Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Samples Collection
2.2. Mosquito Rearing and Molecular Identification
2.3. Determination of Pesticide Residues in Mosquito Breeding Water and Soil Samples
2.4. Estimation of Sporozoite Rate
2.5. Susceptibility Tests and Resistance Intensity
2.6. Synergist Test
2.7. Determination of LLINs Efficacy with Cone Test
2.8. Genotyping of the Target-Site Resistant Markers in An. gambiae s.s. from Mangoum
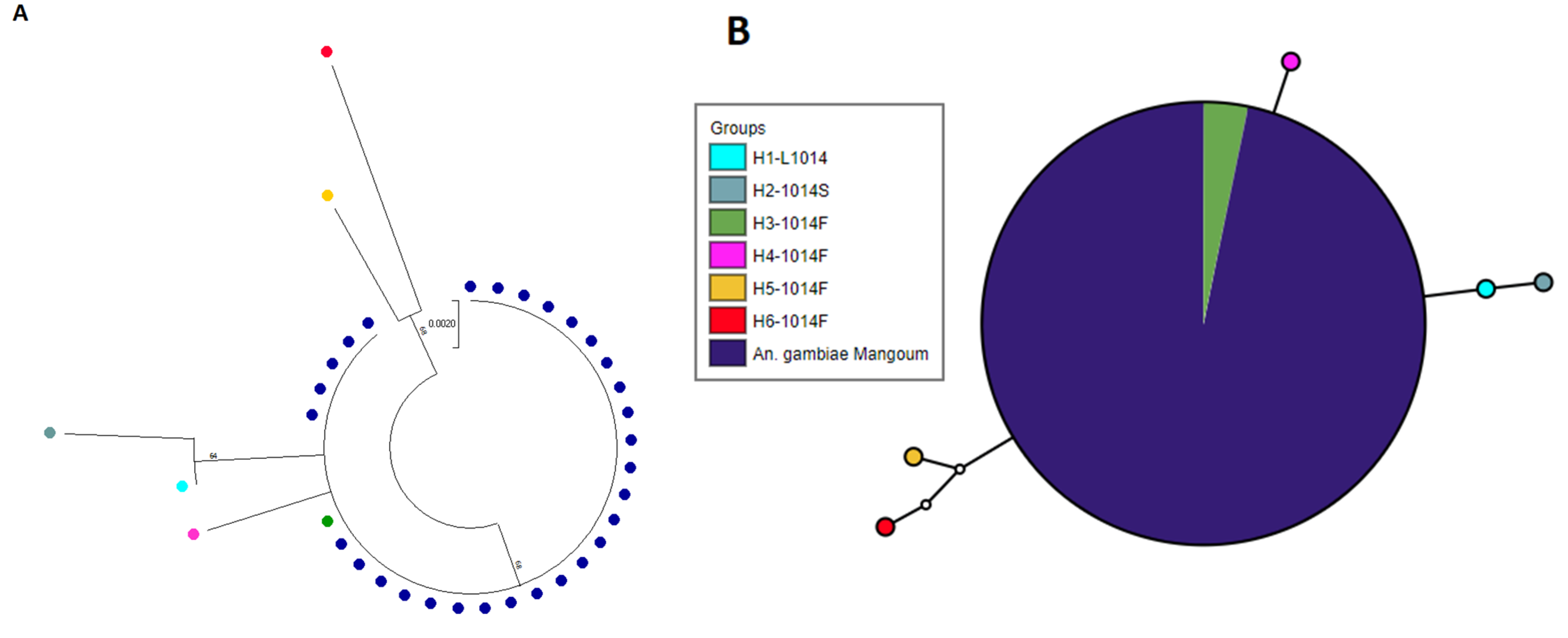
2.9. Polymorphism Analysis of the Voltage-Gated Sodium Channel Gene in An. gambiae from Mangoum
2.10. Transcription Profile of Metabolic Resistance Genes in An. gambiae s.s.
3. Results
3.1. Species Composition and Plasmodium Infection Rate
3.2. Agrochemical’s Residues Concentration in Mosquitoes Breeding Water and Soil
3.3. Insecticide Resistance Profile
