Gene–Environment Correlation over Time: A Longitudinal Analysis of Polygenic Risk Scores for Schizophrenia and Major Depression in Three British Cohorts Studies
Abstract
1. Introduction
1.1. Prevalence of SCZ and MDD
1.2. Genetic Influences
1.3. Environmental Influences
1.4. Psychopathology across Development
1.5. rGE across Development
1.6. The Current Study
1.7. Hypotheses
2. Methods
2.1. Participants
2.2. Measures
2.2.1. Environmental and Psychosocial Risk Factors
2.2.2. Genetic Data Processing and Polygenic Risk Scoring
2.2.3. Data Analysis
3. Results
3.1. rGE across Childhood
3.2. rGE across Adulthood
3.3. Comparison between Childhood and Adulthood
3.4. Sample Size and Power Calculation
4. Discussion
4.1. Change in rGE across Childhood
4.2. Change in rGE across Adulthood
4.3. Childhood vs. Adulthood PRS-by-Time Comparison
4.4. Comparison between SCZ and MDD
4.5. Strengths and Limitations
4.6. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendler, K.S.; Karkowski-Shuman, L. Stressful life events and genetic liability to major depression: Genetic control of exposure to the environment? Psychol. Med. 1997, 27, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, S.R.; Price, T.S. Gene-environment correlations: A review of the evidence and implications for prevention of mental illness. Mol. Psychiatry 2007, 12, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; DeFries, J.C.; Loehlin, J.C. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol. Bull. 1977, 84, 309–322. [Google Scholar] [CrossRef]
- Beam, C.R.; Turkheimer, E. Phenotype-environment correlations in longitudinal twin models. Dev. Psychopathol. 2013, 25, 7–16. [Google Scholar] [CrossRef][Green Version]
- Kruijshaar, M.E.; Barendregt, J.; Vos, T.; de Graaf, R.; Spijker, J.; Andrews, G. Lifetime prevalence estimates of major depression: An indirect estimation method and a quantification of recall bias. Eur. J. Epidemiol. 2005, 20, 103–111. [Google Scholar] [CrossRef]
- Kendler, K.S.; Gatz, M.; Gardner, C.O.; Pedersen, N.L. A Swedish national twin study of lifetime major depression. Am. J. Psychiatry 2006, 163, 109–114. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Gogtay, N.; Vyas, N.S.; Testa, R.; Wood, S.J.; Pantelis, C. Age of onset of schizophrenia: Perspectives from structural neuroimaging studies. Schizophr. Bull. 2011, 37, 504–513. [Google Scholar] [CrossRef]
- Wilson, S.; Hicks, B.M.; Foster, K.; McGue, M.; Iacono, W.G. Age of onset and course of major depressive disorder: Associations with psychosocial functioning outcomes in adulthood. Psychol. Med. 2015, 45, 505–514. [Google Scholar] [CrossRef]
- Gochman, P.; Miller, R.; Rapoport, J.L. Childhood-onset schizophrenia: The challenge of diagnosis. Curr. Psychiatry Rep. 2011, 13, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Mattai, A.K.; Hill, J.L.; Lenroot, R.K. Treatment of early-onset schizophrenia. Curr. Opin. Psychiatry 2010, 23, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Domènech-Llaberia, E.; Viñas, F.; Pla, E.; Jané, M.C.; Mitjavila, M.; Corbella, T.; Canals, J. Prevalence of major depression in preschool children. Eur. Child Adolesc. Psychiatry 2009, 18, 597–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cicchetti, D.; Toth, S.L. The development of depression in children and adolescents. Am. Psychol. 1998, 53, 221–241. [Google Scholar] [CrossRef]
- Costello, E.J.; Erkanli, A.; Angold, A. Is there an epidemic of child or adolescent depression? J. Child Psychol. Psychiatry 2006, 47, 1263–1271. [Google Scholar] [CrossRef]
- Cardno, A.G.; Gottesman, I.I. Twin studies of schizophrenia: From bow-and-arrow concordances to star wars Mx and functional genomics. Am. J. Med. Genet. 2000, 97, 12–17. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Kendler, K.S.; Prescott, C.A. A population-based twin study of lifetime major depression in men and women. Arch. Gen. Psychiatry 1999, 56, 39–44. [Google Scholar] [CrossRef]
