; Li, L.; Su, Y. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour. Biol. 2016, 37, 1683–1691. [Google Scholar] [CrossRef]
- Liu, M.; Jia, J.; Wang, X.; Liu, Y.; Wang, C.; Fan, R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 2018, 19, 391–399. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Jin, X.; Liu, Y.; Yang, Y.; Li, L.; Wang, X.; Li, G. Long non-coding RNA LINC01535 promotes cervical cancer progression via targeting the miR-214/EZH2 feedback loop. J. Cell Mol. Med. 2019, 23, 6098–6111. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Q.; Li, S.; Jiang, S.; Cui, J.; Dang, G. Interference of the long noncoding RNA CDKN2B-AS1 upregulates miR-181a-5p/TGFβI axis to restrain the metastasis and promote apoptosis and senescence of cervical cancer cells. Cancer Med. 2019, 8, 1721–1730. [Google Scholar] [CrossRef]
- Hsu, W.; Liu, L.; Chen, X.; Zhang, Y.; Zhu, W. LncRNA CASC11 promotes the cervical cancer progression by activating Wnt/beta-catenin signaling pathway. Biol. Res. 2019, 52, 33. [Google Scholar] [CrossRef]
- Ma, S.; Deng, X.; Yang, Y.; Zhang, Q.; Zhou, T.; Liu, Z. The lncRNA LINC00675 regulates cell proliferation, migration, and invasion by affecting Wnt/β-catenin signaling in cervical cancer. Biomed. Pharmacother. 2018, 108, 1686–1693. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Fang, S.; Jiang, B.; Qin, C.; Xie, P.; Zhou, G.; Li, G. The role of MALAT1 correlates with HPV in cervical cancer. Oncol. Lett. 2014, 7, 2135–2141. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Liu, Y.J.; He, Y.; Chen, P. Down-regulation of long non-coding RNA ANRIL inhibits the proliferation, migration and invasion of cervical cancer cells. Cancer Biomark 2018, 23, 243–253. [Google Scholar] [CrossRef]
- Shan, D.; Shang, Y.; Hu, T. Long noncoding RNA BLACAT1 promotes cell proliferation and invasion in human cervical cancer. Oncol. Lett. 2018, 15, 3490–3495. [Google Scholar] [CrossRef]
- Chang, Q.Q.; Chen, C.Y.; Chen, Z.; Chang, S. LncRNA PVT1 promotes proliferation and invasion through enhancing Smad3 expression by sponging miR-140-5p in cervical cancer. Radiol. Oncol. 2019, 53, 443–452. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, A.M.; Lu, J.K.; Cen, R.; Liu, L.L. Long noncoding RNA HOXD-AS1 regulates proliferation of cervical cancer cells by activating Ras/ERK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5049–5055. [Google Scholar] [CrossRef]
- Xie, F.; Xie, G.; Sun, Q. Long Noncoding RNA DLX6-AS1 Promotes the Progression in Cervical Cancer by Targeting miR-16-5p/ARPP19 Axis. Cancer Biother Radiopharm. 2020, 35, 129–136. [Google Scholar] [CrossRef]
- Yang, H.Y.; Huang, C.P.; Cao, M.M.; Wang, Y.F.; Liu, Y. Long non-coding RNA CRNDE may be associated with poor prognosis by promoting proliferation and inhibiting apoptosis of cervical cancer cells through targeting PI3K/AKT. Neoplasma 2018, 65, 872–880. [Google Scholar] [CrossRef]
- Sun, N.X.; Ye, C.; Zhao, Q.; Zhang, Q.; Xu, C.; Wang, S.B.; Jin, Z.J.; Sun, S.H.; Wang, F.; Li, W. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS ONE 2014, 9, e100340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, X.; Que, S.; Yang, X.; Fan, H.; Liu, M.; Li, X.; Tang, H. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget 2017, 8, 43768–43781. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.J.; Zou, Y.H.; He, P.J.; Zhang, S.; Sun, X.M.; Li, C.Z. Long non-coding RNA SPRY4-IT1 promotes epithelial-mesenchymal transition of cervical cancer by regulating the miR-101-3p/ZEB1 axis. Biosci. Rep. 2019, 39, BSR20181339. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Jia, W.C.; Li, T.; Li, N.; Wei, W. Knockdown of lncRNA NEAT1 suppresses proliferation and migration, and induces apoptosis of cervical cancer cells by regulating the miR-377/FGFR1 axis. Mol. Med. Rep. 2022, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.A.; Hayes, K.E.; Brownmiller, T.; Harold, A.D.; Jagannathan, R.; Lockman, P.R.; Khan, S.; Martinez, I. Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells. Sci. Rep. 2019, 9, 3662. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Xu, Y.; Jiang, X.; Ye, W.; Huang, Y.; Jiang, J. Long non-coding RNA C5orf66-AS1 promotes cell proliferation in cervical cancer by targeting miR-637/RING1 axis. Cell Death Dis. 2018, 9, 1175. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y. Long non-coding RNA MEG3 inhibits cervical cancer cell growth by promoting degradation of P-STAT3 protein via ubiquitination. Cancer Cell Int. 2019, 19, 175. [Google Scholar] [CrossRef]
- Shao, S.; Wang, C.; Wang, S.; Zhang, H.; Zhang, Y. LncRNA STXBP5-AS1 suppressed cervical cancer progression via targeting miR-96-5p/PTEN axis. Biomed. Pharmacother. 2019, 117, 109082. [Google Scholar] [CrossRef]
- Yang, W.; Hong, L.; Xu, X.; Wang, Q.; Huang, J.; Jiang, L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol 2017, 39, 1010428317711315. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am. J. Transl. Res. 2019, 11, 4909–4921. [Google Scholar]
- Zhu, Y.; Liu, B.; Zhang, P.; Zhang, J.; Wang, L. LncRNA TUSC8 inhibits the invasion and migration of cervical cancer cells via miR-641/PTEN axis. Cell Biol. Int. 2019, 43, 781–788. [Google Scholar] [CrossRef]
- Liao, L.M.; Sun, X.Y.; Liu, A.W.; Wu, J.B.; Cheng, X.L.; Lin, J.X.; Zheng, M.; Huang, L. Low expression of long noncoding XLOC_010588 indicates a poor prognosis and promotes proliferation through upregulation of c-Myc in cervical cancer. Gynecol. Oncol. 2014, 133, 616–623. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ding, X.; Sui, X. LINC00861 inhibits the progression of cervical cancer cells by functioning as a ceRNA for miR-513b-5p and regulating the PTEN/AKT/mTOR signaling pathway. Mol. Med. Rep. 2021, 23, 24. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, Z.; Wang, Y.Y.; Wang, Y. Long noncoding RNA ZNF667-AS1 reduces tumor invasion and metastasis in cervical cancer by counteracting microRNA-93-3p-dependent PEG3 downregulation. Mol. Oncol. 2019, 13, 2375–2392. [Google Scholar] [CrossRef]
- Han, H.F.; Chen, Q.; Zhao, W.W. Long non-coding RNA RP11-284F21.9 functions as a ceRNA regulating PPWD1 by competitively binding to miR-769-3p in cervical carcinoma. Biosci. Rep. 2020, 40, BSR20200784. [Google Scholar] [CrossRef]
- Ding, X.; Jia, X.; Wang, C.; Xu, J.; Gao, S.-J.; Lu, C. A DHX9-lncRNA-MDM2 interaction regulates cell invasion and angiogenesis of cervical cancer. Cell Death Differ. 2019, 26, 1750–1765. [Google Scholar] [CrossRef]
- Xue, C.; Chen, C.; Gu, X.; Li, L. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am. J. Cancer Res. 2021, 11, 1–13. [Google Scholar]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Drak Alsibai, K.; Meseure, D. Tumor microenvironment and noncoding RNAs as co-drivers of epithelial-mesenchymal transition and cancer metastasis. Dev. Dyn. 2018, 247, 405–431. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, J.; Zhang, B.; Wang, W.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. The promising role of noncoding RNAs in cancer-associated fibroblasts: An overview of current status and future perspectives. J. Hematol. Oncol. 2020, 13, 154. [Google Scholar] [CrossRef]
- Fan, D.; Wang, Y.; Qi, P.; Chen, Y.; Xu, P.; Yang, X.; Jin, X.; Tian, X. MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol. Oncol. 2016, 141, 166–174. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Wu, X.G.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Wei, W.F.; Chen, X.J.; Yi, H.Y.; Liang, L.J.; Fan, L.S.; Liang, L.; et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019, 22, 397–410. [Google Scholar] [CrossRef]
