Abstract
Sexual regulation in Apis mellifera is controlled by the complementary sex-determiner (csd) gene: females (queens and workers) are heterozygous at this locus and males (drones) are hemizygous. When homozygous diploid drones develop, they are eaten by worker bees. High csd allelic diversity in honeybee populations is a priority for colony survival. The focus of this study is to investigate csd variability in the genomic sequence of the hypervariable region (HVR) of the csd gene in honeybee subspecies sampled in Italy. During the summer of 2017 and 2018, worker bees belonging to 125 colonies were sampled. The honeybees belonged to seven different A. mellifera subspecies: A. m. ligustica, A. m. sicula, A. m cecropia, A. m. carnica, A. m. mellifera, Buckfast and hybrid Carnica. Illumina genomic resequencing of all samples was performed and used for the characterization of global variability among colonies. In this work, a pipeline using existing resequencing data to explore the csd gene allelic variants present in the subspecies collection, based on de novo assembly of sequences falling within the HVR region, is described. On the whole, 138 allelic sequences were successfully reconstructed. Among these, 88 different alleles were identified, 68 of which match with csd alleles present in the NCBI GenBank database.
1. Introduction
Honeybees, like other hymenopteran insects, are haplodiploid: females (queen and workers) develop from fertilized diploid eggs, while males (drones) develop from unfertilized haploid eggs [1]. The complementary sex-determiner (csd) gene has been identified to be the primary gene involved in sexual regulation in honeybees [2]. Honeybees heterozygous at the csd locus develop into females, while haploid hemizygous bees develop as drones [3]. When diploid eggs are homozygous at the csd gene, diploid males are formed, but subsequently identified and eaten by worker bees [4]. The csd gene derives from its conserved paralog gene feminizer (fem) by gene duplication; fem regulates female development and is located 12 kb upstream of csd [5,6].
The csd gene is composed of nine exons divided into three clusters that encode an SR-type protein with a putative protein-binding function [2,7]. The third cluster (exons six to nine) contains a highly polymorphic region defined as the hypervariable region (HVR), which shows high variability and includes asparagine/tyrosine repeats varying significantly in number among alleles [2,8,9,10]. In 2013, Beye et al. determined that at least five amino acid differences and length variations between the two alleles of the same honeybee are sufficient to regularly induce the female pathway [8]. Initial findings estimated that the number of csd alleles in Apis mellifera populations ranged from 10 to 13 [11,12]. Subsequent studies have been conducted worldwide to identify the number of alleles circulating in the honeybee population, identifying a much higher number of alleles.
In detail, Cho et al. identified 18 distinct alleles by 27 workers sampled from a single hive in Michigan [13]. In Slovenia (Litija) and South Africa (Pretoria), Hasselmann found 15 csd alleles from hundreds of embryos collected from two colonies in each location [14]. In New Zealand, Hyink et al. screened the csd locus by sampling six purple eye drone pupae from 42 hives, obtaining both allele sequences from 35 of the 42 queens, and only one allele sequence from the remaining seven. Based on these 77 amino acid sequences, they identified 16 different alleles, six of which were new alleles that had not been sequenced previously [15]. In 2019, Kaskinova et al. sampled 42 drones belonging to 15 different colonies in Russia and found 20 different alleles [16]. In 2020, Kolics et al. analyzed, with a new bioinformatic approach, 12 bee samples (4 workers, 4 drones and 4 queens) from three different subspecies (A. m. carnica, A. m. ligustica and A. m. caucasica) and 8 samples of honey collected in China, Hungary, Japan and Georgia. Among these samples, they found a total of 25 csd alleles [17]. In 2021, Bovo et al. analyzed DNA from honey samples from 12 different colonies and identified 160 different csd alleles [18].