- Rutter, M.; Kim-Cohen, J.; Maughan, B. Continuities and discontinuities in psychopathology between childhood and adult life. J. Child Psychol. Psychiatry 2006, 47, 276–295. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Krapohl, E.; Euesden, J.; Zabaneh, D.; Pingault, J.-B.; Rimfeld, K.; von Stumm, S.; Dale, P.; Breen, G.; O’Reilly, P.; Plomin, R.J. Phenome-wide analysis of genome-wide polygenic scores. Mol. Psychiatry 2016, 21, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Baker, J.H. Genetic influences on measures of the environment: A systematic review. Psychol. Med. 2007, 37, 615–626. [Google Scholar] [CrossRef]
- Chiu, T.-F.; Yu, T.-M.; Chuang, Y.-W.; Sun, K.-T.; Li, C.-Y.; Su, Y.-C.; Kao, C.-H. Sequential risk of depression in children born prematurely: A nationwide population- based analysis. J. Affect. Disord. 2019, 243, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.; Rantakallio, P.; Hartikainen, A.-L.; Isohanni, M.; Sipila, P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: A 28-year follow-up of the 1966 north Finland general population birth cohort. Am. J. Psychiatry 1998, 155, 355–364. [Google Scholar] [CrossRef]
- Keefe, R.S.; Eesley, C.E.; Poe, M.P. Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry 2005, 57, 688–691. [Google Scholar] [CrossRef]
- Hakulinen, C.; McGrath, J.J.; Timmerman, A.; Skipper, N.; Mortensen, P.B.; Pedersen, C.B.; Agerbo, E. The association between early-onset schizophrenia with employment, income, education, and cohabitation status: Nationwide study with 35 years of follow-up. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1343–1351. [Google Scholar] [CrossRef]
- Cohen, A.K.; Nussbaum, J.; Weintraub, M.L.R.; Nichols, C.R.; Yen, I.H. Association of Adult Depression With Educational Attainment, Aspirations, and Expectations. Prev. Chronic. Dis. 2020, 17, E94. [Google Scholar] [CrossRef]
- Evensen, S.; Wisløff, T.; Lystad, J.U.; Bull, H.; Ueland, T.; Falkum, E. Prevalence, Employment Rate, and Cost of Schizophrenia in a High-Income Welfare Society: A Population-Based Study Using Comprehensive Health and Welfare Registers. Schizophr. Bull. 2016, 42, 476–483. [Google Scholar] [CrossRef]
- Freeman, A.; Tyrovolas, S.; Koyanagi, A.; Chatterji, S.; Leonardi, M.; Ayuso-Mateos, J.L.; Tobiasz-Adamczyk, B.; Koskinen, S.; Rummel-Kluge, C.; Haro, J.M. The role of socio-economic status in depression: Results from the COURAGE (aging survey in Europe). BMC Public Health 2016, 16, 1098. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Gunnell, D.; Glazebrook, C.; Page, K.; Kwiecinski, R. Association between schizophrenia and social inequality at birth: Case-control study. Br. J. Psychiatry 2001, 179, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Domingue, B.W.; Liu, H.; Okbay, A.; Belsky, D.W. Genetic Heterogeneity in Depressive Symptoms Following the Death of a Spouse: Polygenic Score Analysis of the U.S. Health and Retirement Study. Am. J. Psychiatry 2017, 174, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Knafo, A.; Jaffee, S.R. Gene-environment correlation in developmental psychopathology. Dev. Psychopathol. 2013, 25, 1–6. [Google Scholar] [CrossRef]
- Rutter, M.L.; Dunn, J.; Plomin, R.; Simonoff, E.; Pickles, A.; Maughan, B.; Ormel, J.; Meyer, J.; Eaves, L. Integrating nature and nurture: Implications of person-environment correlations and interactions for developmental psychopathology. Dev. Psychopathol. 1997, 9, 335–364. [Google Scholar] [CrossRef]
- Rutter, M.; Quinton, D. Parental psychiatric disorder: Effects on children. Psychol. Med. 1984, 14, 853–880. [Google Scholar] [CrossRef]
- Halfon, N.; Larson, K.; Lu, M.; Tullis, E.; Russ, S. Lifecourse health development: Past, present and future. Matern. Child Health J. 2014, 18, 344–365. [Google Scholar] [CrossRef]
- Thapar, A.; Riglin, L. The importance of a developmental perspective in Psychiatry: What do recent genetic-epidemiological findings show? Mol. Psychiatry 2020, 25, 1631–1639. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Postorino, V.; Siracusano, M.; Riccioni, A.; Armando, M.; Curatolo, P.; Mazzone, L. Autistic Symptoms in Schizophrenia Spectrum Disorders: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 78. [Google Scholar] [CrossRef]
