Abstract
Ectropis oblique is one of the main pests that feed on tea leaves. At present, the main control method is chemical control, but the long-term use of insecticides has been related to the development of insect resistance. One of the resistance mechanisms is the upregulation of relevant detoxification enzymes for defense. In this study, four genes with increased expression were screened from the gene sequences annotated from the transcriptome data of deltamethrin-treated larvae of E. oblique, which are acid phosphatase EoACP138, and cytochrome P450 EoCYP316, carboxylesterase EoCarE592 and acetylcholine esterase EoAchE989, respectively. The fourth instar larvae of E. oblique were stimulated by deltamethrin, chlorpyrifos and fenpropathrin respectively, and the expression levels of the genes were detected by qRT-PCR. The result showed that all four genes’ expression had significantly increased under the stimulation of three insecticides. RNAi technology was used to silence the expression of genes of EoACP138, EoCYP316, EoCarE592 and EoAchE989 in the fourth instar larvae of E. oblique. The change in the expression levels of the above genes in the larvae treated with dsRNA and stimulated with pesticides was determined by qRT-PCR. The target genes have been effectively silenced after feeding on dsRNA and higher sensitivity with higher mortality to pesticides was observed in the larvae interfered with dsRNA. The above genes are related to the detoxification and metabolism of resistance of E. oblique, which lays a foundation for further study on the mechanism of insecticide resistance in E. oblique.
1. Introduction
The tea plant is a popular cash crop that has been grown in more than 60 countries and consumed in more than 100 countries [1]. The leaves are processed into tea and become one of the popular non-alcoholic beverages [2,3]. Tea is beneficial to people’s health owing to its bioactive components including catechins, polyphenols, theanine, caffeine, volatile oil, flavonoids, vitamins and medicinal properties [4,5,6]. E. oblique (Lepidoptera: Geometridae) is one of the most destructive chewing pests of tea trees. In the early stage, the larvae feed on tender leaves and shoots. With a rapid growth, the larvae of the fourth and 5th instars bite the leaves to form notches, which will seriously affect the yield and quality of tea [7,8,9]. There are various control methods for the pest, including manpower, entrapment, a chemical pesticide, microorganism pesticide, biological guided missiles and agriculture expert systems; however, chemical pesticides are the main control measures. The excessive use of chemical pesticides will lead to environmental pollution, the generation of insecticide resistance, and the residual pesticides would endanger human health [10,11,12,13,14]. For these reasons, more innovative and safe methods are needed for the control of E. oblique and other pests.
At present, RNA interference (RNAi) is mainly used to study the function of genes; in insects, this technology has become a research hotspot and has been applied to pest control [15,16,17,18]. RNAi is a gene-silencing technique using double-stranded RNA (dsRNA). DsRNA is mainly introduced into insects by oral administration, immersion or injection [19], digested into siRNA (short interfering RNA) by endonuclease, and finally leads to the specific degradation of messenger RNA through the action of RNA-induced silencing complex (RISC). Since the discovery that double-stranded RNA can mediate specific interference in Caenorhabditis elegans [20], RNAi technology has been successfully applied to the research of many insects. After being injected with dsRNA, the expression of marker genes of the pea aphid Acyrthosiphon pisum was inhibited by about 40% [21]. Jing Lü et al., proved that the survival rate of Henosepilachna vigintioctopunctata was significantly reduced with the target gene effectively silenced after feeding on dsRNA targeting vATPase-B [22].
Many studies have found the resistance mechanisms to insecticide was highly related to the upregulation of detoxifying enzyme genes [23]. In many cases, insects can respond to the stimulation of pesticides through the detoxification metabolic pathway. The metabolic enzymes related to detoxification mainly include esterase (EST), cyto-chrome P450 enzyme family (cytochrome P450, CYP) and glutathione S-transferase (GST) [24,25,26,27]; however, the resistance mechanisms of different pests and the related genes which responded to different pesticides are different. Therefore, RNAi technology can be used to silence the relevant detoxification gene base in insects in order to study its function in detecting the sensitivity of insects to pesticides and their mortality rate.
In this study, four genes EoACP138, EoCYP316, EoCarE592 and EoAchE989 from E. oblique were selected based on the previous studies of transcriptome [28], and the relationship between the genes and pesticide resistance of E. oblique has been further studied by RNAi technology. The results showed that the silencing rate of the four genes was higher than 70%, and the sensitivity of E. oblique to pesticides was improved; it paves the way for potential application in pest control and the development of detoxification drugs in the future.
2. Materials and Methods
2.1. Insect Rearing
E. oblique used in this research was provided by the Tea Research Institute of the Chinese Academy of Agricultural Sciences (Hangzhou, Zhejiang, China), and were reared and propagated with fresh tea leaves in groups by our laboratory. Fresh tea leaves were picked from Jianshan Scenic Spot in Zigong, Sichuan province. The feeding conditions were as follows: temperature 22 ± 2 °C, photoperiod 14:10 (light: dark), relative humidity 65 ± 5%.