However, some studies propose that the number of circulating alleles in the worldwide honeybee population is significantly underestimated. Wang et al. found a total of 79 haplotypes by analyzing 50 workers for each of the six different Apis mellifera subspecies they sampled (A. m. anatolica, A. m. caucasica, A. m. carnica, A. m. carpatica, A. m. ssp. and A. m. ligustica) [19]. Lechner et al., using a dataset of 244 csd sequences sampled worldwide, demonstrated that the total number of csd alleles in A. mellifera ranges from 53 locally to 87 worldwide [9]. In 2017, Zareba et al., in agreement with Wang and Lechner, identified 121 different alleles by analyzing 193 colonies in Poland [20].
A recent comprehensive study conducted on 652 sequences found in GenBank confirmed that the number of circulating alleles is very high. In this set, which represents a vast geographic area and eight subspecies, 225 alleles were found, grouping sequences by identity and numbering them consecutively from the most common to the least (Amelcsd-HVR1-Amelcsd-HVR225) [21].
The high number of csd alleles reflects the high honeybee genetic diversity due to adaptation to different global environmental conditions [22]. However, high attention must be given to the number of circulating alleles in managed breeding honeybee populations.
Over the years, different approaches to determine csd alleles have been explored, usually based on Sanger sequencing. Currently, csd alleles of a diploid female honeybee are frequently determined through the analysis of csd sequences of six to eight male descendants by Sanger sequencing [15,19,20,23].
In 2020, Kolics et al. [17] used a high-throughput sequencing method to determine both csd alleles from honeybee queens and from honey samples. DNA was extracted from clipped wings of queen bees and worker bees, and locus targeted Illumina sequencing was performed, obtaining csd alleles directly from the diploid queens. In 2021, Bovo et al. [18] used a semiconductor-based sequencing technology (Ion Torrent) on honey samples to assess csd allelic variability from 12 different colonies. To further investigate the applicability of bioinformatic approaches for csd allele assessment and variability investigation from NGS data, we propose an alternative method based on local de novo assembly of Illumina reads matching the HVR, integrated by manual inspection and curation. The aim of this work was to exploit the existing Illumina genomic resequencing data collection described in Minozzi et al. [24] and retrieve csd HVR allele-specific sequences from diploid worker bees, thus describing the inter- and intra- genetic variability of seven Apis mellifera subspecies on 125 diploid worker bees sampled in different Italian regions.
2. Materials and Methods
2.1. Sampling
One hundred twenty-five worker colonies were sampled between summer 2017 and summer 2018 [24] in twelve different Italian regions: Abruzzo, Emilia Romagna, Liguria, Lombardy, Marche, Piedmont, Sicily, Trentino, Tuscany, Umbria and Veneto. Newly hatched worker bees were collected from each hive and used to perform a whole genome DNA sequencing analysis. Bees were individually placed in 1.5 mL Eppendorf tubes containing 95% ethanol and kept at −4 °C. The 125 colonies belonged to seven different subspecies [24], two autochthonous A. m. ligustica (n = 61) and A. m. sicula (n = 6); three allochthonous A. m. mellifera (n = 4), A. m. carnica (n = 8) and A. m. cecropia (n = 1); and two hybrids: A. m. carnica by A. m. ligustica hybrids (n = 2), and Buckfast bees (n = 43).
2.2. DNA Extraction, Library Preparation, Sequence Processing and Alignment
All details of DNA extraction to sequence processing and alignment can be found in Minozzi et al. [24]. Based on the existing alignment, a pipeline was set up to perform local de novo assembly of sequences falling within the HVR region and determine the csd gene allelic variants present in the subspecies collection.