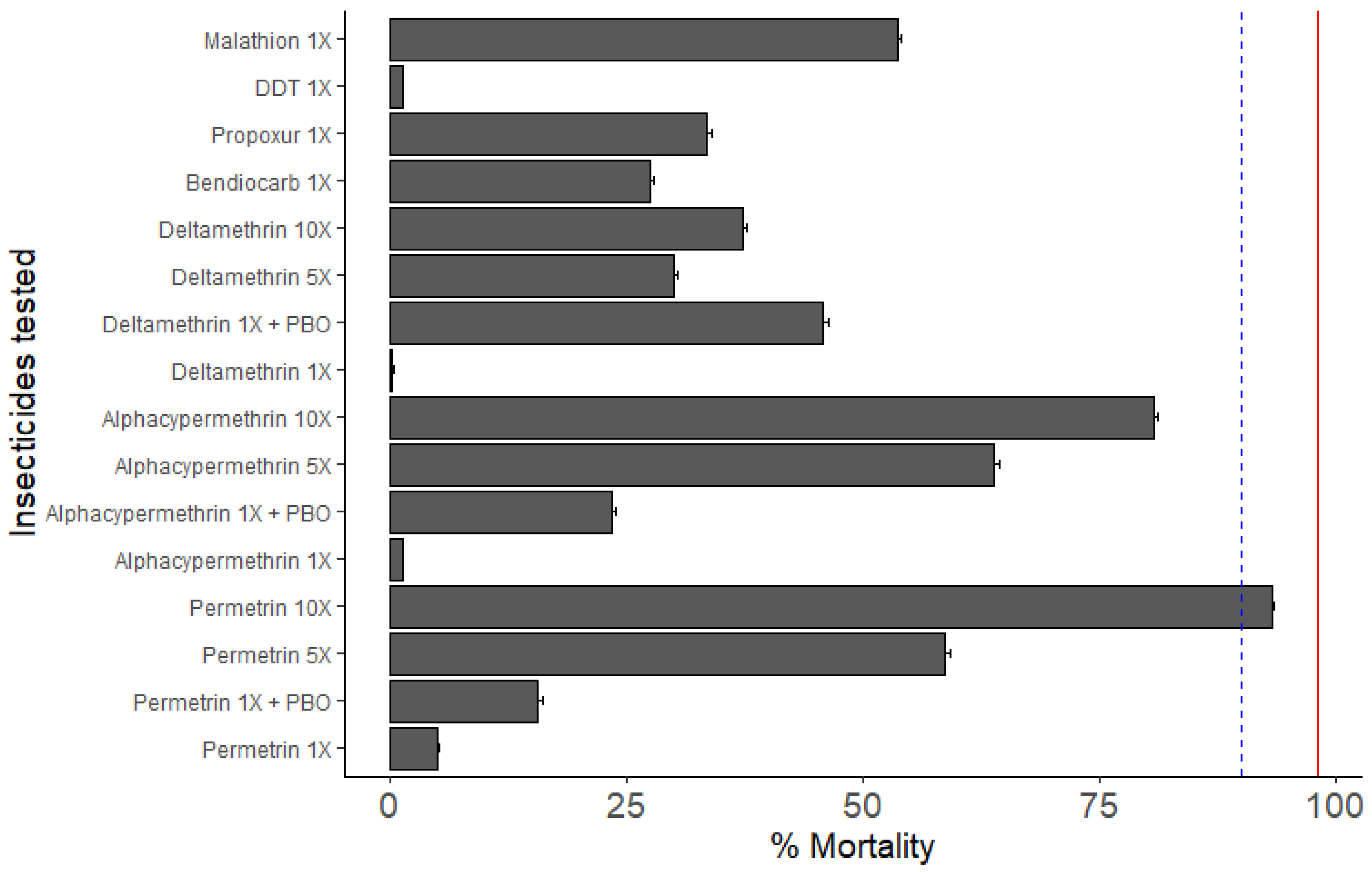
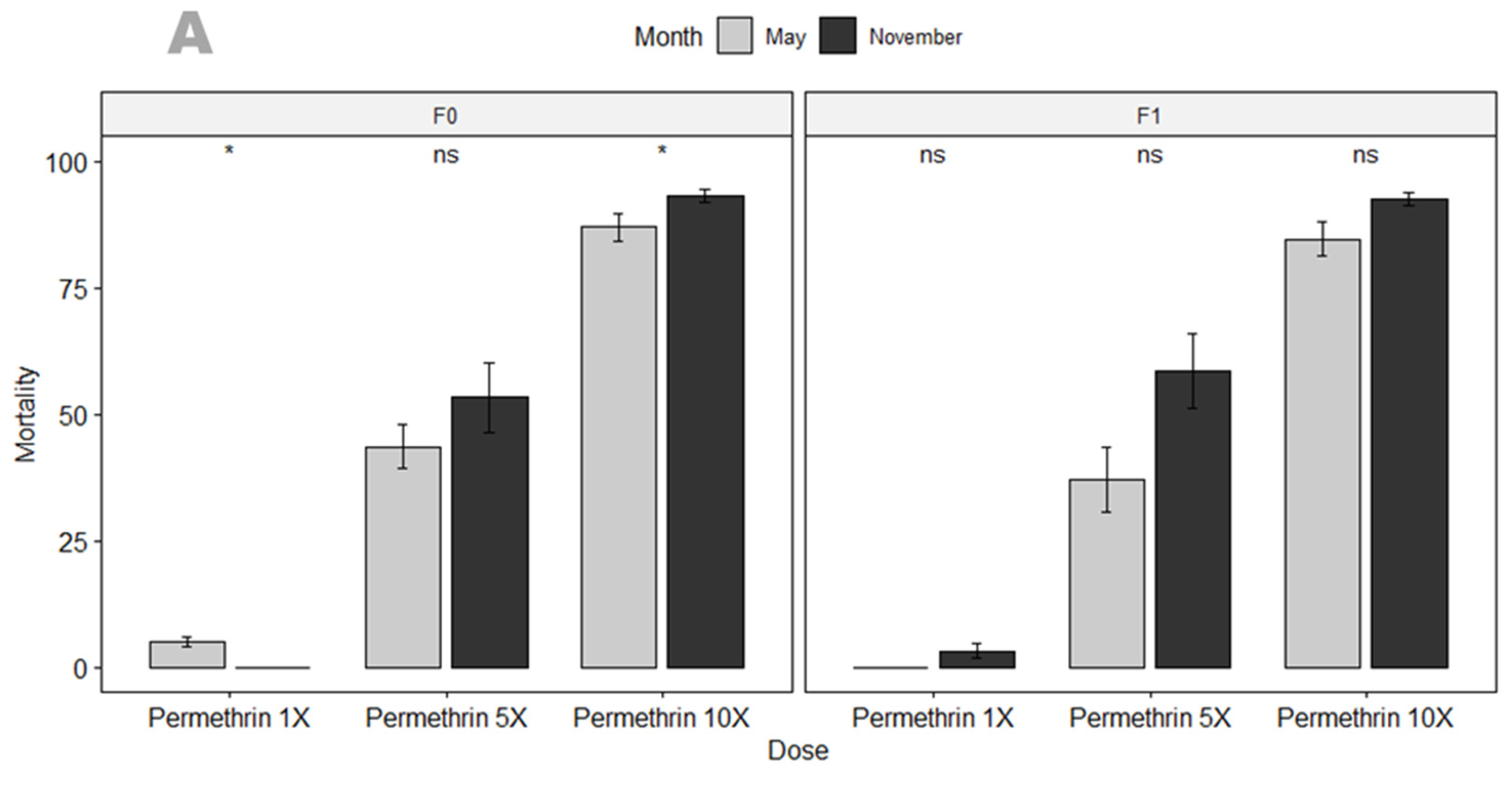
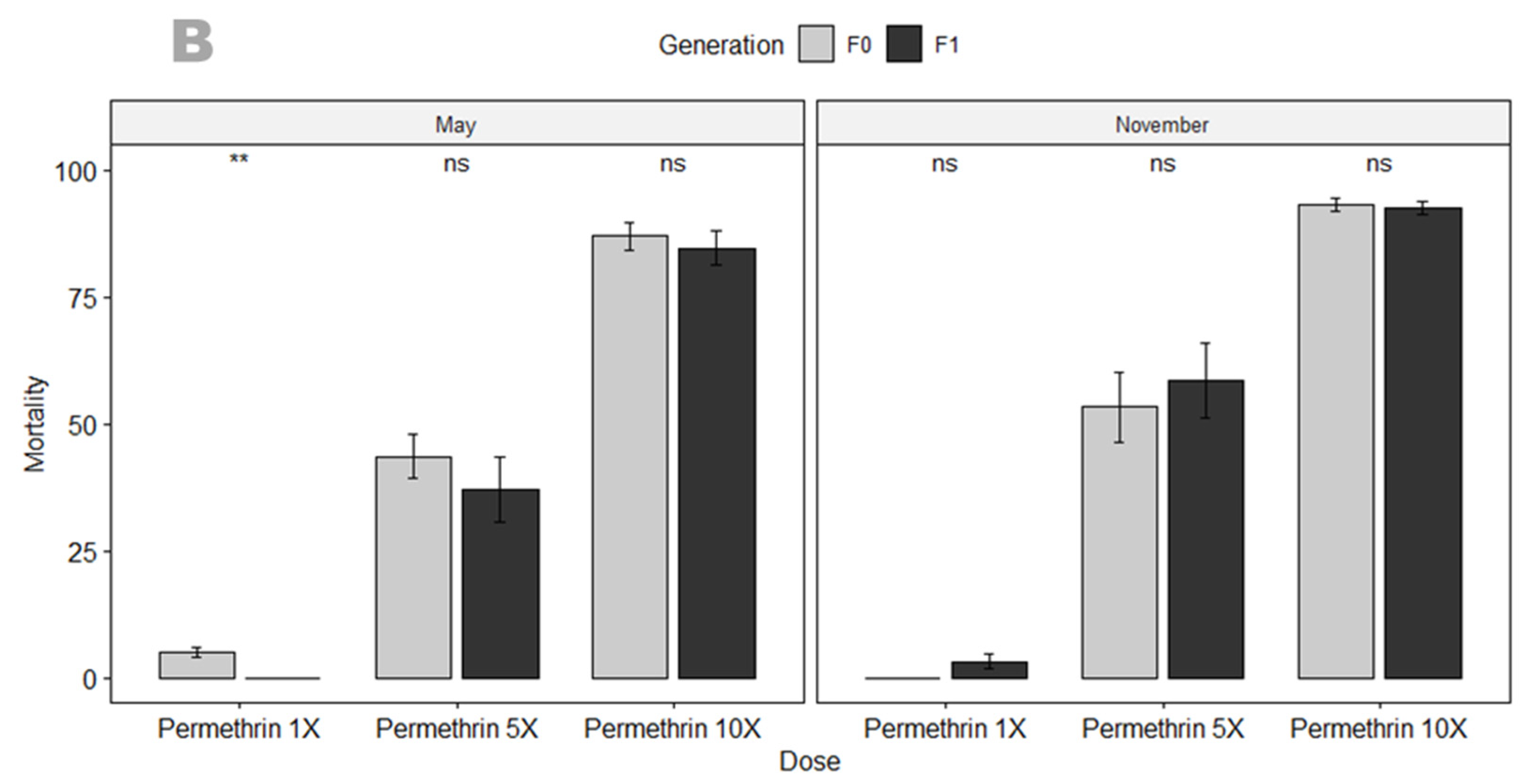
3.3.1. Susceptibility Profile and Resistance Intensity
3.3.2. Synergist Bioassay with PBO
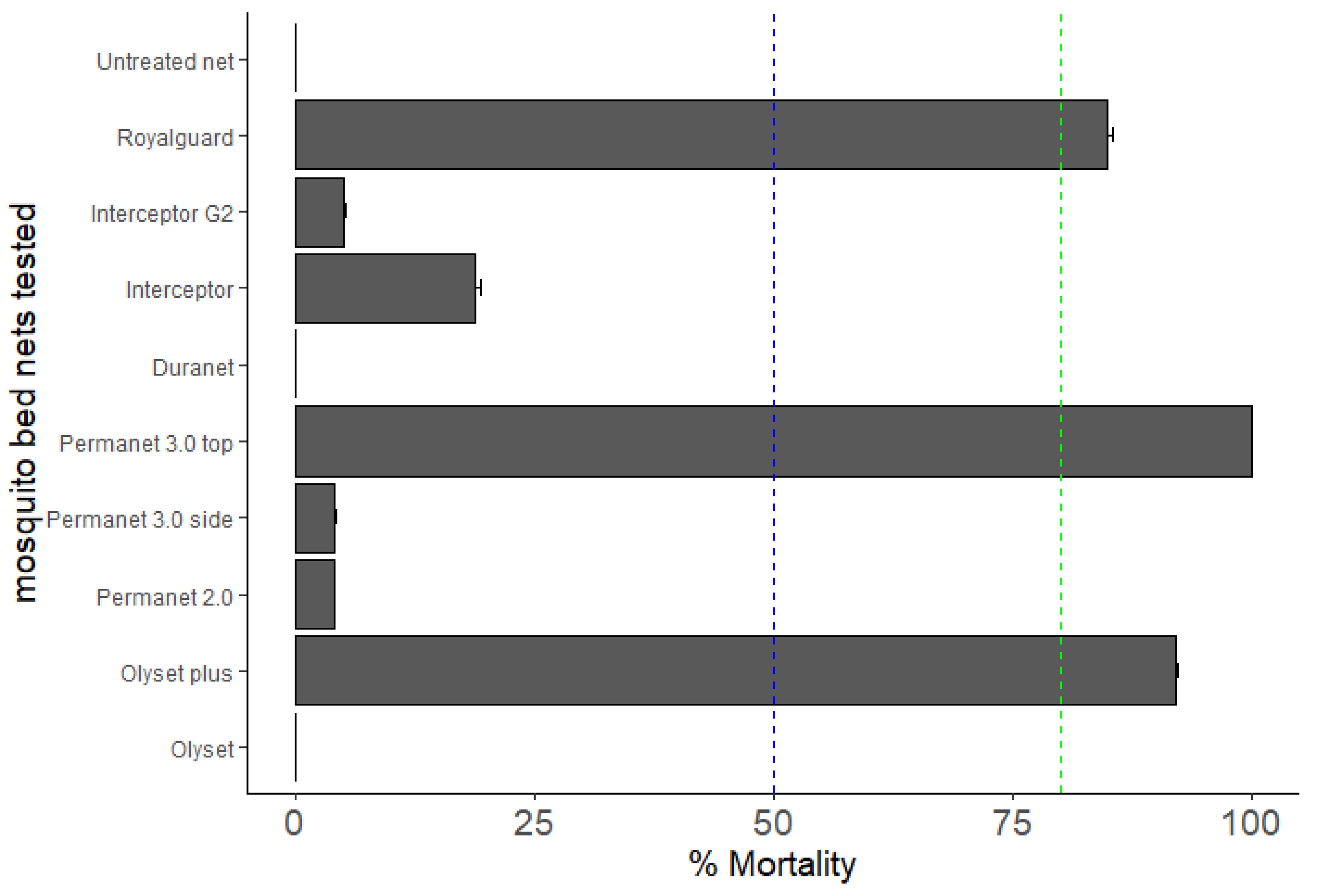
3.4. Bioefficacy of Insecticide-Treated Bed Nets
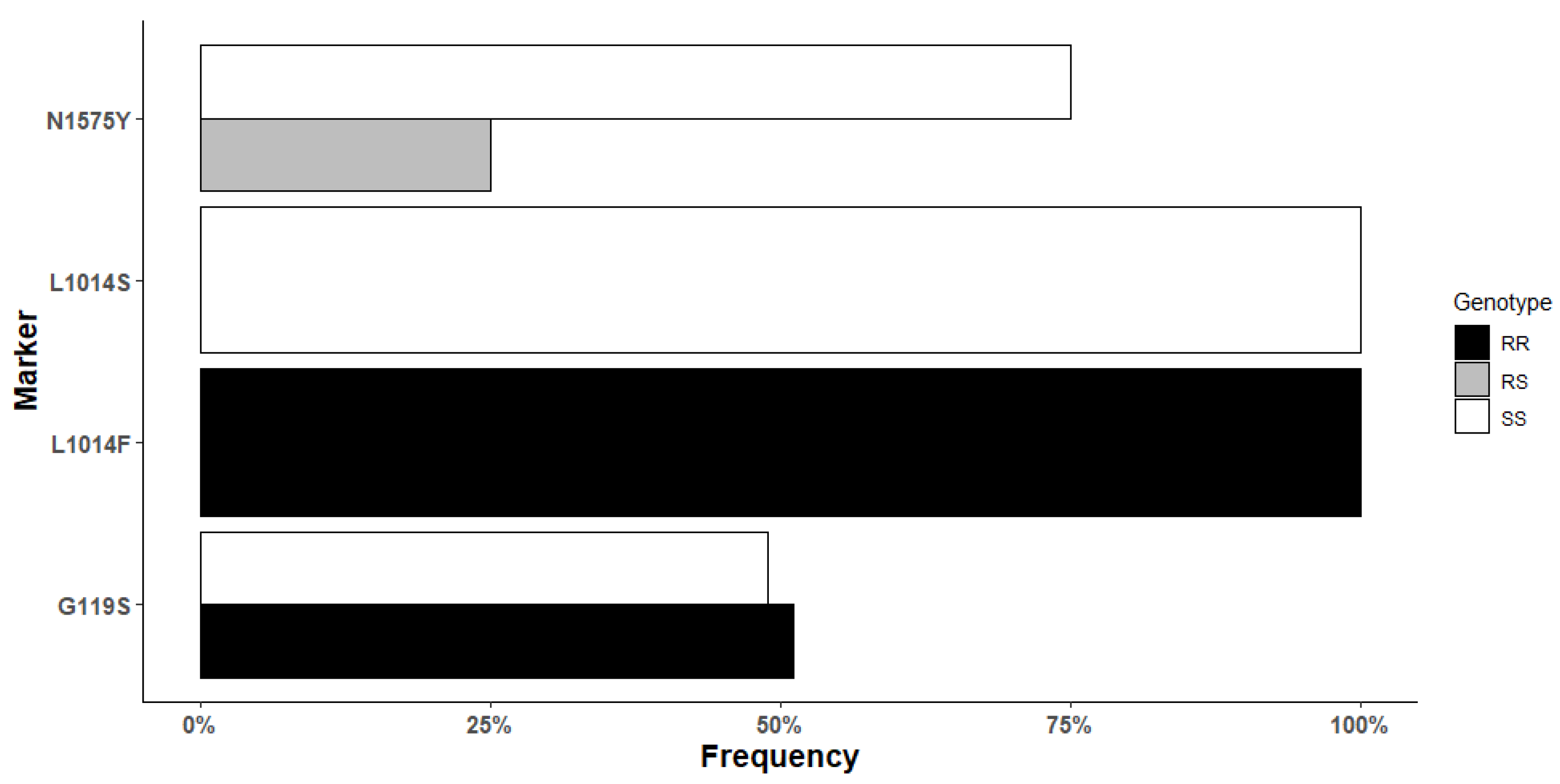
3.5. Target-Site Resistance Markers in An. gambiae from Mangoum
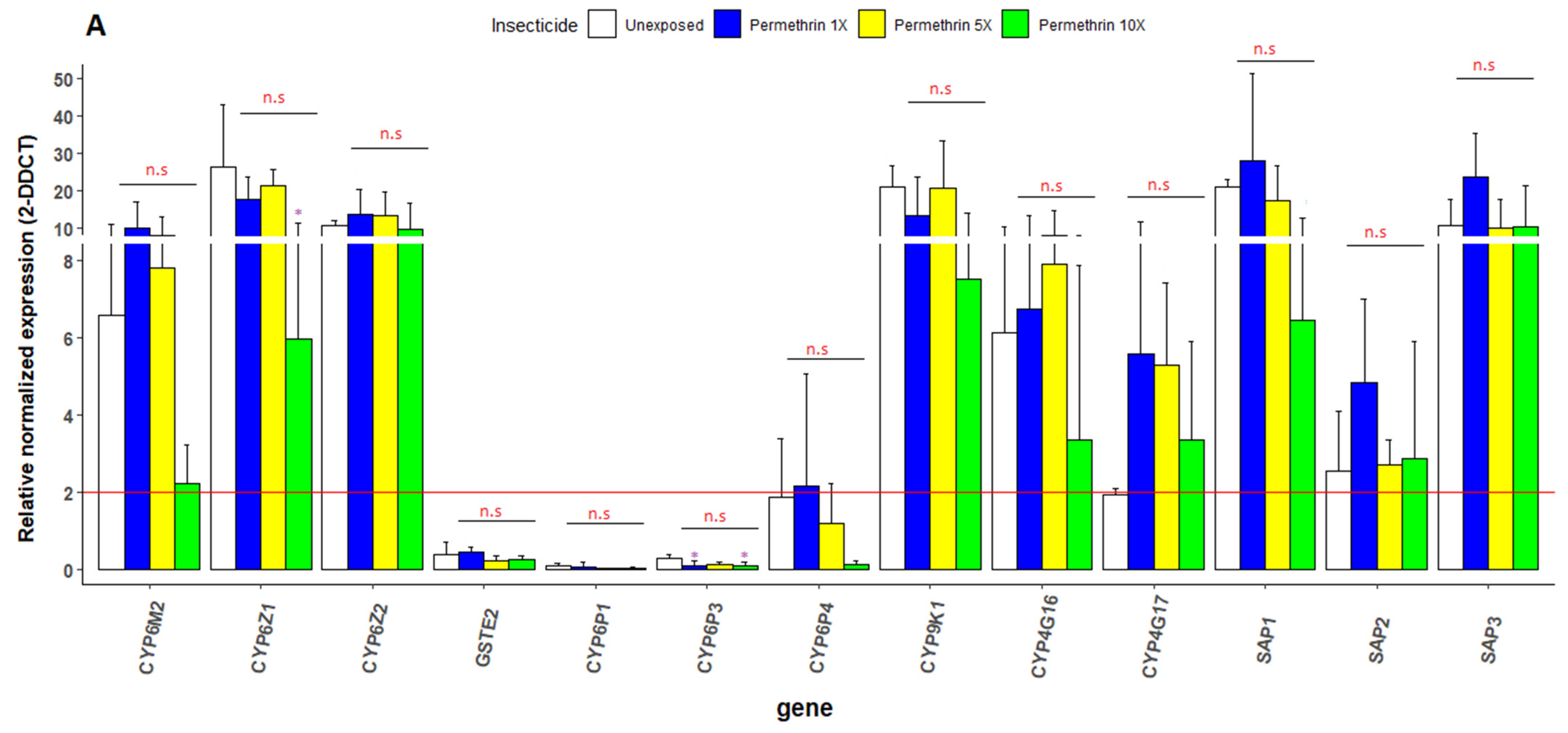
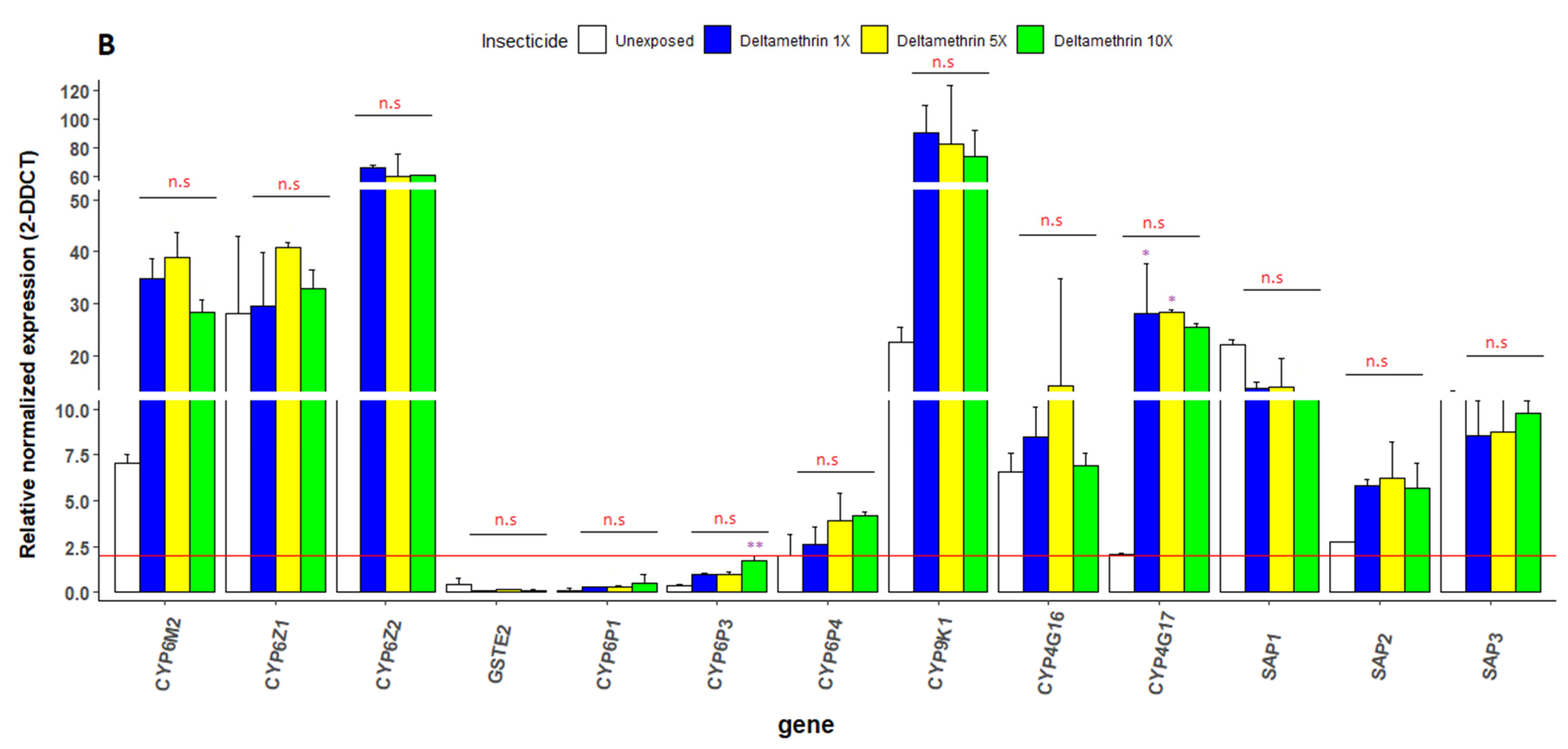
3.6. Transcriptional Profiling of Metabolic Resistance Genes in An. gambiae s.s.
4. Discussion
4.1. An. gambiae Is Driving Malaria Transmission in a Context of Intense Agricultural Activities
4.2. An. gambiae in Mangoum Exhibits High Resistance to the Four Classes of Insecticides