- Chen, D.; Lu, T.; Tan, J.; Li, H.; Wang, Q.; Wei, L. Long Non-coding RNAs as Communicators and Mediators Between the Tumor Microenvironment and Cancer Cells. Front. Oncol. 2019, 9, 739. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, Y.; Wang, Z.; Zheng, J.; Chen, Y.; Li, X.; Wang, Y.; Ming, H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 490, 406–414. [Google Scholar] [CrossRef]
- Hu, Q.; Ye, Y.; Chan, L.C.; Li, Y.; Liang, K.; Lin, A.; Egranov, S.D.; Zhang, Y.; Xia, W.; Gong, J.; et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 2019, 20, 835–851. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, X.; Su, X. Noncoding RNAs in cancer immunity: Functions, regulatory mechanisms, and clinical application. Mol. Cancer 2020, 19, 48. [Google Scholar] [CrossRef]
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- McCune, J.S. Rapid Advances in Immunotherapy to Treat Cancer. Clin. Pharmacol. Ther. 2018, 103, 540–544. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Q.; Yao, F.; Qing, C.; Ye, J.; Deng, Y.; Li, J. Identification of Differentially Expressed Long Non-coding RNAs in Polarized Macrophages. Sci. Rep. 2016, 6, 19705. [Google Scholar] [CrossRef]
- Squadrito Mario, L.; Pucci, F.; Magri, L.; Moi, D.; Gilfillan Gregor, D.; Ranghetti, A.; Casazza, A.; Mazzone, M.; Lyle, R.; Naldini, L.; et al. miR-511-3p Modulates Genetic Programs of Tumor-Associated Macrophages. Cell Rep. 2012, 1, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Caescu, C.I.; Guo, X.; Tesfa, L.; Bhagat, T.D.; Verma, A.; Zheng, D.; Stanley, E.R. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood 2015, 125, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, M.; Xu, Z.; Li, Y.; Guo, H.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2016, 7, 13502–13519. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- He, S.; Chu, J.; Wu, L.-C.; Mao, H.; Peng, Y.; Alvarez-Breckenridge, C.A.; Hughes, T.; Wei, M.; Zhang, J.; Yuan, S.; et al. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood 2013, 121, 4663–4671. [Google Scholar] [CrossRef]
- Dou, R.; Nishihara, R.; Cao, Y.; Hamada, T.; Mima, K.; Masuda, A.; Masugi, Y.; Shi, Y.; Gu, M.; Li, W.; et al. MicroRNA let-7, T Cells, and Patient Survival in Colorectal Cancer. Cancer Immunol. Res. 2016, 4, 927–935. [Google Scholar] [CrossRef]
- Yao, H.; Jiang, X.; Fu, H.; Yang, Y.; Jin, Q.; Zhang, W.; Cao, W.; Gao, W.; Wang, S.; Zhu, Y.; et al. Exploration of the Immune-Related Long Noncoding RNA Prognostic Signature and Inflammatory Microenvironment for Cervical Cancer. Front. Pharmacol. 2022, 13, 870221. [Google Scholar] [CrossRef]
- Zuccherato, L.W.; Machado, C.M.T.; Magalhães, W.C.S.; Martins, P.R.; Campos, L.S.; Braga, L.C.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Franco, T.; Paula, S.O.C.; et al. Cervical Cancer Stem-Like Cell Transcriptome Profiles Predict Response to Chemoradiotherapy. Front. Oncol. 2021, 11, 639339. [Google Scholar] [CrossRef]
- Huang, R.; Rofstad, E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 2017, 8, 35351–35367. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Q.; Liu, S.; Ning, N.; Zhang, X.; Xu, Y.; Chang, A.E.; Wicha, M.S. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem. Cells 2015, 33, 2085–2092. [Google Scholar] [CrossRef]
- Xia, M.; Duan, L.J.; Lu, B.N.; Pang, Y.Z.; Pang, Z.R. LncRNA AFAP1-AS1/miR-27b-3p/VEGF-C axis modulates stemness characteristics in cervical cancer cells. Chin. Med. J. 2021, 134, 2091–2101. [Google Scholar] [CrossRef]