2.3. De Novo Assembly and Analysis of Sample-Specific HVR Allele Consensus Sequences
Samtools was used to extract reads belonging to each sample, which mapped in the genomic interval corresponding to exon 7 of the csd gene, harboring the HVR (Hav3.1_NC_037640.1:11771976-11772119) [10]. Sample-specific fasta files containing all the sequences falling within the HVR were created, and the CAP3 Sequence Assembly Program (−o 40, −p 90) [25] was used to cluster sequences and generate contigs. The CAP3 output was filtered, and only samples assembled in 2 contigs and less than two singletons were kept. As no sequencing depth cut-off was imposed prior to the assembly procedure, manual inspection was performed on all assemblies to verify and validate the performance of the procedure and discard poorly supported assemblies. The two contigs sequences retrieved for each sample, supposed to represent the sequences of the two alleles, were translated into all six frames with EMBOSS transeq (https://www.ebi.ac.uk/Tools/st/emboss_transeq/, accessed on 28 April 2021), and the two in-frame translations matching the csd amino acid sequences were retrieved [10,13,15]. In-frame translations resulting in truncated polypeptides were discarded. Putative HVR amino acid allele sequences obtained for all samples where de novo allele reconstruction was successful were aligned with Clustal Omega [26]. The HVR sequence from Apis cerana (GenBank QKY64514.1) was added to the dataset to be used for tree rooting. PhyML 3.0 [27] was used to produce a phylogenetic tree using the CpREV model of amino acid substitution. ITOL [28] was used to display the tree.
CAP3-derived putative HVR amino acid allele sequences were used to blast the Apis mellifera NCBI nr database and retrieve csd HVR known sequences through tBLASTn (amino acid query versus translated database). Only identical hits (100% coverage and 100% similarity) were retained.
3. Results
3.1. Csd HVR Allele Reconstruction and Classification
The pipeline aiming at the reconstruction of csd alleles using genomic resequencing data was applied to sequences from 125 honeybee diploid worker bees. In detail, quality-trimmed reads were mapped to the Amel_Hav3.1 honeybee genome, and 5310 mapped reads falling within the HVR genome coordinates (Hav3.1_NC_037640.1:11771976-11772119) were extracted and split according to the sample of origin. Sample-specific CAP3 generated contigs were inspected, and only sequences from the 87 samples for which the assembly procedure resulted in two contigs and not more than one singleton were retained. The two contigs sequences retrieved for each sample, supposed to represent the sequences of the two alleles, were translated into proteins. One hundred seventy-four sequences gained from 87 diploid samples were then filtered to 154 sequences due to 20 polypeptide sequences being truncated. Thus, among these 154 sequences, only those belonging to the 69 samples showing both alleles successfully reconstructed were kept, leading to 138 sequences.
Among the 138 reconstructed sequences, we found 88 different alleles, 32 were shared in more than one sample, while 56 were unique in our pool of sequences. These 88 sequences, which encompass all the csd alleles represented in our colonies collection that were successfully reconstructed, are reported along with their sequence length in Table 1. Among the 88 alleles, 68 were found in the NCBI GenBank database by BLAST analysis, leading to the identification of 20 novel csd alleles (Supplementary File S1). Further, all the sequences retrieved from GenBank showing 100% identity to our 68 alleles are given in Table 1.

Table 1.
Amino acid sequences of alleles belonging to the hypervariable region of the csd gene, their lengths (L = nr of aa) and frequencies. If the allele was identified in GenBank, the accession numbers are shown (GenBank ID). In bold are the novel alleles identified in the study.
3.2. Phylogenetic Tree
A phylogenetic tree was constructed based on the 138 csd HVR amino acid sequences retrieved from the seven A. mellifera subspecies represented in our collection (Figure 1). The seven subspecies are from three different evolutionary lineages: in detail, A. m. sicula belongs to the African type (A); A. m. mellifera belongs to the western and northern Europe lineage (M); A. m. carnica, A. m. cecropia and A. m. ligustica belong to the eastern Europe group (C); Buckfast and hybrid Carnica are crosses of European subspecies belonging to C type (EC) [29]. A. cerana was used to root the tree. The honeybee samples sub-grouping does not strictly reflect the evolutionary lineages.
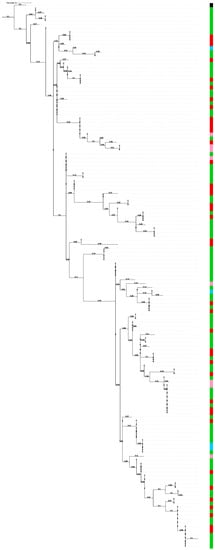
Figure 1.