2.2. Total RNA Extraction and cDNA Synthesis
The total RNA of larvae was extracted using TriZol Reagent (Sangon Biotech, Shanghai, China) following the manufacturer’s manual. Total RNA was treated with DNase I after the extraction procedure to remove genome DNA from the samples. The concentration and purity of 1 μL RNA solution were measured by Nanodrop, and the quality of RNA was further determined by 1.5% agarose gel electrophoresis. First-strand cDNA was synthesized with 2 μL total RNA as a template using M-MuLV First Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China). The reaction conditions of PCR were as follows: 42 °C for 30–60 min, then the reaction was terminated at 70 °C for 10 min. The obtained cDNA can be stored at −20 °C for standby.
2.3. Quantitative Real-Time PCR Analysis
Based on the base sequences of four genes, EoACP138 (GenBank accession number ON110137), EoCYP316 (GenBank accession number ON110138), EoCarE592 (GenBank accession number ON110139) and EoAchE989 (GenBank accession number ON110140), and the design principles of the qRT-PCR primers, Primer premier 5 was used to design the corresponding primers, while a fragment of E. oblique 18S rRNA was used as an internal reference for qRT-PCR. The primers for each gene are listed in Table 1.

Table 1.
Sequences of the primers for quantitative real-time PCR.
2.3.1. Treatment of E. oblique with Deltamethrin
In the preliminary work of the laboratory, the pesticides were diluted to different concentrations, and the fresh tea leaves were immersed in the diluent for 10 s, taken out, and dried in air. The control group was immersed in deionized water. Then, the starved larvae were fed for 48 h, and the mortality rate was recorded. The corresponding LC50 value was calculated based on the deaths at different concentrations. Where the LC50 of deltamethrin was 25 μg/mL, it was diluted with water to 25 μg/mL, and the picked intact leaves (oval) with similar leaf age were completely immersed in the deltamethrin diluent for 10 s, and taken out and naturally air-dried. The fourth instar larvae were selected and divided into 4 groups (10 larvae per group). The larvae were fed for 0 h, 12 h, 24 h, and 48 h and frozen in liquid nitrogen for RNA extraction. Three biological replicates and three technical replicates were set. The transcription levels of EoCYP316, EoACP138, EoCarE592 and EoAchE989 in E. oblique larvae at different time points after deltamethrin exposure were detected by quantitative real-time PCR. The qRT-PCR reaction system includes 2 × transstart tip green qPCR Supermix 10 μL, the forward and reverse primers (10 μM/L) each 0.4 μL, cDNA template 1 μL, Nuclease-free Water 8.2 μL. QRT-PCR conditions were: 95 °C for 2 min; followed by 40 cycles of 95 °C for 10 s, and 60 °C for 20–30 s. 2−∆∆CT method was used to calculate the relative expression of genes.
2.3.2. Fenpropathrin and Chlorpyrifos Treatment for E. oblique
Fenpropathrin was diluted to LC50 (0.861 mg/mL) and chlorpyrifos to LC50 (0.725 mg/mL) based on preliminary laboratory calculations. Tea leaves with similar leaf ages were completely immersed in the dilution of fenpropathrin and chlorpyrifos respectively for 10 s and removed for air drying. Fourth instar larvae were selected and divided into 4 groups (10 larvae per group) and fed on leaves treated with the diluted fenpropathrin and chlorpyrifos respectively. The larvae were treated with liquid nitrogen at 0 h and 24 h after feeding for RNA extraction (10 larvae per group). Three biological replicates and three technical replicates were set. The transcription levels of EoCYP316, EoACP138, EoCarE592 and EoAchE989 in the larvae were detected by qRT-PCR. The relative expression of genes was calculated using the 2−∆∆CT method.
2.4. dsRNA Synthesis
Based on the base sequences of the four genes, the primers were designed including the T7 promoter (TAATACGACTCACTATAGGG) and GATCAC promotor group. The green fluorescence protein gene (GFP) was used as a control gene and the primers were designed as listed in Table 2. A large amount of the purified DNA template was obtained by PCR using the plasmid DNA containing the target gene as the template. Double-strand RNA was synthesized according to the In Vitro Transcription T7 Kit specification from Japan’s TaKaRa Company. The dsRNA was purified by using Easy Pure® RNA Purification Kit from Beijing Quan-Shi Jin Company, and dsRNA homogeneity and concentration were determined by agarose gel electrophoresis and Nanodrop.

Table 2.
Sequences of the primers for dsRNAs.
2.5. RNA Interference
The dsRNA was delivered to E. oblique larvae by injection. The purified dsGFP, dsEoCYP316, dsEoACP138, dsEoCarE592 and dsEoAchE989 were diluted with RNase-free water to 200 ng/μL, 100 ng/μL, 50 ng/μL and 25 ng/μL, respectively. The larvae of the fourth instar with appropriate size were selected and divided into eight groups, six for each group. Each larva was injected with 2 μL dsRNA diluent to yield 50 ng, 100 ng, 200 ng, and 400 ng. After the injection, the larvae were fed with tea leaves for 1 day. Then, the larvae were rapidly frozen with liquid nitrogen for the extraction of total RNA. The change in the target gene’s expression would be detected by qRT-PCR.