- Frangou, S. Cognitive function in early onset schizophrenia: A selective review. Front. Hum. Neurosci. 2010, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J.; Stergiakouli, E.; Tansey, K.E.; Hubbard, L.; Heron, J.; Cannon, M.; Holmans, P.; Lewis, G.; Linden, D.E.J.; Jones, P.B.; et al. Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry 2016, 73, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.N.; Shankman, S.A.; Lewinsohn, P.M.; Seeley, J.R. Subthreshold depressive disorder in adolescents: Predictors of escalation to full-syndrome depressive disorders. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 703–710. [Google Scholar] [CrossRef]
- Scarr, S.; McCartney, K. How people make their own environments: A theory of genotype greater than environment effects. Child Dev. 1983, 54, 424–435. [Google Scholar]
- Newbury, J.B.; Arseneault, L.; Caspi, A.; Moffitt, T.E.; Odgers, C.L.; Belsky, D.W.; Sugden, K.; Williams, B.; Ambler, A.P.; Matthews, T.; et al. Association between genetic and socioenvironmental risk for schizophrenia during upbringing in a UK longitudinal cohort. Psychol. Med. 2020, 1–11. [Google Scholar] [CrossRef]
- Machlitt-Northen, S.; Keers, R.; Munroe, P.B.; Howard, D.M.; Pluess, M. Polygenic Risk Scores for Schizophrenia and Major Depression Are Associated with Socio-Economic Indicators of Adversity in Two British Community Samples; Department of Biological and Experimental Psychology, Queen Mary University of London: London, UK, 2022. [Google Scholar]
- Machlitt-Northen, S.; Keers, R.; Munroe, P.; Howard, D.; Trubetskoy, V.; Pluess, M. Polygenic Scores for Schizophrenia and Major Depression are Associated with Psychosocial Risk Factors in Children: Evidence of Gene-Environment Correlation. J. Child Psychol. Psychiatry 2022, in press. [Google Scholar]
- Nasir, M.; Bloch, M.H. Editorial: Money cannot buy happiness—But can it prevent depression? A commentary on Su et al. J. Child Psychol. Psychiatry 2021, 62, 1047–1049. [Google Scholar] [CrossRef]
- Joshi, H.; Fitzsimons, E. The Millennium Cohort Study: The making of a multi-purpose resource for social science and policy. Longitud. Life Course Stud. 2016, 7, 409–430. [Google Scholar] [CrossRef]
- Gutman, L.M.; Joshi, H.; Parsonage, M.; Schoon, I. Children of the New Century: Mental Health Findings from the Millennium Cohort Study; Centre for Mental Health: London, UK, 2015. [Google Scholar]
- Fitzsimons, E.; Moulton, V.; Hughes, D.A.; Neaves, S.; Ho, K.; Hemani, G.; Timpson, N.; Calderwood, L.; Gilbert, E.; Ring, S. Collection of DNA samples and genetic data at scale in the UK Millennium Cohort Study. In CLS Working Paper Number 2020/7; UCL Centre for Longitudinal Studies: London, UK, 2020. [Google Scholar]
- University of Essex, Institute for Social and Economic Research. Understanding Society: Waves 1–9, 2009–2018 and Harmonised BHPS: Waves 1–18, 1991–2009 [Data Collection]; UK Data Service: Colchester, UK, 2019. [Google Scholar]
- Buck, N.; McFall, S. Understanding Society: Design Overview. Longitud. Life Course Stud. 2011, 3, 5–17. [Google Scholar]
- University of Essex; Institute for Social and Economic Research; National Centre for Social Research. Understanding Society: Waves 2 and 3 Nurse Health Assessment, 2010–2012 [Data Collection]; UK Data Service: Colchester, UK, 2014. [Google Scholar]
- Brown, M.; Goodman, A. National Child Development Study (or 1958 Birth Cohort). J. Open Health Data 2014, 2, e5. [Google Scholar] [CrossRef]
- Power, C.; Elliott, J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int. J. Epidemiol. 2006, 35, 34–41. [Google Scholar] [CrossRef] [PubMed]
- University of London; Institute of Education; Centre for Longitudinal Studies. National Child Development Study: Biomedical Survey, 2002–2004 [Data Collection]; UK Data Service: London, UK, 2020. [Google Scholar]
- Prins, B.P.; Kuchenbaecker, K.B.; Bao, Y.; Smart, M.; Zabaneh, D.; Fatemifar, G.; Luan, J.A.; Wareham, N.J.; Scott, R.A.; Perry, J.R.; et al. Genome-wide analysis of health-related biomarkers in the UK Household Longitudinal Study reveals novel associations. Sci. Rep. 2017, 7, 11008. [Google Scholar] [CrossRef] [PubMed]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef]
- WTCCC2. Available online: www.wtccc.org.uk/ccc2/ (accessed on 23 September 2021).