Phylogenetic tree based on the amino acid sequence of the csd alleles HVR region in 7 different Italian honeybee subspecies. Colors are given according to evolutionary lineages: A. m. sicula belongs to the African type (pink); A. m. mellifera belongs to the western and northern Europe lineage (blue); A. m. carnica, A. m. cecropia and A. m. ligustica belong to the eastern Europe group (green); Buckfast and hybrid Carnica are crosses of European subspecies belonging to C type (red). The tree was rooted on Apis cerana (black).
3.3. Frequency Analysis
Figure 2 shows the Venn diagram of the allele distribution among subspecies. Of the 88 alleles (numbered from Allele 1 to Allele 88) reconstructed in our study, 70 are private alleles; 16 alleles are shared in two subspecies (2, 4, 7, 8, 9, 17, 18, 19, 21, 22, 24, 26, 27, 28, 29, 31), while 2 alleles (13, 16) are present in three subspecies (A. m. ligustica, A. m. carnica and Buckfast).
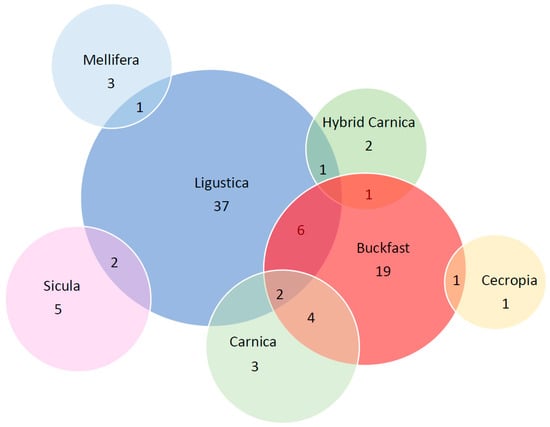
Figure 2.
Venn diagram of the csd alleles among the 7 honeybee subspecies.
Allele frequencies of alleles were computed and reported in Table 1. In detail, the most frequent allele in the entire population (allele 9, frequency 3.62%) is carried by five bees: four Buckfast and one A. m. ligustica.
The most frequent alleles within each subspecies were further explored:
- Among the 68 sequences belonging to 34 A. m. ligustica specimens, we identified 49 different alleles. Among these, alleles 1, 11 and 32 were the most frequent in A. m. ligustica.
- Among the 42 sequences belonging to 21 Buckfast individuals, we identified 33 different alleles. Allele 9 was the most frequent, with a frequency of 9.52%.
- Among the eight sequences belonging to four A. m. sicula, seven different alleles were identified; allele 30 was the most frequent, with a frequency of 25%,
- Among the 10 sequences belonging to five A. m. carnica, we identified nine different alleles with a frequency of 20%; allele 25 was the most frequent.
- The remaining three subspecies (hybrid Carnica, A. m. mellifera, A. m. cecropia) had a limited number of reconstructed csd HVR sequences (4, 4 and 2, respectively), each bearing a different allele.
4. Discussion
A reduction of the number of csd alleles in the population and, consequently, an increase in colony losses can cause problems to honeybee biodiversity conservation strategies as well as in honeybee-managed colonies. It is therefore essential to analyze, quantify and conserve the highest number and diversity of csd alleles in the honeybee populations worldwide.
In the first studies, csd alleles were determined by Sanger sequencing based on haploid drone samples [19,20] or by high-resolution melting analysis [15]. The first study to use high-throughput sequencing focusing on this locus showed an alternate method to Sanger sequencing: sample-specific amplified csd alleles were Illumina sequenced, clustered, compared to the reference honeybee csd sequence, filtered and translated to yield a discrete number of alleles [17]. The results, based on deep sequencing of the region under analysis, were supported by GenBank data gained using Sanger technology showing 100% identity. A recent study used Ion Torrent on honey samples to extract, trim and cluster reads, which were then translated to obtain csd alleles [18].