2.6. Effect of RNAi on the Survival of E. oblique
In order to further study whether the injection of dsRNA of different genes will affect the survival of E. oblique, 2 μL of dsRNA at a concentration of 100 ng/μL was injected into the abdomen of larvae. Groups treated with dsEoACP138, dsEoCYP316, dsEoCarE592 and dsEoAchE989 were experimental groups, and the group treated with dsGFP and TE buffer was used as the control group (30 larvae per group). After injection for 2 days, the physiological conditions of insects were observed, and the mortality rate was calculated. The experiment was repeated three times.
2.7. Expression of Detoxification Related Genes in RNAi E. oblique Stimulated by Insecticides
The fourth instar larvae were injected with 200 ng dsGFP or dsEoACP138, respectively, and fed with untreated fresh tea leaves for one day (54 larvae per group). Then, 54 larvae were injected with the same dsRNA of each group, divided into six groups, and fed with tea leaves treated with deltamethrin, fenpropathrin and chlorpyrifos (LC50), respectively, for 0 h or 24 h, respectively. After the treatment was completed, the insects were snap-frozen with liquid nitrogen. Total RNA was extracted, and cDNA was obtained by reverse transcription. Three biological replicates and three technical replicates were set. The transcript levels of EoCYP316, EoACP138, EoCarE592 and EoAchE989 of E. oblique larvae stimulated by different insecticides were detected by qRT-PCR. The reaction procedure was the same as that in the Section 2.3.1. The same process was applied to the test groups for dsEoCYP316, dsEoCarE592 and dsEoAchE989.
2.8. Determination of Toxicity of Three Insecticides after RNA Interference
Larvae of the fourth instar were collected and divided into six groups (30 larvae per group), five of which were injected with 200 ng of dsGFP, dsEoACP138, dsEoCYP316, dsEoCarE592 and dsEoAchE989, respectively. One group with no injection was used as a control. After the injection, the larvae were fed with untreated fresh tea leaves for one day. Then, each group were fed tea leaves treated with deltamethrin (17.5 μg/mL), Chlorpyrifos (0.363 mg/mL) or fenpropathrin (0.43 mg/mL) and mortality was calculated after 48 h.
2.9. Statistical Analysis
The relative gene expression was calculated by the 2−ΔΔCT method. The expression levels of EoACP138, EoCYP316, EoCarE592 and EoAchE989 genes were the relative expression levels compared with the reference gene 18S rRNA. Statistical significance of results was assessed using a one-way analysis of variance followed by Tukey’s multiple comparison test or were analyzed by an independent sample T-test in the biostatistical software SPSS, depending on the number of experimental groups under analysis.
3. Results
3.1. Quantitative Real-Time PCR Analysis
3.1.1. Treatment of E. oblique with Deltamethrin
Results of quantitative real-time PCR showed that the expression level of EoACP138 (Figure 1A) and EoCYP316 (Figure 1B) in the fourth instar larvae was significantly up-regulated at 24 h under deltamethrin treatment, then, it was down-regulated at 48 h for EoACP138 while consistent up-regulation was observed for EoCYP316 at 48 h. The expression of gene EoCarE529 (Figure 1C) and EoAchE989 (Figure 1D); however, was significantly up-regulated at 12 h. Compared to the consistent down-regulation for the gene of EoAchE989 at 24 h and 48 h, it was persistently up-regulated at 24 h and down-regulated at 48 h for the gene of EoCarE529; these results indicated that the expression levels of four detoxification-related genes were significantly increased when the larvae were stimulated by the pesticide, especially after 24 h.
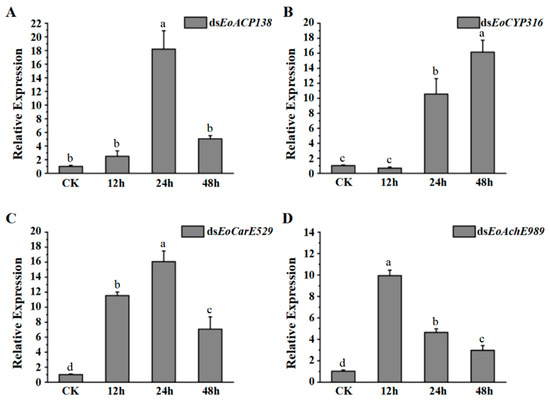
Figure 1.
The gene expression of fourth instar larvae after deltamethrin was stimulated. (A) EoACP138 (B) EoCYP316 (C) EoCarE592 (D) EoAchE989. Where CK was the 0 h treatment control, and 18S rRNA was used as the internal reference gene for qRT-PCR with three replicates per treatment. Each bar represents the mean ± SD. Different letters indicate significant differences (p < 0.05) by using the Tukey test to compare in ANOVA.