- Shang, C.; Guo, Y.; Zhang, J.; Huang, B. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer. Cancer Chemother. Pharmacol. 2016, 77, 1061–1067. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, X.; Chen, S.; Li, W.; Li, D.; Singer, R.; Gu, W. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. 2018, 20, 32. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Q.; Ji, M.; Guo, X.; Li, L.; Su, X. Exosomal lncRNA UCA1 modulates cervical cancer stem cell self-renewal and differentiation through microRNA-122-5p/SOX2 axis. J. Transl. Med. 2021, 19, 229. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Panigrahi, M.; Challa, S.; Mahadevan, A.; Babu, P.P. Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem. Int. 2009, 55, 307–317. [Google Scholar] [CrossRef]
- Babashah, S.; Sadeghizadeh, M.; Hajifathali, A.; Tavirani, M.R.; Zomorod, M.S.; Ghadiani, M.; Soleimani, M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int. J. Cancer 2013, 133, 579–589. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Wang, K.; Xie, Y.; Yang, Z.; Qie, M.; Liao, Z.; Zheng, Z. miR-326 inhibits the cell proliferation and cancer stem cell-like property of cervical cancer in vitro and oncogenesis in vivo via targeting TCF4. Ann. Transl. Med. 2020, 8, 1638. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, B.; Zhang, Y.; Wang, S.; Zhu, X. Human bone marrow mesenchymal stem cell-derived extracellular vesicles impede the progression of cervical cancer via the miR-144-3p/CEP55 pathway. J. Cell. Mol. Med. 2021, 25, 1867–1883. [Google Scholar] [CrossRef]
- Leung, C.O.N.; Deng, W.; Ye, T.M.; Ngan, H.Y.S.; Tsao, S.W.; Cheung, A.N.Y.; Ziru, N.; Yuen, D.C.K.; Pang, R.T.K.; Yeung, W.S.B. MicroRNA-135a-induced formation of CD133+ subpopulation with cancer stem cell properties in cervical cancer. Carcinogenesis 2020, 41, 1592–1604. [Google Scholar] [CrossRef]
- Dong, Z.; Yu, C.; Rezhiya, K.; Gulijiahan, A.; Wang, X. Downregulation of miR-146a promotes tumorigenesis of cervical cancer stem cells via VEGF/CDC42/PAK1 signaling pathway. Artif Cells Nanomed. Biotechnol. 2019, 47, 3711–3719. [Google Scholar] [CrossRef]
- Akerman, G.S.; Tolleson, W.H.; Brown, K.L.; Zyzak, L.L.; Mourateva, E.; Engin, T.S.; Basaraba, A.; Coker, A.L.; Creek, K.E.; Pirisi, L. Human papillomavirus type 16 E6 and E7 cooperate to increase epidermal growth factor receptor (EGFR) mRNA levels, overcoming mechanisms by which excessive EGFR signaling shortens the life span of normal human keratinocytes. Cancer Res. 2001, 61, 3837–3843. [Google Scholar]
- Chhabra, R. let-7i-5p, miR-181a-2-3p and EGF/PI3K/SOX2 axis coordinate to maintain cancer stem cell population in cervical cancer. Sci. Rep. 2018, 8, 7840. [Google Scholar] [CrossRef]
- Mainguy, G.; Koster, J.; Woltering, J.; Jansen, H.; Durston, A. Extensive polycistronism and antisense transcription in the mammalian Hox clusters. PLoS ONE 2007, 2, e356. [Google Scholar] [CrossRef]
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef]
- Kim, H.J.; Eoh, K.J.; Kim, L.K.; Nam, E.J.; Yoon, S.O.; Kim, K.H.; Lee, J.K.; Kim, S.W.; Kim, Y.T. The long noncoding RNA HOXA11 antisense induces tumor progression and stemness maintenance in cervical cancer. Oncotarget 2016, 7, 83001–83016. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, W.; Su, B.; Yu, B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed. Res. Int. 2013, 2013, 251098. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Cui, Y.H.; Wang, T.; Luo, Y. Long non-coding RNA HOTAIR in cervical cancer: Molecular marker, mechanistic insight, and therapeutic target. Adv. Clin. Chem. 2020, 97, 117–140. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wu, Q.; Liu, Y.; Ma, C. HOTAIR Contributes to Stemness Acquisition of Cervical Cancer through Regulating miR-203 Interaction with ZEB1 on Epithelial-Mesenchymal Transition. J. Oncol. 2021, 2021, 4190764. [Google Scholar] [CrossRef]