- Coleman, J.R.I.; Euesden, J.; Patel, H.; Folarin, A.A.; Newhouse, S.; Breen, G. Quality control, imputation and analysis of genome-wide genotyping data from the Illumina HumanCoreExome microarray. Brief. Funct. Genom. 2016, 15, 298–304. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Bakken Stovner, E. Snpflip; GitHub: San Francisco, CA, USA, 2017. [Google Scholar]
- Ritchie, S. LiftOverPlink; GitHub: San Francisco, CA, USA, 2014. [Google Scholar]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Euesden, J.; Lewis, C.M.; O’Reilly, P.F. PRSice: Polygenic Risk Score software. Bioinformatics 2015, 31, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 12; StataCorp LP: College Station, TX, USA, 2011. [Google Scholar]
- University of London; Institute of Education; Centre for Longitudinal Studies. National Child Development Study: Age 55, Sweep 9, 2013 [Data Collection]; UK Data Service: London, UK, 2020. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Aalbers, M.B. The Financialization of Home and the Mortgage Market Crisis. Compet. Chang. 2008, 12, 148–166. [Google Scholar]
- Atingdui, N. Cohort Effect. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; p. 389. [Google Scholar]
- Pedersen, C.B.; Mortensen, P.B. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch. Gen. Psychiatry 2001, 58, 1039–1046. [Google Scholar] [CrossRef]
- Branje, S.; Geeraerts, S.; de Zeeuw, E.L.; Oerlemans, A.M.; Koopman-Verhoeff, M.E.; Schulz, S.; Nelemans, S.; Meeus, W.; Hartman, C.A.; Hillegers, M.H.; et al. Intergenerational transmission: Theoretical and methodological issues and an introduction to four Dutch cohorts. Dev. Cogn. Neurosci. 2020, 45, 100835. [Google Scholar] [CrossRef]
- Baselmans, B.M.L.; Willems, Y.E.; van Beijsterveldt, C.E.M.; Ligthart, L.; Willemsen, G.; Dolan, C.V.; Boomsma, D.I.; Bartels, M. Unraveling the Genetic and Environmental Relationship Between Well-Being and Depressive Symptoms Throughout the Lifespan. Front. Psychiatry 2018, 9, 261. [Google Scholar] [CrossRef]
- Tunstall, R. ‘Mixed tenure’ policy in the UK: Privatisation, pluralism or euphemism? Hous. Theory Soc. 2003, 20, 153–159. [Google Scholar] [CrossRef]
- Palk, A.C.; Dalvie, S.; De Vries, J.; Martin, A.R.; Stein, D.J. Potential use of clinical polygenic risk scores in psychiatry—Ethical implications and communicating high polygenic risk. Philos. Ethics Humanit. Med. 2019, 14, 4. [Google Scholar] [CrossRef]
- Shepherd, P.; Gilbert, E. Millennium Cohort Study—Ethical Review and Consent; Centre for Longitudinal Studies: London, UK, 2019. [Google Scholar]
- Centre for Longitudinal Studies. National Child Development Study—Ethical Review and Consent. 2014. Available online: https://cls.ucl.ac.uk/wp-content/uploads/2017/07/NCDS-Ethical-review-and-Consent-2014.pdf (accessed on 12 May 2022).