3.1.2. Fenpropathrin and Chlorpyrifos Treatment for E. oblique
The gene expression of the fourth instar larva after fenpropathrin and chlorpyrifos stimulated at 24 h was detected by qRT-PCR. Combined with the results of deltamethrin treatment, the gene expression of EoACP138, EoCYP316, EoCarE592 and EoAchE989 was significantly upregulated after exposure to each insecticide for 24 h compared with the control at 0 h (Figure 2A–D). The up-regulated expression levels of EoACP138, EoCYP316 and EoCarE592 for the different pesticide treatments showed the same trend (Figure 2A–C): the lower level for the chlorpyrifos treatment and higher level for fenpropathrin and deltamethrin treatment. For EoAchE989, the fenpropathrin treatment exhibited higher relative expression, followed by chlorpyrifos and deltamethrin (Figure 2D).
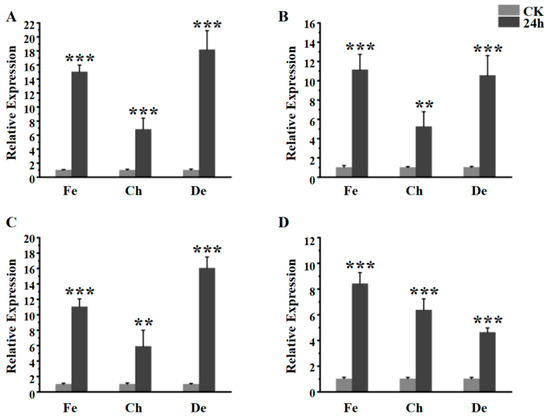
Figure 2.
The gene expression of fourth instar larva after Chlorpyrifos, deltamethrin and fenpropathrin stimulated. (A) EoACP138 gene (B) EoCYP316 gene (C) EoCarE592 gene (D) EoAchE989 gene, where CK was the 0 h treatment control, Fe was fenpropathrin, Ch was chlorpyrifos, and De was deltamethrin. Each bar represents the mean ± SD. Asterisks indicate significant differences between 0 h and 24 h expression. ** = p < 0.01, and *** = p < 0.001.
3.2. dsRNA Synthesis
Results of agarose gel electrophoresis showed that purified dsRNA of EoACP138, EoCYP316, EoCarE592, EoAchE989, and GFP as control synthesized in vitro had a single band in the gel, indicating the high purity of synthesized dsRNA (Figure 3). The dsRNA concentrations, measured by Nanodrop, are as follows: 365.3 ng/µL of GFP, 427.1 ng/µL of EoACP138, 378.4 ng/µL of EoCYP316, 478.2 ng/µL of EoCarE592, and 586.4 ng/µL of EoAchE989.
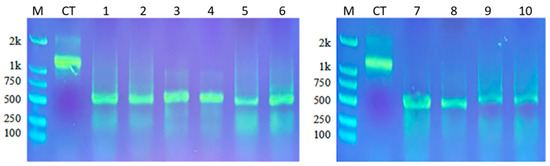
Figure 3.
Results of dsRNA synthesis. M: Marker CT: Control Template 1–2: EoACP138 dsRNA 3–4: EoCYP316 dsRNA 5–6: EoCarE592 dsRNA 7–8: EoAchE989 dsRNA 9–10: GFP dsRNA.
3.3. RNA Interference
The efficiency of RNA interference depends on the concentration of dsRNA and the selection of dsRNA fragments. The fragments of dsRNA have been determined, therefore, the selection of the appropriate amount of dsRNA becomes important for the silencing of genes. As shown in Figure 4A, the gene expression levels were decreased by 22%, 52%, 79%, and 84% after injection of 50 ng, 100 ng, 200 ng, and 400 ng dsEoACP138, respectively. With the increasing dsRNA amount, the interference of dsEoACP138 on the target gene became more effective. The silencing effect of dsEoCYP316 was broadly similar to that of dsEoACP138, resulting in 31%, 53%, 82% and 87% gene knockdown of EoCYP316 with the dsRNA amount increasing from 50 ng, 100 ng, 200 ng to 400 ng (Figure 4B).
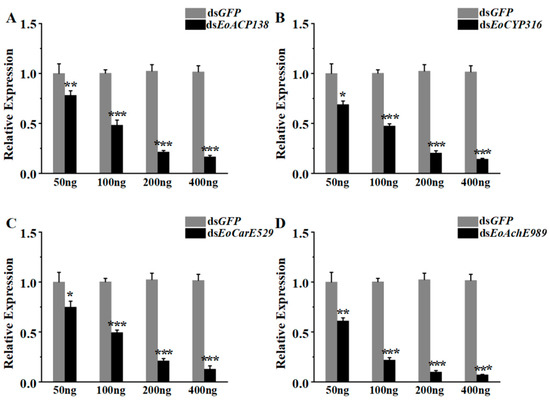
Figure 4.