Our focus was to study csd variability by analyzing existing next-generation resequencing data through a semi-automated pipeline to reconstruct both csd alleles from diploid honey bees. The pipeline encompasses manual curation steps, which are essential due to the nature of the available data, which were produced with different aims and did not provide a sequencing depth compliant with conventional de novo assembly procedures. Nonetheless, in many situations, curated reference-independent sequence assembly was possible. In our study, csd HVR allele reconstruction was obtained for 70.4% of the total samples in our collection. Our results are supported by comparison to known alleles present in GenBank, showing 100% identity of 68 out of 88 alleles, the 20 remaining alleles representing variability at the csd HVR locus explored for the first time.
The 88 different csd alleles identified among the 138 reconstructed amino acid sequences belong to seven different honeybee subspecies; this number of alleles is in agreement with previous studies on large collections of bees, in which a large number of alleles were observed [9,19,20,21]. If we consider the ratio between the observed alleles and the total number of sequences analyzed, our result of 0.63 is comparable with other studies that report results on smaller sample sets. Cho et al. identified 18 distinct alleles, sampling 27 workers from a single hive; for this reason, they hypothesized that the total number of circulating csd alleles should be very high [13]. In 2019, Kaskinova et al. analyzed 15 different colonies (sampling 2-3 haploid drones per colony), finding 20 different HVR sequences with a ratio of 0.66 (20/30), which is comparable with our results [16]. Moreover, the 88 alleles found in our study differ in amino acid sequence and length. As shown in Table 1, the length varies from 27 to 50 amino acids, in agreement with Kaskinova et al. [16].
The phylogenetic tree (Figure 1) generated from all the reconstructed sequences at our disposal from the seven subspecies belonging to three different evolutionary lineages shows mixed distribution of the subspecies, forming clades that do not reflect the evolutionary lineages. Our result is similar to the phylogenetic tree constructed by Wang et al. on six different subspecies (A. m. anatolica, A. m. caucasica, A. m. carnica, A. m. carpatica, A. m. ssp. and A. m. ligustica) belonging to two different geographical types [19]. This result might be related to the high frequency of mutations of this locus that continuously create new variability [20].
However, it is interesting to note and observe from the Venn diagram that two alleles (alleles 13 and 16) are shared between three different subspecies A. m. ligustica, A. m. carnica and Buckfast. It is known that the three subspecies belong to the same evolutionary lineage C type (Figure 2) and often share the same geographical area. However, a phylogenetic tree based on a whole genome SNP dataset of the same sample set, excluding the A. m. cecropia sample [24], identified a distinctive subgrouping of samples based on their subspecies. Furthermore, as already stated by Kaskinova et al. [16], no correspondence was observed between the structure of the phylogenetic tree of csd haplotypes and the structure of the phylogenetic tree of honeybee subspecies obtained on the basis of the analysis of microsatellite loci and mitochondrial DNA. Our results further support the observation that this specific region, although being very variable, does not allow for discriminating among honeybee subspecies and consequently is not suitable for inferring phylogenetic relationships at the population level [14].
Furthermore, our results confirm the uneven distribution of csd alleles in Apis mellifera populations with many alleles present in a single bee (infrequent alleles) and other alleles that show a high frequency [20]. In detail, Allele 9 was identified in our study as the most frequent allele present in five samples belonging to two different subspecies. The same allele was identified in more than one sample in a consolidated report on csd alleles by Bilodeau et al. in 2021 (named Allele Amel Csd-HVR57 in their report) [21].
5. Conclusions
In conclusion, we used existing 150 bp Illumina whole genome resequencing data to explore the hypervariable region (HVR) of the csd gene of 125 diploid worker bees and investigated allelic variability in the complementary sex determiner gene (csd). We identified 88 different alleles, 68 of which match with csd alleles already present in the NCBI GenBank database, while 20 are newly described alleles. Given that the viability of the brood requires a high number of circulating alleles, high allelic variation should be a priority in both subspecies as well as in highly selected honeybee breeding lines. A representation of circulating alleles can be obtained by both traditional Sanger and NGS-based sequencing approaches. To this end, NGS-based csd assessment offers the availability of a method to rapidly generate csd alleles population information, starting from whole genome data created for selection. This information can enable beekeepers to monitor csd variability in selective breeding programs and to perform traditional selection at the same time. This solution should be actively promoted among bee-breeders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13060991/s1, Supplementary File S1: Nucleotide sequences of the novel csd hypervariable regions.