4.3. Molecular Drivers of Resistance Escalation Are Likely Complex Combining Several Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. World Malaria Report 2019; World Health Organisation: Geneva, Switzerland, 2019; ISBN 978-92-4-156572-1. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The Effect of Malaria Control on Plasmodium Falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Bagi, J.; Grisales, N.; Corkill, R.; Morgan, J.C.; N’Falé, S.; Brogdon, W.G.; Ranson, H. When a Discriminating Dose Assay Is Not Enough: Measuring the Intensity of Insecticide Resistance in Malaria Vectors. Malar. J. 2015, 14, 210. [Google Scholar] [CrossRef] [Green Version]
- Toé, K.H.; Jones, C.M.; N’Fale, S.; Ismail, H.M.; Dabiré, R.K.; Ranson, H. Increased Pyrethroid Resistance in Malaria Vectors and Decreased Bed Net Effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014, 20, 1691–1696. [Google Scholar] [CrossRef]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.D.; Mzilahowa, T.; Wondji, C.S. Rise of Multiple Insecticide Resistance in Anopheles Funestus in Malawi: A Major Concern for Malaria Vector Control. Malar. J. 2015, 14, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. The Effects of Larval Organic Fertiliser Exposure on the Larval Development, Adult Longevity and Insecticide Tolerance of Zoophilic Members of the Anopheles Gambiae Complex (Diptera: Culicidae). PLoS ONE 2019, 14, e0215552. [Google Scholar] [CrossRef]
- Gueye, O.K.; Tchouakui, M.; Dia, A.K.; Faye, M.B.; Ahmed, A.A.; Wondji, M.J.; Nguiffo, D.N.; Mugenzi, L.M.J.; Tripet, F.; Konaté, L.; et al. Insecticide Resistance Profiling of Anopheles Coluzzii and Anopheles Gambiae Populations in the Southern Senegal: Role of Target-sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chouaïbou, M.S.; Fodjo, B.K.; Fokou, G.; Allassane, O.F.; Koudou, B.G.; David, J.