RNAi efficiency analysis of genes related to detoxification from E. oblique after injection of dsRNA. (A) The expression analysis of EoACP138 at 24 h after injection of dsEoACP138 by qRT-PCR. (B) The expression analysis of EoCYP316 at 24 h after injection of dsEoCYP316 by qRT-PCR. (C) The expression analysis of EoCarE592 at 24 h after injection of dsEoCarE592 by qRT-PCR. (D) The expression analysis of EoAchE989 at 24 h after injection of dsEoAchE989 by qRT-PCR. Each bar represents the mean ± SD. Asterisks indicate significant differences in expression level between GFP and four different genes after RNAi. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
The silencing effects of dsEoCarE592 (Figure 4C) and dsEoAchE989 (Figure 4D) for the fourth instar showed roughly the same trend. After injection doses of 50 ng, 100 ng, 200 ng, and 400 ng, 25%, 51%, 79% and 87% of gene knockdown was observed for EoCarE592 and 39%, 78%, 92% and 94% of knockdown was observed for EoAchE989, respectively. At the injection dsRNA amounts of 200 ng, the suppression effect on the target genes researched 79% of EoACP138, 82% of EoCYP316, 79% of EoCarE592, and 92% of EoAchE989. Therefore, dsRNA of 200 ng was determined as the interference injection amount for the following studies.
3.4. Effects of RNAi on the Survival of E. oblique
The mortality of the larvae was recorded at 48 h after injection of 200 ng of dsRNA. Compared with the mortality of 15.3% for the control group injected with TE buffer and 21.7% mortality in larvae injected with ds GFP, the mortality of tested groups treated with dsEoACP138, dsEoCYP316, dsEoCarE592, and dsEoAchE989 were 31%, 23.7%, 19.7% and 31.7%, respectively (Figure 5). The survival of larvae interfered with dsEoACP138 and dsEoAchE989 was significantly decreased for 48 h after injection compared to that of the larvae injected with GFP dsRNA.
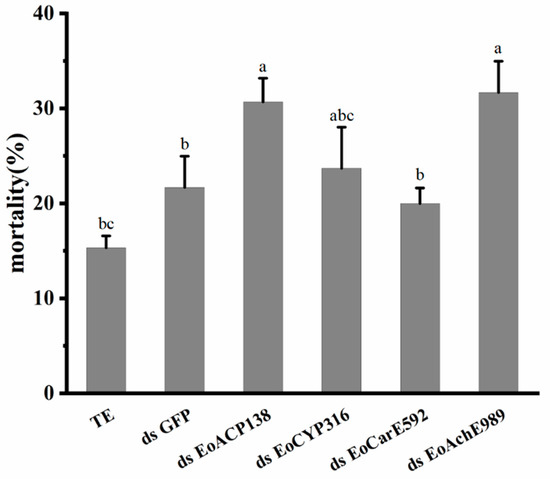
Figure 5.
Effect of 200 ng dsRNA of four Genes on mortality of E. oblique. Three replicates per treatment. Each bar represents the mean ± SD. Different letters indicate significant differences (p < 0.05) by using a Tukey test to compare in ANOVA.
3.5. Expression of Detoxification Related Genes in RNAi E. oblique Stimulated by Insecticides
Twenty hours after injecting 200 ng of dsRNA, the expression levels of EoACP138, EoCYP316, EoCarE592 and EoAchE989 had been significantly decreased when compared to the control (Figure 6). The transcript levels of the detoxification-related genes of RNAi larvae stimulated by different insecticides for 24 h were detected by qRT-PCR. Although the gene expression was up-regulated after pesticide stimulation, the degree of up-regulation was significantly lower than that of the control group, which was in line with the experimental expectation. As shown in Figure 6, after treatment with chlorpyrifos, 35%, 30%, 24% and 39% of gene knockdown was observed for EoACP138, EoCYP316, EoCarE592 and EoAchE989 genes, respectively. After treatment with fenpropathrin, 34%, 44%, 27% and 34% of gene knockdown was observed for EoACP138, EoCYP316, EoCarE592 and EoAchE989 gene, respectively. After treatment with deltamethrin, 59%, 53%, 55% and 56% of gene knockdown was observed for EoACP138, EoCYP316, EoCarE592 and EoAchE989 gene, respectively.
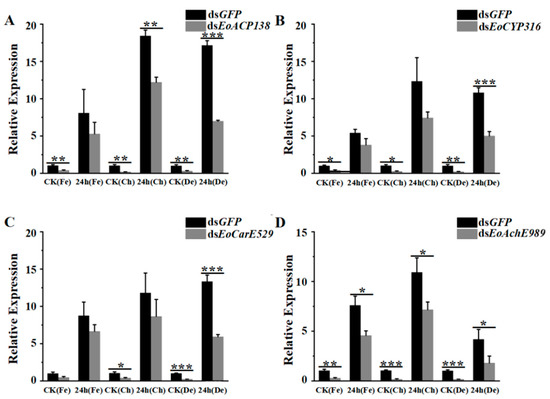
Figure 6.
