Dominant Stickler Syndrome
Abstract
1. Introduction
2. Historical Overview
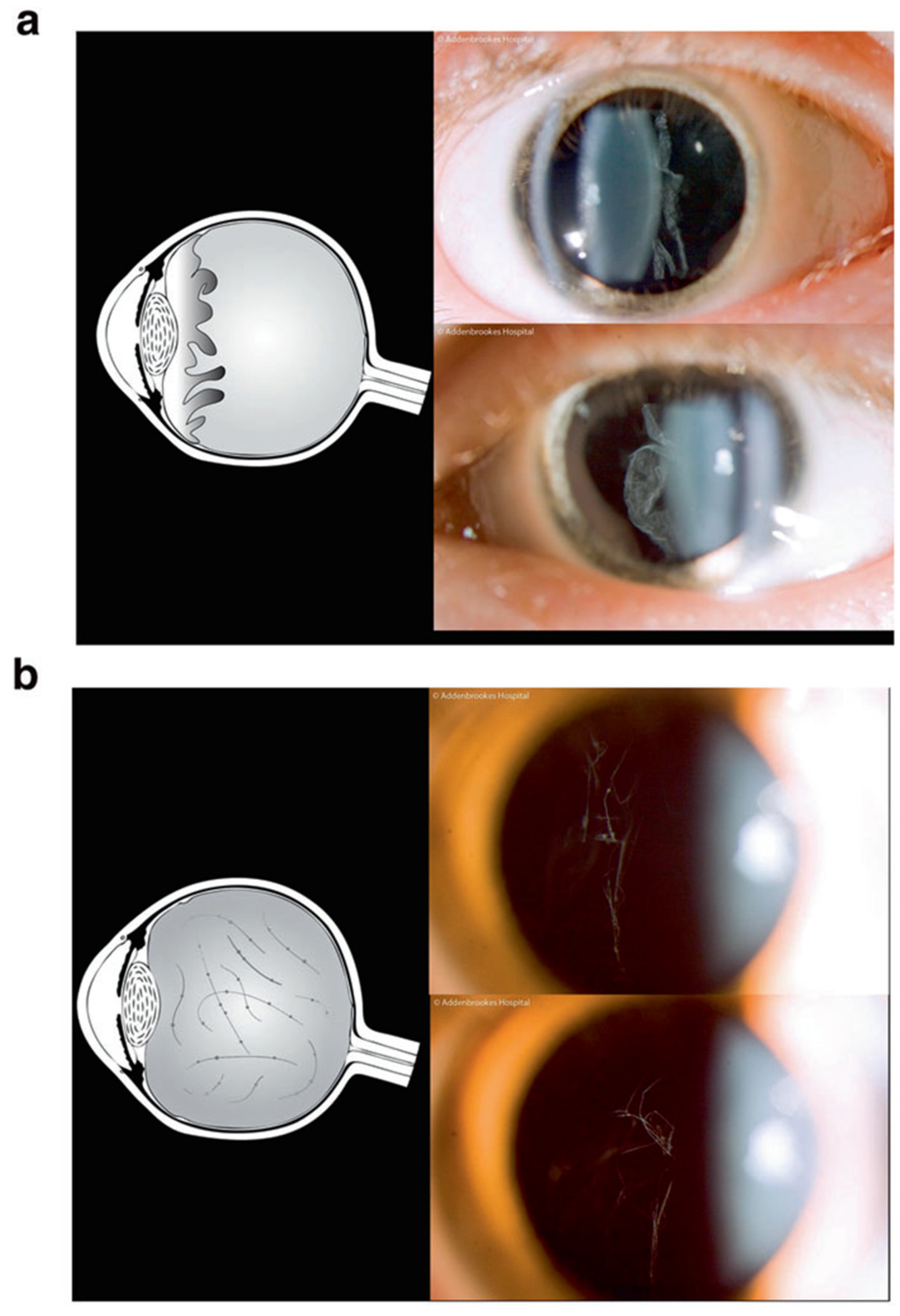
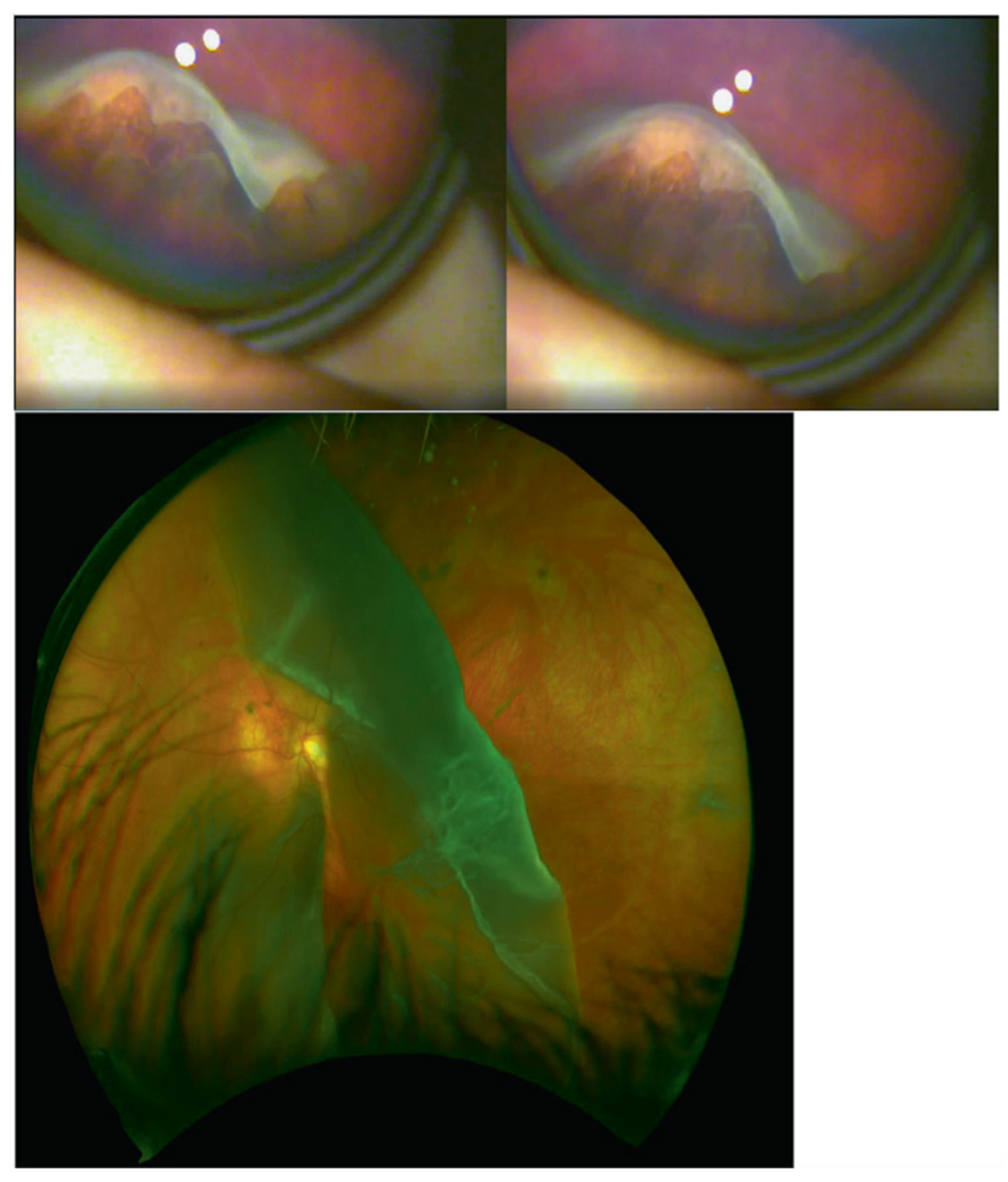
3. Clinical Features
- (i)
- Infants with a history of congenital myopia in association with deafness
- (ii)
- Infants born with cleft palate or Pierre Robin Sequence in association with myopia
- (iii)
- Infants with joint hypermobility and/or epiphyseal dysplasia in association with myopia
- (iv)
- Individuals suffering rhegmatogenous retinal detachment with a family history of rhegmatogenous retinal detachment.
4. Differential Diagnosis
5. Genotype and Phenotype
5.1. Type 1 AD Stickler Syndrome
5.1.1. Nonsense-Mediated Decay (Haploinsufficiency)
5.1.2. Effects on Phenotype
5.2. Ocular-Only AD Stickler
5.3. Type 2 AD Stickler Syndrome
5.3.1. Exon Skipping and Missense Mutations (Dominant Negative Effect)
5.3.2. Effects on Phenotype
5.4. Type 3 AD Stickler Syndrome (Non-Ocular)
5.4.1. Exon Skipping and Missense Mutations (Dominant Negative Effect)
5.4.2. Effects on Phenotype
5.5. Type 7 AD Stickler Syndrome
5.5.1. Severe Truncation (Haploinsufficiency)
5.5.2. Effects on Phenotype
6. Conclusions
- Creation of premature stop codon through nonsense or frameshift mutations (possibly through affecting splicing) leading to nonsense-mediated decay;
- Loss-of-function mutations that prevent the mutant protein from interacting with other proteins.
- 3.
- Missense or nucleotide adding/deleting mutations that produce an in-frame transcript.
- 4.