-P.; Antonio-Nkondjio, C.; Ranson, H.; Bonfoh, B. Influence of the Agrochemicals Used for Rice and Vegetable Cultivation on Insecticide Resistance in Malaria Vectors in Southern Côte d’Ivoire. Malar. J. 2016, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Piameu, M.; Nwane, P.; Toussile, W.; Mavridis, K.; Wipf, N.C.; Kouadio, P.F.; Mbakop, L.R.; Mandeng, S.; Ekoko, W.E.; Toto, J.C.; et al. Pyrethroid and Etofenprox Resistance in Anopheles Gambiae and Anopheles Coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis. Molecules 2021, 26, 5543. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004049-6. [Google Scholar]
- Toé, K.H.; N’Falé, S.; Dabiré, R.K.; Ranson, H.; Jones, C.M. The Recent Escalation in Strength of Pyrethroid Resistance in Anopheles Coluzzi in West Africa Is Linked to Increased Expression of Multiple Gene Families. BMC Genom. 2015, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Tchouakui, M.; Mugenzi, L.M.J.; Menze, B.D.; Khaukha, J.N.T.; Tchapga, W.; Tchoupo, M.; Wondji, M.J.; Wondji, C.S. Pyrethroid Resistance Aggravation in Ugandan Malaria Vectors Is Reducing Bednet Efficacy. Pathogens 2021, 10, 415. [Google Scholar] [CrossRef]
- Stica, C.; Jeffries, C.L.; Irish, S.R.; Barry, Y.; Camara, D.; Yansane, I.; Kristan, M.; Walker, T.; Messenger, L.A. Characterizing the Molecular and Metabolic Mechanisms of Insecticide Resistance in Anopheles Gambiae in Faranah, Guinea. Malar. J. 2019, 18, 244. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.T.; de Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). S. Afr. Inst. Med. Res. 1968, 54, 1–343. Available online: https://www.cabdirect.org/cabdirect/abstract/19692900946.
- Gillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). S. Afr. Inst. Med. Res. 1987, 55, 1–143. Available online: https://www.cabdirect.org/cabdirect/abstract/19880590772.
- Morgan, J.C.; Irving, H.; Okedi, L.M.; Steven, A.; Wondji, C.S. Pyrethroid Resistance in an Anopheles Funestus Population from Uganda. PLoS ONE 2010, 5, e11872. [Google Scholar] [CrossRef]
- Livak, K.J. Organization and Mapping of a Sequence on the Drosophila Melanogaster X and Y Chromosomes That Is Transcribed during Spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion Polymorphisms of SINE200 Retrotransposons within Speciation Islands of Anopheles Gambiae Molecular Forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Djouaka, R.; Zeukeng, F.; Bigoga, J.D.; Kakou-Ngazoa, S.E.; Akoton, R.; Tchigossou, G.; Coulibaly, D.N.; Tchebe, S.J.-E.; Aboubacar, S.; Nguepdjo, C.N.; et al. Domestic Animals Infected with Mycobacterium Ulcerans-Implications for Transmission to Humans. PLoS Negl. Trop. Dis. 2018, 12, e0006572. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Chai, M.-K. Sample Preparation in the Analysis of Pesticides Residue in Food by Chromatographic Techniques. In Pesticides-Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-460-3. [Google Scholar]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Blagborough, A.M.; Vontas, J.; Sinden, R.E.; Williamson, M.S.; Field, L.M. PCR-Based Detection of Plasmodium in Anopheles Mosquitoes: A Comparison of a New High-Throughput Assay with Existing Methods. Malar. J. 2008, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High Sensitivity of Detection of Human Malaria Parasites by the Use of Nested Polymerase Chain Reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- World Health Organisation. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Feyereisen, R. Insect P450 Inhibitors and Insecticides: Challenges and Opportunities. Pest Manag. Sci. 2015, 71, 793–800. [Google Scholar] [CrossRef]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory Selection for and Characteristics of Pyrethroid Resistance in the Malaria Vector Anopheles Funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef]
- World Health Organisation. Guidelines for Laboratory and Field Testing of Long-Lasting Insecticidal Mosquito Nets; World Health Organisation: Geneva, Switzerland, 2005. [Google Scholar]
- Fadel, A.N.; Ibrahim, S.S.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Wanji, S.; Wondji, C.S. A Combination of Metabolic Resistance and High Frequency of the 1014F Kdr Mutation Is Driving Pyrethroid Resistance in Anopheles Coluzzii Population from Guinea Savanna of Cameroon. Parasit. Vectors 2019, 12, 263. [Google Scholar] [CrossRef]
- Pinto, J.; Lynd, A.; Vicente, J.L.; Santolamazza, F.; Randle, N.P.; Gentile, G.; Moreno, M.; Simard, F.; Charlwood, J.D.; do Rosário, V.E.; et al. Multiple Origins of Knockdown Resistance Mutations in the Afrotropical Mosquito Vector Anopheles Gambiae. PLoS ONE 2007, 2, e1243. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Fadel, A.N.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Kérah-Hinzoumbé, C.; Wanji, S.; Wondji, C.S. High Insecticide Resistance in the Major Malaria Vector Anopheles Coluzzii in Chad Republic. Infect. Dis. Poverty 2019, 8, 39–50. [Google Scholar] [CrossRef]
- Kamgang, B.; Tchapga, W.; Ngoagouni, C.; Sangbakembi-Ngounou, C.; Wondji, M.; Riveron, J.M.; Wondji, C.S. Exploring Insecticide Resistance Mechanisms in Three Major Malaria Vectors from Bangui in Central African Republic. Pathog. Glob. Health 2018, 112, 349–359. [Google Scholar] [CrossRef]
- Ochomo, E.; Subramaniam, K.; Kemei, B.; Rippon, E.; Bayoh, N.M.; Kamau, L.; Atieli, F.; Vulule, J.M.; Ouma, C.; Gimnig, J.; et al. Presence of the Knockdown Resistance Mutation, Vgsc-1014F in Anopheles Gambiae and An. Arabiensis in Western Kenya. Parasit. Vectors 2015, 8, 616. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.M.; Ibrahim, S.S.; Chanda, E.; Mzilahowa, T.; Cuamba, N.; Irving, H.; Barnes, K.G.; Ndula, M.; Wondji, C.S. The Highly Polymorphic CYP6M7 Cytochrome P450 Gene Partners with the Directionally Selected CYP6P9a and CYP6P9b Genes to Expand the Pyrethroid Resistance Front in the Malaria Vector Anopheles Funestus in Africa. BMC Genom. 2014, 15, 817. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C T Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Nkya, T.E.; Akhouayri, I.; Poupardin, R.; Batengana, B.; Mosha, F.; Magesa, S.; Kisinza, W.; David, J.-P. Insecticide Resistance Mechanisms Associated with Different Environments in the Malaria Vector Anopheles Gambiae: A Case Study in Tanzania. Malar. J. 2014, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Atangana, J.; Bigoga, J.D.; Patchoké, S.; Ndjemaï, M.N.H.; Tabue, R.N.; Nem, T.E.; Fondjo, E. Anopheline Fauna and Malaria Transmission in Four Ecologically Distinct Zones in Cameroon. Acta Trop. 2010, 115, 131–136. [Google Scholar] [CrossRef]