Author Contributions
Conceptualization, G.M. and B.L.; data curation, B.L.; formal analysis, G.P. (Gianluigi Paolillo); funding acquisition, G.P. (Giulio Pagnacco), G.M.; sampling, G.M.; methodology, A.S.; writing original draft, G.M., F.R., M.G.D.I. and B.L.; writing, review and editing, A.S., G.G. and J.F.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the BEENOMIX and BEENOMIX 2.0 projects funded by the Lombardy Region (FEASR program), PSR 2014–2020 (grant number 2016/00361532-G42F16000540002) and PSR (grant number 201801057971—G44I19001910002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Amino acid sequences of the csd alleles are given in the manuscript. Nucleotide sequences of the novel csd hypervariable regions described in this work are available in fasta format (Supplementary File S1), while raw genome data and phenotypes are part of a reference population used for selection by commercial breeders and have commercial value. Therefore, restrictions apply to the availability of these data, which are not publicly available. The authors can be contacted for a specific request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dzierzon, J. Neue verbesserte Bienen-Zucht des Pfarrers Dzierzon zu Carlsmarkt in Schlesien; Ernst’schen: Quedlinburg, Germany, 1861. [Google Scholar]
- Beye, M.; Hasselmann, M.; Fondrk, M.; Page, R.E.; Omholt, S.W. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 2003, 114, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Mackensen, O. Viability and sex determination in the honeybee (Apis mellifera L.). Genetics 1951, 36, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Woyke, J. What happens to diploid drone larvae in a honeybee colony. J. Apic. Res. 1963, 2, 73–75. [Google Scholar] [CrossRef]
- Hasselmann, M.; Gempe, T.; Schiott, M.; Nunes-Silva, C.G.; Otte, M.; Beye, M. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 2008, 454, 519–522. [Google Scholar] [CrossRef]
- Gempe, T.; Hasselmann, M.; Schiøtt, M.; Hause, G.; Otte, M.; Beye, M. Sex determination in honeybees: Two separate mechanisms induce and maintain the female pathway. PLoS Biol. 2009, 7, e1000222. [Google Scholar] [CrossRef] [Green Version]
- Hasselmann, M.; Beye, M. Signatures of selection among sex-determining alleles of the honey bee. Proc. Natl. Acad. Sci. USA 2004, 101, 4888–4893. [Google Scholar] [CrossRef] [Green Version]
- Beye, M.; Seelmann, C.; Gempe, T.; Hasselmann, M.; Vekemans, X.; Fondrk, M.K.; Page, R.E., Jr. Gradual molecular evolution of a sex determination switch through incomplete penetrance of femaleness. Curr. Biol. 2013, 23, 2559–2564. [Google Scholar] [CrossRef] [Green Version]
- Lechner, S.; Ferretti, L.; Schöning, C.; Kinuthia, W.; Willemsen, D.; Hasselmann, M. Nucleotide variability at its limit? Insights into the number and evolutionary dynamics of the sex-determining specificities of the honey bee Apis mellifera. Mol. Biol. Evol. 2014, 31, 272–287. [Google Scholar]
- Kaskinova, M.D.; Nikolenko, A.G. Csd gene of honeybee: Genetic structure, functioning, and evolution. Russ. J. Genet. 2017, 53, 297–301. [Google Scholar] [CrossRef]
- Laidlaw, H.H.; Gomes, F.P.; Kerr, W.E. Estimation of the number of lethal alleles in a panmitic population of Apis mellifera L. Genetics 1956, 41, 179–188. [Google Scholar] [CrossRef]