- Walder, R.Y.; Walder, J.A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc. Natl. Acad. Sci. USA 1988, 85, 5011–5015. [Google Scholar] [CrossRef]
- Chiriboga, C.A. Nusinersen for the treatment of spinal muscular atrophy. Expert. Rev. Neurother. 2017, 17, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Jaschinski, F.; Rothhammer, T.; Jachimczak, P.; Seitz, C.; Schneider, A.; Schlingensiepen, K.H. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-β2. Curr. Pharm. Biotechnol. 2011, 12, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Teng, X.; Li, J.; Liang, X.-J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jinek, M. Cas9 versus Cas12a/Cpf1: Structure-function comparisons and implications for genome editing. Wiley Interdiscip. Rev. RNA 2018, 9, e1481. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Zhang, T.; Li, F.; Yang, W.; Kaminski, R.; Fagan, P.R.; Putatunda, R.; Young, W.B.; Khalili, K.; et al. CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Sci. Rep. 2015, 5, 16277. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef]
- Shen, L.; Wu, C.; Zhang, J.; Xu, H.; Liu, X.; Wu, X.; Wang, T.; Mao, L. Roles and potential applications of lncRNAs in HIV infection. Int. J. Infect. Dis. 2020, 92, 97–104. [Google Scholar] [CrossRef]
- Sharma, S.; Mandal, P.; Sadhukhan, T.; Roy Chowdhury, R.; Ranjan Mondal, N.; Chakravarty, B.; Chatterjee, T.; Roy, S.; Sengupta, S. Bridging Links between Long Noncoding RNA HOTAIR and HPV Oncoprotein E7 in Cervical Cancer Pathogenesis. Sci. Rep. 2015, 5, 11724. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Tong, J.; Gao, J.; Guo, Q.; Zhang, L.; Wang, B.; Zhao, H.; Wang, H.; Jiang, E.; et al. Long non-coding RNA-dependent mechanism to regulate heme biosynthesis and erythrocyte development. Nat. Commun. 2018, 9, 4386. [Google Scholar] [CrossRef]
- Yang, J.; Meng, X.; Pan, J.; Jiang, N.; Zhou, C.; Wu, Z.; Gong, Z. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018, 15, 35–43. [Google Scholar] [CrossRef]
- Gupta, P.; Kadamberi, I.P.; Mittal, S.; Tsaih, S.W.; George, J.; Kumar, S.; Vijayan, D.K.; Geethadevi, A.; Parashar, D.; Topchyan, P.; et al. Tumor Derived Extracellular Vesicles Drive T Cell Exhaustion in Tumor Microenvironment through Sphingosine Mediated Signaling and Impacting Immunotherapy Outcomes in Ovarian Cancer. Adv. Sci. 2022, 9, e2104452. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Baumann, V.; Winkler, J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 2014, 6, 1967–1984. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Lee, J.; Haemmerle, M.; Ling, H.; Previs, R.A.; Pradeep, S.; Wu, S.Y.; Ivan, C.; Ferracin, M.; Dennison, J.B.; et al. Long Noncoding RNA Ceruloplasmin Promotes Cancer Growth by Altering Glycolysis. Cell Rep. 2015, 13, 2395–2402. [Google Scholar] [CrossRef]
- Arora, S.; Swaminathan, S.K.; Kirtane, A.; Srivastava, S.K.; Bhardwaj, A.; Singh, S.; Panyam, J.; Singh, A.P. Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: Potential implications for pancreatic cancer therapy. Int. J. Nanomed. 2014, 9, 2933–2942. [Google Scholar] [CrossRef]
- Zhou, Z.; Kennell, C.; Lee, J.Y.; Leung, Y.K.; Tarapore, P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine 2017, 13, 403–410. [Google Scholar] [CrossRef]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. miRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Fresta, M.; Paolino, D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Weirauch, U.; Thomas, M.; Grünweller, A.; Hartmann, R.K.; Aigner, A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011, 71, 5214–5224. [Google Scholar] [CrossRef]