Transcriptional levels of detoxification related genes stimulated by fenprothrin, chlorpyrifos and deltamethrin respectively in E. oblique after RNAi. (A) EoACP138. (B) EoCYP316. (C) EoCarE592. (D) EoAchE989. Where CK was the 0 h treatment control, Fe was fenpropathrin, Ch was chlorpyrifos, and De was deltamethrin. Each bar represents the mean ± SD. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
3.6. Determination of Toxicity of Insecticides after RNA Interference
As the four genes of EoACP138, EoCYP316, EoCarE592 and EoAchE989 were related to the detoxification ability of E. oblique, after the four genes of EoACP138, EoCYP316, EoCarE592 and EoAchE989 were silenced, the mortality of the fourth instar larvae after treatment with deltamethrin, fenpropathrin and chlorpyrifos was counted respectively to test whether the resistance of E. oblique larvae to pesticides was affected after the genes were silenced. The mortality of larvae after injection with dsRNAs targeting detoxifying genes was significantly greater than mortality in controls (CK and GFP dsRNA) and ranged from 44% to 59% when compared to the 12–19% mortality in larvae injected with CK and 25–33% mortality in larvae injected with GFP dsRNA (Figure 7).
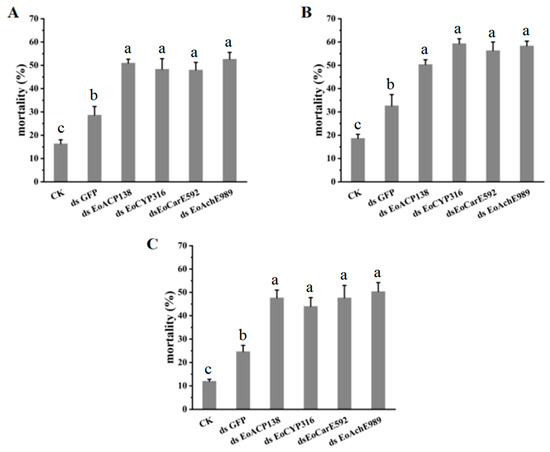
Figure 7.
Pesticides tolerance of E. oblique after RNAi. (A) deltamethrin (B) chlorpyrifos (C). Fenpropathrin CK means no injection. Each bar represents the mean ± SD. Different letters indicate significant differences (p < 0.05) by using a Tukey test to compare in ANOVA.
4. Discussion
Pests cause significant crop losses worldwide. The use of broad-spectrum chemical pesticides to control pest damage is popular, but the following development of drug resistance has affected the sustainable use of pesticides. Therefore, it is very important for pest control to understand the expression levels of candidate genes potentially involved in insecticide resistance; however, with the rapid development of RNAi technology in insect gene function research and pest control [29], it has been found that the technology can be used to screen genes related to insecticide resistance and target genes for pest control [30].
In this study, firstly, the expression changes of genes of acid phosphatase EoACP138, cytochrome P450 EoCYP316, carboxylesterase EoCarE592 and acetylcholinesterase EoAchE989 were detected at 12 h, 24 h and 48 h under the stimulation of deltamethrin. The same trend of expression changes of EoACP138 and EoCarE592 genes reached the peak at 24 h, and then decreased; however, the changing trends of EoCYP316 and EoAchE989 are just the opposite. The expression of EoCYP316 is the highest at 48 h, which is 15.73 times higher than that of the control group. EoAchE989 is significantly increased at 12 h, and the expression is increased by 9.67 times. It is suggested that four genes may be involved in the detoxification process of E. oblique. It has been reported that arginine kinase TcAK1 in Tribolium castaneum significantly increased its mRNA level at 2 h and 4 h after deltamethrin stimulation, but recovered to the control level at 12 h, which indicated that different enzyme genes had different response mechanisms to deltamethrin stimulation [31].
Secondly, the expression levels of EoACP138, EoCYP316, EoCarE592 and EoAchE989 in the fourth instar larvae after 24 h treatment with chlorpyrifos and fenpropathrin were detected. The results showed that the expression levels of four genes were significantly increased at 24 h after treatment with chlorpyrifos and fenpropathrin, and the expression levels were increased by 5.14–6.74 times. The expression levels were increased by 8.23–14.64 times after treatment with fenpropathrin. It was speculated that the content of the above genes could be increased in 24 h after the treatment of pesticides in the worms to resist the pesticide damage. It has been reported that imidacloprid and β-cypermethrin could significantly induce the expression of RpCSP gene of Rhopalosiphum padi, suggesting that RpCSPs might be related to the response to exogenous toxic pesticides [32]. In this study, the expression levels of four genes (EoACP138, EoCYP316, EoCarE592 and EoAchE989) were up-regulated after treatment with deltamethrin, chlorpyrifos and fenpropathrin, indicating that these four genes might be related to the detoxification metabolism of three pesticides by E. oblique.
RNAi is an indispensable means for gene function research in modern molecular biology experiments. At present, RNAi research on insects has involved gene function research on many common insects, such as Spodoptera litura [33], Hyposidra talaca [34], Leptinotarsa decemlineata [35], etc. The RNAi targeting EoACP138, EoCYP316, EoCarE592 and EoAchE989 resulted in significantly reduced expression of four target genes in E. oblique; this result was consistent with that observed in the diamondback moth, plutella xylostella, after oral administration of dsRNA for 24 h [36].