- Adams, J.; Rothman, E.D.; Kerr, W.E.; Paulino, Z.L. Estimation of the number of sex alleles and queen matings from diploid male frequencies in a population of Apis mellifera. Genetics 1977, 86, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Huang, Z.Y.; Green, D.R.; Smith, D.R.; Zhang, J. Evolution of the complementary sex-determination gene of honey bees: Balancing selection and trans-species polymorphisms. Genome Res. 2006, 16, 1366–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselmann, M.; Vekemans, X.; Pflugfelder, J.; Koeniger, N.; Koeniger, G.; Tingek, S.; Beye, M. Evidence for convergent nucleotide evolution and high allelic turnover rates at the complementary sex-determiner gene of western and asian honeybees. Mol. Biol. Evol. 2008, 25, 696–708. [Google Scholar] [CrossRef] [Green Version]
- Hyink, O.; Laas, F.; Dearden, P.K. Genetic tests for alleles of complementary sex-determiner to support honeybee breeding programmes. Apidologie 2013, 44, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Kaskinova, M.D.; Gataullina, A.R.; Saltykova, E.S.; Gaifullinaa, L.R.; Poskryakov, A.V.; Nikolenko, A.G. Polymorphism of the Hypervariable Region of the csd gene in the Apis mellifera L. population in Southern Ural. Russian J. Gen. 2019, 55, 267–270. [Google Scholar]
- Kolics, É.; Parrag, T.; Házi, F.; Szepesi, K.; Heltai, B.; Mátyás, K.; Kutasy, B.; Virág, E.; Taller, J.; Orbán, L.; et al. An alternative, high throughput method to identify csd alleles of the honey bee. Insects 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Bovo, S.; Ribani, A.; Utzeri, V.J.; Taurisano, V.; Schiavo, G.; Bolner, M.; Fontanesi, L. Application of Next Generation semiconductor-based sequencing for the identification of Apis mellifera complementary sex-determiner (csd) alleles from honey DNA. Insects 2021, 12, 868. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Wu, X.; Yan, W.; Zeng, Z. Polymorphism analysis of csd gene in six Apis mellifera subspecies. Mol. Biol. Rep. 2012, 39, 3067–3071. [Google Scholar] [CrossRef]
- Zareba, J.; Blazej, P.; Laszkiewicz, A.; Sniezewski, L.; Majkowski, M.; Janik, S.; Cebrat, M. Uneven distribution of complementary sex-determiner (csd) alleles in Apis mellifera population. Sci. Rep. 2017, 7, 2317. [Google Scholar] [CrossRef]
- Bilodeau, L.; Elsik, C. A scientific note defining allelic nomenclature standards for the highly diverse complementary sex-determiner (csd) locus in honey bees. Apidologie 2021, 52, 749–754. [Google Scholar] [CrossRef]
- Wallberg, A.; Han, F.; Wellhagen, G.; Dahle, B.; Kawata, M.; Haddad, N.; Simões, Z.L.P.; Allsopp, M.H.; Kandemir, I.; De La Rúa, P.; et al. Worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis Mellifera. Nat. Genet. 2014, 46, 1081–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridi, R.; Tabet Aoul, N.; Catays, G.; Basso, B.; Bienefeld, K.; Gregorc, A.; Vignal, A.; Canale-Tabet, K. Genetic diversity and population genetic structure analysis of Apis mellifera subspecies in Algeria and Europe based on complementary sex-determiner (csd) gene. Apidologie 2022, 53, 4. [Google Scholar] [CrossRef]
- Minozzi, G.; Lazzari, B.; De Iorio, M.G.; Costa, C.; Carpana, E.; Crepaldi, P.; Rizzi, R.; Facchini, E.; Gandini, G.; Stella, A.; et al. Whole-Genome sequence analysis of Italian honeybees (Apis mellifera). Animals 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. Meth. Mol. Biol. 2014, 1079, 105–116. [Google Scholar]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Franck, P.; Garnery, L.; Celebrano, G.; Solignac, M.; Cornuet, J.M. Hybrid origins of honeybees from Italy (Apis mellifera ligustica) and Sicily (A. m. sicula). Mol. Ecol. 2000, 9, 907–921. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).