- Ramot, Y.; Rotkopf, S.; Gabai, R.M.; Zorde Khvalevsky, E.; Muravnik, S.; Marzoli, G.A.; Domb, A.J.; Shemi, A.; Nyska, A. Preclinical Safety Evaluation in Rats of a Polymeric Matrix Containing an siRNA Drug Used as a Local and Prolonged Delivery System for Pancreatic Cancer Therapy. Toxicol. Pathol. 2016, 44, 856–865. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Østergaard, M.E.; Yu, J.; Kinberger, G.A.; Wan, W.B.; Migawa, M.T.; Vasquez, G.; Schmidt, K.; Gaus, H.J.; Murray, H.M.; Low, A.; et al. Efficient Synthesis and Biological Evaluation of 5’-GalNAc Conjugated Antisense Oligonucleotides. Bioconjug Chem. 2015, 26, 1451–1455. [Google Scholar] [CrossRef]
- Song, T.-T.; Xu, F.; Wang, W. Inhibiting ubiquitin conjugating enzyme E2 N by microRNA-590-3p reduced cell growth of cervical carcinoma. Kaohsiung J. Med. Sci. 2020, 36, 501–507. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, D.; Cheng, G.; Yung, B.C.; Liu, Y.; Li, H.; Kang, C.; Fang, X.; Tian, S.; Zhou, X.; et al. T7 Peptide-Conjugated Lipid Nanoparticles for Dual Modulation of Bcl-2 and Akt-1 in Lung and Cervical Carcinomas. Mol. Pharm. 2018, 15, 4722–4732. [Google Scholar] [CrossRef]
- Dugal-Tessier, J.; Thirumalairajan, S.; Jain, N. Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugates. J. Clin. Med. 2021, 10, 838. [Google Scholar] [CrossRef]
- Dan, N.; Setua, S.; Kashyap, V.K.; Khan, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Antibody-Drug Conjugates for Cancer Therapy: Chemistry to Clinical Implications. Pharmaceuticals 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Huggins, I.J.; Medina, C.A.; Springer, A.D.; van den Berg, A.; Jadhav, S.; Cui, X.; Dowdy, S.F. Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase. Molecules 2019, 24, 3287. [Google Scholar] [CrossRef] [PubMed]
- Nanna, A.R.; Kel’in, A.V.; Theile, C.; Pierson, J.M.; Voo, Z.X.; Garg, A.; Nair, J.K.; Maier, M.A.; Fitzgerald, K.; Rader, C. Generation and validation of structurally defined antibody-siRNA conjugates. Nucleic. Acids Res. 2020, 48, 5281–5293. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Shao, L.; Wang, R.; Sun, Y.; Yue, Z.; Sun, H.; Wang, X.; Wang, P.; Sun, G.; Hu, J.; Sun, H.; et al. Delivery of MicroRNA-let-7c-5p by Biodegradable Silica Nanoparticles Suppresses Human Cervical Carcinoma Cell Proliferation and Migration. J. Biomed. Nanotechnol. 2020, 16, 1600–1611. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T.T. CD59 receptor targeted delivery of miRNA-1284 and cisplatin-loaded liposomes for effective therapeutic efficacy against cervical cancer cells. AMB Express 2020, 10, 54. [Google Scholar] [CrossRef]

| MiRNAs | miRNA Expression Profile | Target Gene/Pathway | Biological Function of Oncogenic miRNAs | Ref. |
|---|---|---|---|---|
| miR-17-5p | Upregulated | TGFBR2 | Promotes CC cell metastasis and proliferation | [68] |
| miR-9-5p | Upregulated | SOCS5. | Promotes angiogenesis, cell proliferation, and invasion | [69] |
| miR-96 | Upregulated | CAV-1 | Promotes cell proliferation, migration, and invasion | [70] |
| miR-21 | Upregulated | RASA1 | Promotes metastasis and enhances the invasiveness of CC cells | [71] |
| miR-221-3p | Upregulated | TWIST2/THBS2 | Promotes metastases of the lymph nodes in CC | [72] |
| miR-199 b-5p | Upregulated | KLK10 | Promotes cell proliferation, migration, and inhibits apoptosis | [73] |
| miR-106b | Upregulated | DAB2/TGF-β1 | Induces migration of CC cells | [74] |
| miR-519d | Upregulated | Smad7 | Promotes invasiveness and migration abilities of CC cells and prevent cell autophagy | [75] |
| miR-378 | Upregulated | ATG12 | Promotes metastases and inhibits apoptosis | [76] |