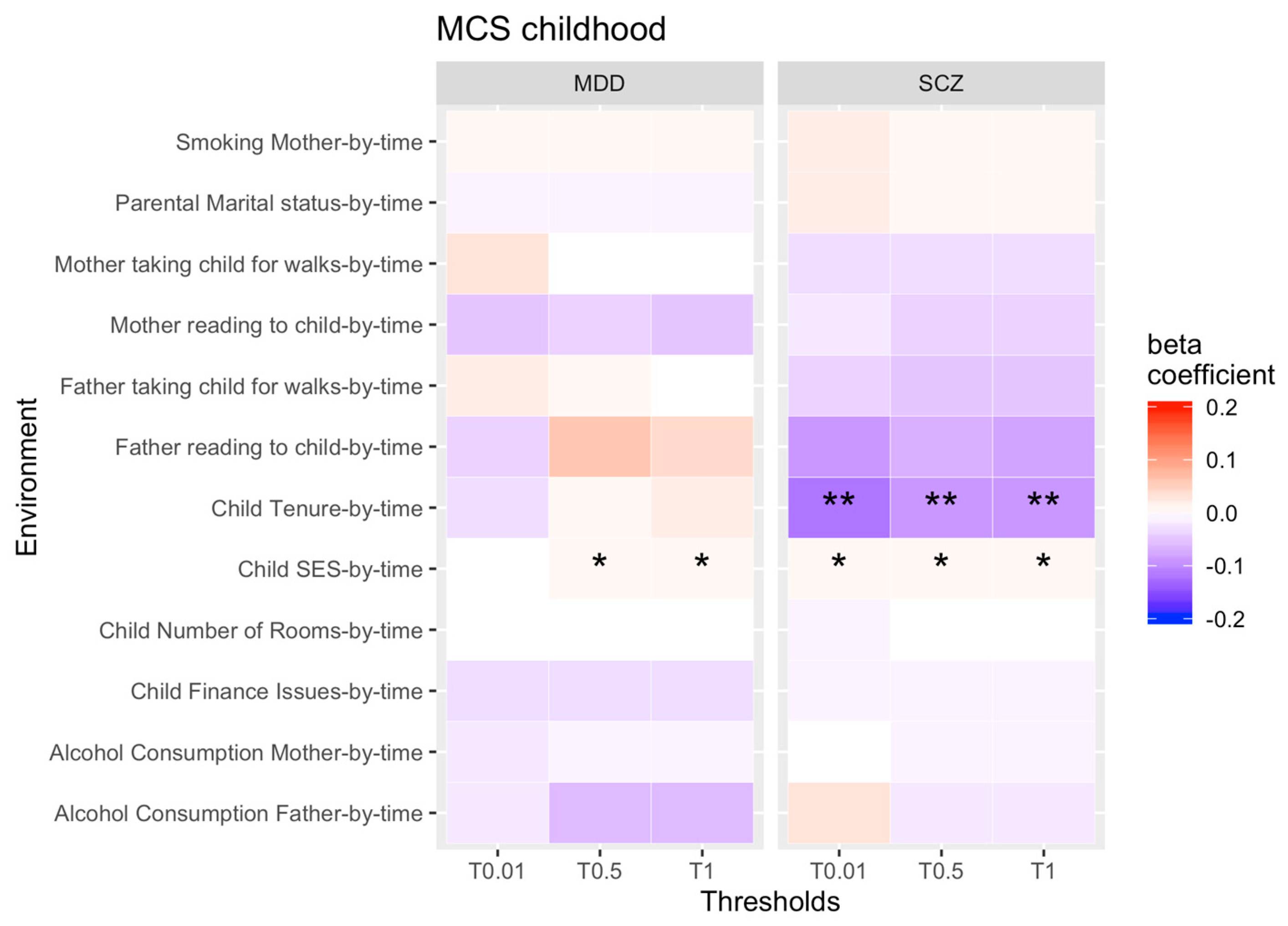
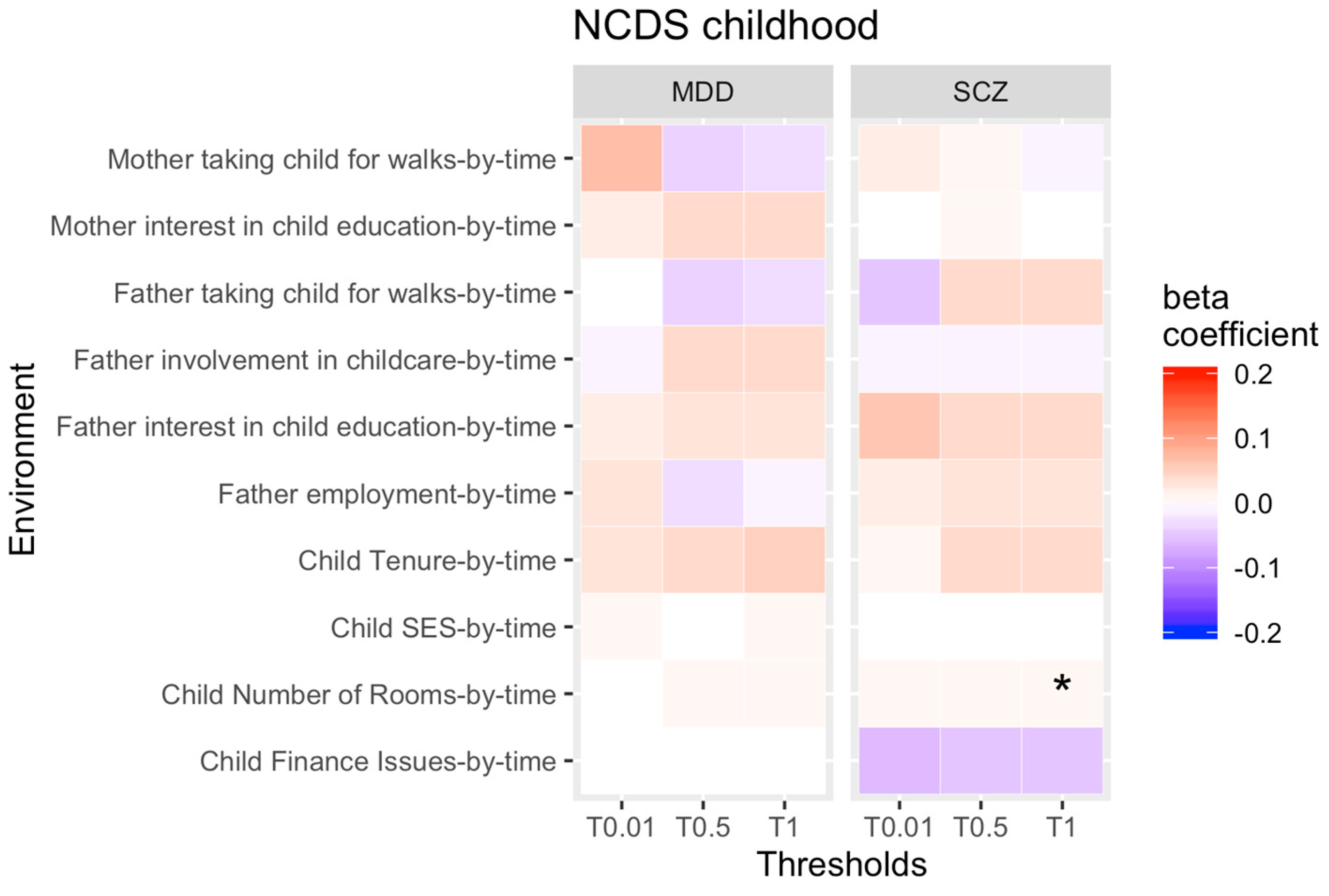
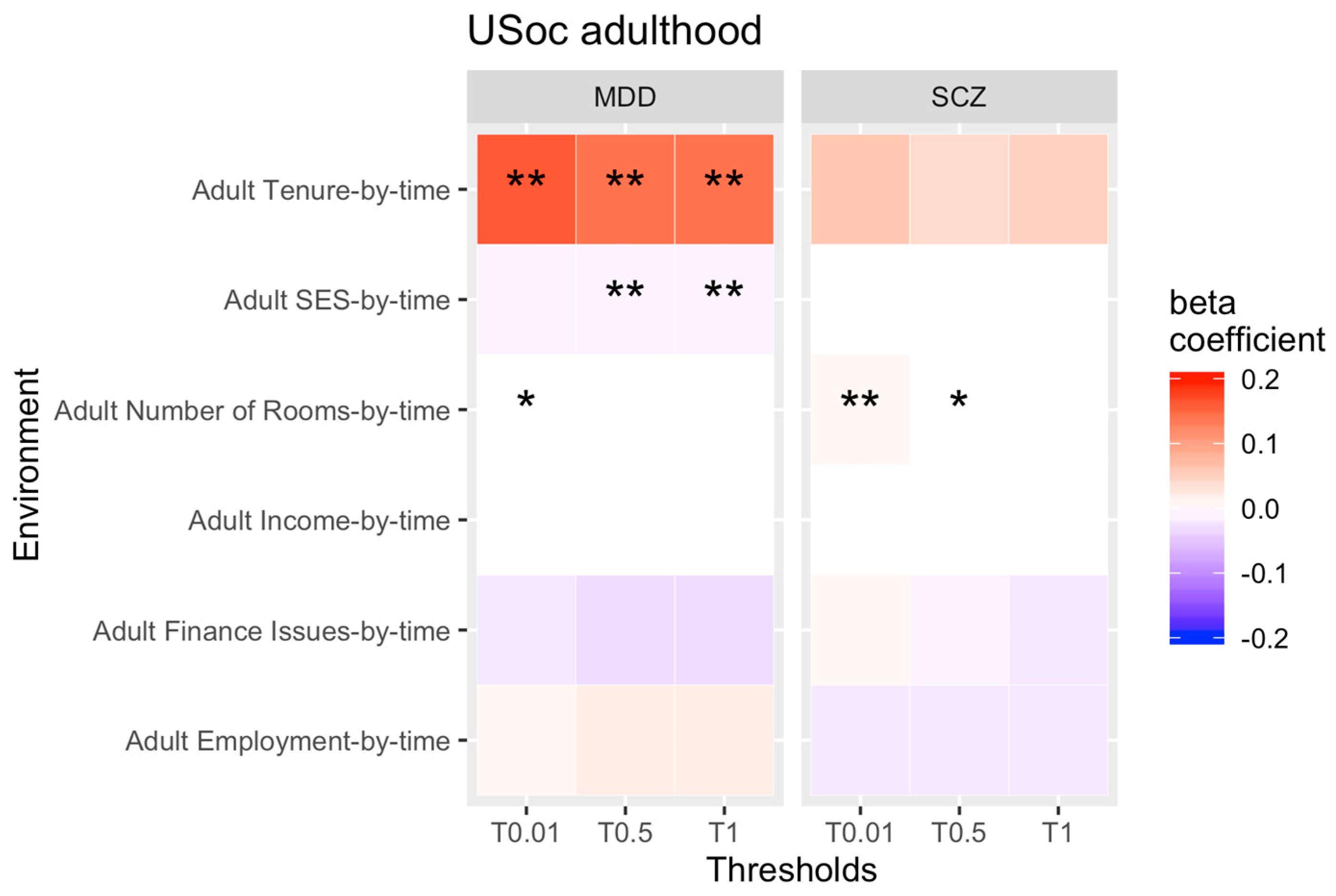

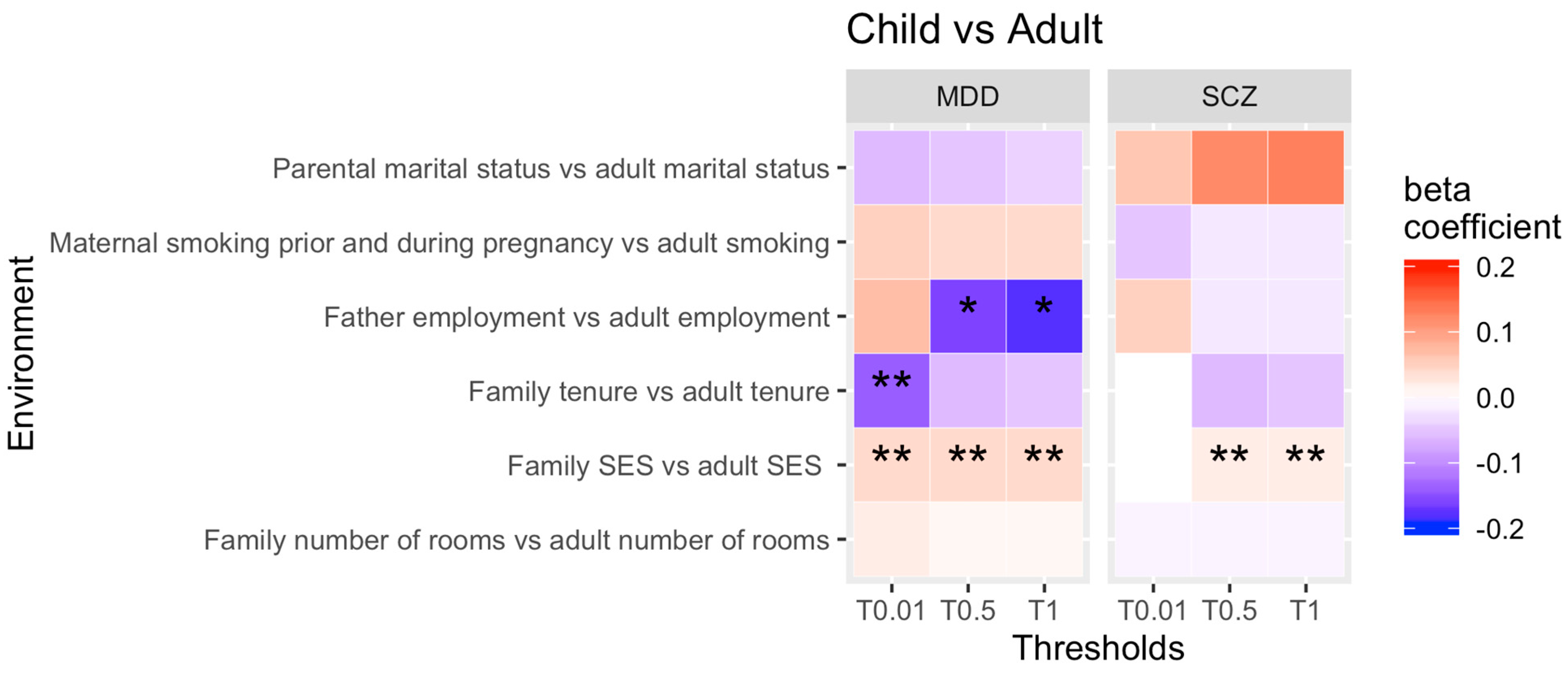
| Analysis | Economic Situation | Substance Abuse | Psychosocial Outcomes |
|---|---|---|---|
| Childhood rGE by time analysis | -SES, -tenure, -financial issues, -number of bedrooms, -employment | -maternal smoking, -maternal alcohol consumption, -paternal alcohol consumption | -parental marital status, -father’s involvement in the child’s upbringing, -maternal interest in the child’s education, -paternal interest in the child’s education, -mother takes child for walks, -father takes child for walks, -mother reads to child, -father reads to child |