After injecting 200 ng of dsRNA, E. oblique were exposed to three pesticides, and a significant increase in larval mortality compared with the control was observed, indicating that RNAi increased the susceptibility of E. oblique to the three pesticides. The same phenomenon was also confirmed in Nilaparvata lugens [37]. In addition, after feeding Spodoptera litura dsRNA targeting SlGOBP2, reduced susceptibility to chrolorpyrifos was reported [38]. Similar results were observed in organophosphorus pesticide toxicity tests conducted by Meng et al. Following the injection of dsRNA of Pp-AChE1 and Pp-AChE2 encoded two acetylcholinesterase into the abdomen of Pardosa pseudoannulata [39]. This study demonstrated that the RNAi technology could be used to silence the detoxification-related genes to verify their function and improve the sensitivity of insects to pesticides, providing new ideas for the prevention and control of insects.
5. Conclusions
In this study, we observed that after pesticide stimulation, the expression levels of acidic phosphatase gene EoACP138, cytochrome P450 gene EoCYP316, carboxylesterase gene EoCarE592, and acetylcholinesterase gene EoAchE989 were all significantly increased. After silencing the above genes by RNAi, it was found that the toxicity of the pesticide to E. oblique was significantly increased. The results indicated that the genes of EoACP138, EoCYP316, EoCarE592 and EoAchE989 were involved in metabolic detoxification pathways, which might be candidate genes for the biological control of E. oblique by RNAi. The results of this study will contribute to the implementation of more effective pest control strategies and the development of resistance to metabolic pesticides.
Author Contributions
Data curation, C.P. and H.Y.; software, Y.L.; Methodology, Z.-Y.L., C.P. and H.Y.; investigation, C.P. and H.Y.; writing—original draft preparation, C.P. and H.Y.; writing—review and editing, X.-F.M. and Z.-Y.L.; project administration, Z.-Y.L.; funding acquisition, Z.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Sichuan application basic research project (No. 2019YJ0459) and Thousand talents project from the Sichuan Province of China (No. E90112).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest with respect to authorship, research, and publishing of this article.
References
- Su, T. Studies on Determination Method and Degradation Dynamic of Acetamiprid Residues in Tea. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2012. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, Y.X.; Han, T.; Yu, G.W.; Sun, X.L. Research progress of induced defense against insect pests in tea plant. Acta Entomol. Sin. 2022, 65, 399–408. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Feng, X.B.; Wang, Y.; Xu, W.W.; Huang, K.; Hu, M.H.; Zhang, C.; Yuan, H.Y. Advances in research on functional genes of tea plant. Gene 2019, 711, 143940. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Suh, J.H. Multi-omics approach in tea polyphenol research regarding tea plant growth, development and tea processing: Current technologies and perspectives. Food Sci. Hum. Wellness 2022, 11, 524–536. [Google Scholar] [CrossRef]
- Wei, C.L.; Yang, H.; Wang, S.B.; Zhao, J.; Liu, C.; Gao, L.P.; Xia, E.H.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Wheeler, W.J. The medicinal chemistry of tea. Drug Dev. Res. 2004, 61, 45–65. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.A.; Li, L.B.; Li, F.D.; He, Y.X.; Wu, J.Q.; Wei, C. Transcriptomic and phytochemical analyses reveal root-mediated resource-based defense response to leaf herbivory by Ectropis oblique in tea plant (Camellia sinensis). J. Agric. Food Chem. 2019, 67, 5465–5476. [Google Scholar] [CrossRef]
- Lin, S.H. The occurrence regularity and control measures of Ectropis obliqua. Fujian Agric. Sci. Technol. 2003, 1, 52–53. [Google Scholar] [CrossRef]
- Wang, Y.N.; Tang, L.; Hou, Y.; Wang, P.; Yang, H.; Wei, C.L. Differential transcriptome analysis of leaves of tea plant (Camellia sinensis) provides comprehensive insights into the defense responses to Ectropis oblique attack using RNA-Seq. Funct. Integr. Genom. 2016, 16, 383–398. [Google Scholar] [CrossRef]
- Hazarika, L.K.; Puzari, K.C.; Wahab, S. Biological Control of Tea Pests. In Biocontrol Potential and Its Exploitation in Sustainable Agriculture; Upadhyay, R.K., Mukerji, K.G., Chamola, B.P., Eds.; Springer: Boston, MA, USA, 2001; pp. 159–180. [Google Scholar] [CrossRef]
- Ye, G.Y.; Xiao, Q.; Chen, M.; Chen, X.X.; Yuan, Z.J.; Stanley, D.W.; Hu, C. Tea: Biological control of insect and mite pests in China. Biol. Control 2014, 68, 73–91. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Sun, M.; Zhang, B.; Fan, C. Use of a headspace solid-phase microextraction-based methodology followed by gas chromatography–tandem mass spectrometry for pesticide multiresidue determination in teas. Chromatographia 2018, 81, 809–821. [Google Scholar] [CrossRef]