| miR-20a | Upregulated | TIMP2 and ATG7 | Increases histopathological grade, tumor size, and distant metastases | [77] |
| miR-106a | Upregulated | TIMP2 | Promotes the cell migration and invasion | [78] |
| miR-150 | Upregulated | PDCD4 | Promotes cell invasion and migration | [79] |
| miR-31 | Upregulated | BAP1 | Promotes cell proliferation and modulates the EMT | [80] |
| miR-155 | Upregulated | LKB1 | Promotes CC cell proliferation | [81] |
| MiR-RNA | Expression Pattern of miRNAs | Target Gene/Pathway /Molecule | Biological Function of Tumor Suppressor miRNAs | Ref. |
|---|---|---|---|---|
| miR-520d-5p | Downregulated | PTK2 | Promotes apoptosis and inhibits CC cell proliferation, invasion, and migration | [83] |
| miR-125 | Downregulated | VEGF and PI3K/AKT | Inhibits CC cell growth and tumor progression | [92] |
| miR-23b | Downregulated | AKT/mTOR | Inhibits CC cell multiplication invasion and migration abilities | [93] |
| miR-29a | Downregulated | DNMT1-SOCS1/NF-κB | Inhibits proliferation, migration, and invasion and promotes CC cell apoptosis | [94] |
| miR-543 | Downregulated | P13K/AKT, p38/MAPK and TRPM7 | Inhibits cell proliferation, migration, and invasion; induces cell cycle arrest and boost apoptosis | [95] |
| miR-497 | Downregulated | IGF-1R | Inhibits cell proliferation and arrest cells at S phase of cell cycle | [96] |
| miR-218 | Downregulated | Survivin (BIRC5) | Inhibits clonogenicity, invasion, and migration | [97] |
| miR-200b | Downregulated | Rho-E | Inhibits migration potential of CC cells and therefore their ability to metastasize | [98] |
| miR-124 | Downregulated | AmotL1 | Inhibits angiogenesis, migration, and invasion | [99] |
| miR-214 | Downregulated | EZH2 | Inhibits proliferation of CC cells | [100] |
| miR-203 | Downregulated | VEGFA | Inhibits cell proliferation, tumor development, and angiogenesis | [84] |
| miR-143 | Downregulated | Bcl-2 | Inhibits cell proliferation and promoted apoptosis | [101] |
| miR-101-5p | Downregulated | CXCL6 | Inhibits colony formation, invasion, and migration | [102] |
| miR-132 | Downregulated | SMAD2 | Inhibits lymph node metastasis | [103] |
| miR-129-5p | Upregulation | ZIC2 | Inhibits tumorigenesis and angiogenesis | [104] |
| miR-138-5p | Downregulated | SIRT1 | Inhibits the tumorigenesis and metastasis | [105] |
| miR-142-3p | Downregulated | CDC25C | Inhibits cell proliferation | [106] |
| miR-148b | Downregulated | CASP3 | Inhibits cell proliferation and promoted apoptosis | [107] |
| miR-182 | Downregulated | DBMT3a | Induces apoptosis and inhibits cell proliferation | [108] |
| miR-195 | Downregulated | Smad3 | Inhibits cell proliferation, migration, and invasion | [109] |
| miR-196b | Downregulated | VEGF | Inhibits angiogenesis | [110] |
| LncRNA | Expression Pattern lncRNA | Target Gene /Pathways/Molecules | Biological Function of Oncogenic lncRNA | Ref. |
|---|---|---|---|---|
| HOTAIR | Upregulated | BCL2, miR-143-3p | Promotes CC cell growth | [117] |
| LINC01535 | Upregulated | miR-214/EZH2 feedback loop | Promotes progression and metastasis of CC | [118] |
| CDKN2B-AS1 | Upregulated | miR-181a-5p/TGFβI axis | Promotes tumor cell growth and inhibits apoptosis | [119] |
| CASC11 | Upregulated | Wnt/β-catenin | Promotes cell proliferation | [120] |
| LINC00675 | Upregulated | Wnt/β-catenin | Promotes cancer cell growth, invasiveness, migration, and repressed cell apoptosis | [121] |
| MALAT-1 | Upregulated | HPV16 E6/E7 | Promotes cell proliferation, migration, and invasion and modulates EMT expression | [122] |
| ANRIL | Upregulated | Cyclin D1, CDK4, CDK6, E-cadherin, vimentin, and N-cadherin. | Promotes cell proliferation, migration, and invasion and inhibits apoptosis | [123] |