- Bashar, K.; Tuno, N.; Ahmed, T.U.; Howlader, A.J. False Positivity of Circumsporozoite Protein (CSP)–ELISA in Zoophilic Anophelines in Bangladesh. Acta Trop. 2013, 125, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Bamou, R.; Kopya, E.; Nkahe, L.D.; Menze, B.D.; Awono-Ambene, P.; Tchuinkam, T.; Njiokou, F.; Wondji, C.S.; Antonio-Nkondjio, C. Increased Prevalence of Insecticide Resistance in Anopheles Coluzzii Populations in the City of Yaoundé, Cameroon and Influence on Pyrethroid-Only Treated Bed Net Efficacy. Parasite 2021, 28, 8. [Google Scholar] [CrossRef]
- Nkemngo, F.N.; Mugenzi, L.M.J.; Terence, E.; Niang, A.; Wondji, M.J.; Tchoupo, M.; Nguete, N.D.; Tchapga, W.; Irving, H.; Ntabi, J.D.M.; et al. Multiple Insecticide Resistance and Plasmodium Infection in the Principal Malaria Vectors Anopheles Funestus and Anopheles Gambiae in a Forested Locality Close to the Yaoundé Airport, Cameroon. Wellcome Open Res. 2020, 5, 146. [Google Scholar] [CrossRef]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles Funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef]
- Keïta, M.; Sogoba, N.; Kané, F.; Traoré, B.; Zeukeng, F.; Coulibaly, B.; Sodio, A.B.; Traoré, S.F.; Djouaka, R.; Doumbia, S. Multiple Resistance Mechanisms to Pyrethroids Insecticides in Anopheles Gambiae Sensu Lato Population From Mali, West Africa. J. Infect. Dis. 2021, 223, S81–S90. [Google Scholar] [CrossRef]
- Lissenden, N.; Kont, M.D.; Essandoh, J.; Ismail, H.M.; Churcher, T.S.; Lambert, B.; Lenhart, A.; McCall, P.J.; Moyes, C.L.; Paine, M.J.I.; et al. Review and Meta-Analysis of the Evidence for Choosing between Specific Pyrethroids for Programmatic Purposes. Insects 2021, 12, 826. [Google Scholar] [CrossRef]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. Plant Health Instr. 2006, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Lynd, A.; Oruni, A.; van’t Hof, A.E.; Morgan, J.C.; Naego, L.B.; Pipini, D.; O’Kines, K.A.; Bobanga, T.L.; Donnelly, M.J.; Weetman, D. Insecticide Resistance in Anopheles Gambiae from the Northern Democratic Republic of Congo, with Extreme Knockdown Resistance (Kdr) Mutation Frequencies Revealed by a New Diagnostic Assay. Malar. J. 2018, 17, 412. [Google Scholar] [CrossRef]
- Jones, C.M.; Liyanapathirana, M.; Agossa, F.R.; Weetman, D.; Ranson, H.; Donnelly, M.J.; Wilding, C.S. Footprints of Positive Selection Associated with a Mutation (N1575Y) in the Voltage-Gated Sodium Channel of Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 2012, 109, 6614–6619. [Google Scholar] [CrossRef] [Green Version]
- Edi, C.V.A.; Koudou, B.G.; Jones, C.M.; Weetman, D.; Ranson, H. Multiple-Insecticide Resistance in Anopheles Gambiae Mosquitoes, Southern Côte d’Ivoire. Emerg. Infect. Dis. 2012, 18, 1508–1511. [Google Scholar] [CrossRef]
- Kwiatkowska, R.M.; Platt, N.; Poupardin, R.; Irving, H.; Dabire, R.K.; Mitchell, S.; Jones, C.M.; Diabaté, A.; Ranson, H.; Wondji, C.S. Dissecting the Mechanisms Responsible for the Multiple Insecticide Resistance Phenotype in Anopheles Gambiae s.s., M Form, from Vallée Du Kou, Burkina Faso. Gene 2013, 519, 98–106. [Google Scholar] [CrossRef] [Green Version]
| Alpha-Cypermethrin LOD = 0.563; LOQ = 52.918 | Deltamethrin LOD = 1.899; LOQ = 5.754 | |||
|---|---|---|---|---|
| Breeding Water | Soil Sediment | Breeding Water | Soil SEDIMENT | |
| µg/mL | µg/mL | µg/mL | µg/mL | |
| IRAD | 2.903 ± 0.06 | ND | ND | ND |
| Djincha | 0.443 ± 0.002 | ND | ND | ND |
| Insecticides | Alive | Death | OR (95% CI) | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| NY | NN | Frequency (%) | NY | NN | Frequency (%) | |||
| Permethrin 5× | 10 | 20 | 16.67% | 12 | 18 | 20.00% | 0.75 (0.25–2.19) | 0.79 |
| Deltamethrin 5× | 14 | 14 | 25.00% | 6 | 25 | 9.68% | 4.01 (1.29–13.90) | 0.03 |
| Deltamethrin 10× | 11 | 17 | 19.64% | 10 | 21 | 16.13% | 1.35 (0.46–4.04) | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepa, A.; Kengne-Ouafo, J.A.; Djova, V.S.; Tchouakui, M.; Mugenzi, L.M.J.; Djouaka, R.; Pieme, C.A.; Wondji, C.S. Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon. Genes 2022, 13, 1206. https://doi.org/10.3390/genes13071206
Tepa A, Kengne-Ouafo JA, Djova VS, Tchouakui M, Mugenzi LMJ, Djouaka R, Pieme CA, Wondji CS. Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon. Genes. 2022; 13(7):1206. https://doi.org/10.3390/genes13071206
Chicago/Turabian StyleTepa, Arnaud, Jonas A. Kengne-Ouafo, Valdi S. Djova, Magellan Tchouakui, Leon M. J. Mugenzi, Rousseau Djouaka, Constant A. Pieme, and Charles S. Wondji. 2022. "Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon" Genes 13, no. 7: 1206. https://doi.org/10.3390/genes13071206
APA StyleTepa, A., Kengne-Ouafo, J. A., Djova, V. S., Tchouakui, M., Mugenzi, L. M. J., Djouaka, R., Pieme, C. A., & Wondji, C. S. (2022). Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon. Genes, 13(7), 1206. https://doi.org/10.3390/genes13071206