- Colapinto, C.K.; Arbuckle, T.E.; Dubois, L.; Fraser, W. Tea consumption in pregnancy as a predictor of pesticide exposure and adverse birth outcomes: The MIREC study. Environ. Res. 2015, 142, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denholm, I.; Devine, G.J.; Williamson, M.S. Insecticide resistance on the move. Science 2002, 297, 2222–2223. [Google Scholar] [CrossRef]
- Kyre, B.R.; Bentz, B.J.; Rieske, L.K. Susceptibility of mountain pine beetle (Dendroctonus ponderosae Hopkins) to gene silencing through RNAi provides potential as a novel management tool. For. Ecol. Manag. 2020, 473, 118322. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rossi, J.J. RNAi mechanisms and applications. Biotechniques 2008, 44, 613–616. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Jaubert-Possamai, S.; Le Trionnaire, G.; Bonhomme, J.; Christophides, G.K.; Rispe, C.; Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007, 7, 63. [Google Scholar] [CrossRef]
- Lü, J.; Guo, M.; Chen, S.; Noland, J.E.; Guo, W.; Sang, W.; Qi, Y.; Qiu, B.; Zhang, Y.; Yang, C.; et al. Double-stranded RNA targeting vATPase B reveals a potential target for pest management of Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2020, 165, 104555. [Google Scholar] [CrossRef]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R.; Dermauw, W.; Van Leeuwen, T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015, 121, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, Q.; Li, Q.; Wang, R.; Xu, C.; Wang, H.; Ran, C.; Song, Y.; Zeng, R. Identification of a cytochrome P450 CYP6AB60 gene associated with tolerance to multi-plant allelochemicals from a polyphagous caterpillar tobacco cutworm (Spodoptera litura). Pestic. Biochem. Physiol. 2019, 154, 60–66. [Google Scholar] [CrossRef]
- Yang, B.; Lin, X.; Yu, N.; Gao, H.; Zhang, Y.; Liu, W.; Liu, Z. Contribution of glutathione S-transferases to imidacloprid resistance in Nilaparvata lugens. J. Agric. Food Chem. 2020, 68, 15403–15408. [Google Scholar] [CrossRef]
- Yin, H.; Fu, Z.Z.; Yang, X.X.; Zhou, Y.Q.; Mao, X.F.; Liu, Z.Y.; Fu, J.Y. Functional annotation of Ectropis obliqua transcriptome in the treatment of pyrethroid insecticides. Meta Gene 2021, 28, 100860. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Guan, R.; Li, H.; Miao, X. RNAi pest control and enhanced BT insecticidal efficiency achieved by dsRNA of chymotrypsin-like genes in Ostrinia furnacalis. J. Pest Sci. 2017, 90, 745–757. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, J.; Jiang, H.; Ge, H.; Zheng, Y.; Meng, X.; Qian, K.; Wang, J. Knockdown or inhibition of arginine kinases enhances susceptibility of Tribolium castaneum to deltamethrin. Pestic. Biochem. Physiol. 2022, 183, 105080. [Google Scholar] [CrossRef]
- Peng, X.; Qu, M.J.; Wang, S.J.; Huang, Y.X.; Chen, C.; Chen, M.H. Chemosensory proteins participate in insecticide susceptibility in Rhopalosiphum padi, a serious pest on wheat crops. Insect Mol. Biol. 2021, 30, 138–151. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, H.; Zhu, X.Z.; Wang, L.; Zhang, K.X.; Li, D.Y.; Ji, J.C.; Niu, L.; Gao, X.K.; Luo, J.Y.; et al. Silencing of cytochrome P450 gene CYP321A1 effects tannin detoxification and metabolism in Spodoptera litura. Int. J. Biol. Macromol. 2022, 194, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Babu, A.; Handique, G.; Dutta, R.; Bora, A.; Das, P. Stage specific differential expression of three detoxifying enzymes of larvae of tea defoliator, Hyposidra talaca Walker (Geometridae: Lepidoptera) and its bearing on their insecticide tolerance status. Int. J. Trop. Insect Sci. 2021, 41, 541–545. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.A.M.; Miyata, T.; Miura, K.; Tanaka, T. RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect Biochem. Mol. Biol. 2009, 39, 38–46. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, Z.C.; Li, Y.; Li, W.R.; Zhou, Q. Characterization and functional analysis of a carboxylesterase gene associated with chlorpyrifos resistance in Nilaparvata lugens (Stl). Comp. Biochem. Physiol. Toxicol. Pharmacol. 2017, 203, 12–20. [Google Scholar] [CrossRef]
- Sun, Z.X.; Wang, R.M.; Du, Y.F.; Gao, B.Y.; Gui, F.R.; Lu, K. Olfactory perception of herbicide butachlor by GOBP2 elicits ecdysone biosynthesis and detoxification enzyme responsible for chlorpyrifos tolerance in Spodoptera litura. Environ. Pollut. 2021, 285, 117409. [Google Scholar] [CrossRef]
- Meng, X.; Li, C.; Bao, H.; Fang, J.; Liu, Z.; Zhang, Y. Validating the importance of two acetylcholinesterases in insecticide sensitivities by RNAi in Pardosa pseudoannulata, an important predatory enemy against several insect pests. Pestic. Biochem. Physiol. 2015, 125, 26–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).