| BLACAT1 | Upregulated | Cyclin B1, and CDC25C, N-Cadherin, E-Cadherin | Enhances CC cell proliferation and invasion | [124] |
| PVT1 | Upregulated | Smad3, miR-140-5p sponging | Promotes cell proliferation and metastasis | [125] |
| HOXD-AS1 | Upregulated | Ras/ERK, | Enhances cell proliferation, migration, and invasion | [126] |
| DLX6-AS1 | Upregulated | miR-16-5p/ARPP19 axis | Increases cell proliferation and invasion | [127] |
| CRNDE | Upregulated | PI3K/AKT | Promotes cell proliferation and inhibits apoptosis | [128] |
| CCAT2 | Upregulated | Cell cycle | Promotes cell multiplication and penetration | [114] |
| EBIC | Upregulated | EZH2, E-cadherin | Promotes metastasis and invasion | [129] |
| RSU1P2 | Upregulated | IGF1R, N-myc, let-7a, EphA4 | Promotes tumor development | [130] |
| SPRY4-IT1 | Upregulated | miR-101-3p, ZEB1 | Promotes cell proliferation, migration, and invasion and modulates EMT expression | [131] |
| NEAT1 | Upregulated | miR-377/FGFR1 axis | Increases CC cell survival and motility and inhibits apoptosis | [132] |
| FAM83H-AS1 | Upregulated | E6-p300 pathway | Promotes cell proliferation and migration and inhibits apoptosis | [133] |
| C5orf66-AS1 | Upregulated | miR-637/RING1 axis | Promotes progression and proliferation of CC cells | [134] |
| LncRNAs | Expression Pattern | Target Genes /Pathways/Molecule | Biological Function of Tumor Suppressor lncRNA | Ref. |
|---|---|---|---|---|
| MEG3 | Downregulated | p-STAT3 | Inhibits cell proliferation and increases apoptosis | [135] |
| GAS5 | Downregulated | miR-205, miR-196a | Inhibits growth and metastases | [137] |
| GAS5-AS1 | Downregulated | Increase GAS5 stability by epigenetic modulation | Suppresses growth and metastasis | [138] |
| STXBP5-AS1 | Downregulated | miR-96-5p/PTEN axis | Inhibits cell proliferation and invasiveness of CC cells | [136] |
| TUSC8 | Downregulated | miR-641/PTEN axis | Inhibits migration and invasion | [139] |
| XLOC_010588 | Downregulated | c-Myc | Inhibits proliferation | [140] |
| LINC00861 | Downregulated | PTEN/AKT/mTOR miR-513b-5p | Inhibit the progression of CC cells | [141] |
| ZNF667-AS1 | Downregulated | Sponge miR-93-3p and upregulate PEG3 | Inhibits cell proliferation, invasion, and metastasis | [142] |
| RP11-284F21.9 | Downregulated | PPWD1, miR-769-3p | Inhibits cell proliferation, migration, and invasion | [143] |
| Lnc-CCDST | Downregulated | DHX9-MDM2 | Inhibits angiogenesis and invasion | [144] |
| DGCR5 | Downregulated | WNT signaling | Suppresses migration and invasion | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parashar, D.; Singh, A.; Gupta, S.; Sharma, A.; Sharma, M.K.; Roy, K.K.; Chauhan, S.C.; Kashyap, V.K. Emerging Roles and Potential Applications of Non-Coding RNAs in Cervical Cancer. Genes 2022, 13, 1254. https://doi.org/10.3390/genes13071254
Parashar D, Singh A, Gupta S, Sharma A, Sharma MK, Roy KK, Chauhan SC, Kashyap VK. Emerging Roles and Potential Applications of Non-Coding RNAs in Cervical Cancer. Genes. 2022; 13(7):1254. https://doi.org/10.3390/genes13071254
Chicago/Turabian StyleParashar, Deepak, Anupam Singh, Saurabh Gupta, Aishwarya Sharma, Manish K. Sharma, Kuldeep K. Roy, Subhash C. Chauhan, and Vivek K. Kashyap. 2022. "Emerging Roles and Potential Applications of Non-Coding RNAs in Cervical Cancer" Genes 13, no. 7: 1254. https://doi.org/10.3390/genes13071254
APA StyleParashar, D., Singh, A., Gupta, S., Sharma, A., Sharma, M. K., Roy, K. K., Chauhan, S. C., & Kashyap, V. K. (2022). Emerging Roles and Potential Applications of Non-Coding RNAs in Cervical Cancer. Genes, 13(7), 1254. https://doi.org/10.3390/genes13071254