| Adulthood rGE by time analysis | -SES, -tenure, -financial issues, -number of bedrooms, -employment, -income | -smoking | -marital status |
| Childhood vs. adulthood rGE by time analysis | -family SES in childhood vs. SES of individual in adulthood, -family tenure in childhood vs. tenure of individual in adulthood, -family number of bedrooms in childhood vs. number of bedrooms of individual in adulthood, -father’s employment in childhood vs. employment of individual in adulthood | -mother’s smoking behaviour prior and during pregnancy vs. smoking behaviour of individual during adulthood | -marital status of mother at birth vs. marital status of individual in adulthood |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machlitt-Northen, S.; Keers, R.; Munroe, P.B.; Howard, D.M.; Pluess, M. Gene–Environment Correlation over Time: A Longitudinal Analysis of Polygenic Risk Scores for Schizophrenia and Major Depression in Three British Cohorts Studies. Genes 2022, 13, 1136. https://doi.org/10.3390/genes13071136
Machlitt-Northen S, Keers R, Munroe PB, Howard DM, Pluess M. Gene–Environment Correlation over Time: A Longitudinal Analysis of Polygenic Risk Scores for Schizophrenia and Major Depression in Three British Cohorts Studies. Genes. 2022; 13(7):1136. https://doi.org/10.3390/genes13071136
Chicago/Turabian StyleMachlitt-Northen, Sandra, Robert Keers, Patricia B. Munroe, David M. Howard, and Michael Pluess. 2022. "Gene–Environment Correlation over Time: A Longitudinal Analysis of Polygenic Risk Scores for Schizophrenia and Major Depression in Three British Cohorts Studies" Genes 13, no. 7: 1136. https://doi.org/10.3390/genes13071136
APA StyleMachlitt-Northen, S., Keers, R., Munroe, P. B., Howard, D. M., & Pluess, M. (2022). Gene–Environment Correlation over Time: A Longitudinal Analysis of Polygenic Risk Scores for Schizophrenia and Major Depression in Three British Cohorts Studies. Genes, 13(7), 1136. https://doi.org/10.3390/genes13071136