- Nonsense mutations that lead to nonsense-associated altered splicing and thus exon skipping.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robin, N.H.; Moran, R.T.; Ala-Kokko, L. Stickler Syndrome. In GeneReviews(®); University of Washington: Washington, DC, USA, 2021. [Google Scholar]
- Snead, M.; Martin, H.; Bale, P.; Shenker, N.; Baguley, D.; Alexander, P.; McNinch, A.; Poulson, A. Therapeutic and diagnostic advances in Stickler syndrome. Ther. Adv. Rare Disease 2020, 1, 2633004020978661. [Google Scholar] [CrossRef]
- Stickler, G.B.; Belau, P.G.; Farrell, F.J.; Jones, J.D.; Pugh, D.G.; Steinberg, A.G.; Ward, L.E. Hereditary Progressive Arthro-Ophthalmopathy. Mayo Clin. Proc. 1965, 40, 433–455. [Google Scholar] [PubMed]
- Francomano, C.A.; Liberfarb, R.M.; Hirose, T.; Maumenee, I.H.; Streeten, E.A.; Meyers, D.A.; Pyeritz, R.E. The Stickler syndrome: Evidence for close linkage to the structural gene for type II collagen. Genomics 1987, 1, 293–296. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Ala-Kokko, L.; Knowlton, R.G.; Jimenez, S.A.; Weaver, E.J.; Maguire, J.I.; Tasman, W.; Prockop, D.J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc. Natl. Acad. Sci. USA 1991, 88, 6624–6627. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Ganguly, A.; Considine, E.; McCarron, S.; Prockop, D.J.; Walsh-Vockley, C.; Michels, V.V. A-2-->G transition at the 3’ acceptor splice site of IVS17 characterizes the COL2A1 gene mutation in the original Stickler syndrome kindred. Am J. Med. Genet. 1996, 63, 461–467. [Google Scholar] [CrossRef]
- Snead, M.P.; Payne, S.J.; Barton, D.E.; Yates, J.R.; al-Imara, L.; Pope, F.M.; Scott, J.D. Stickler syndrome: Correlation between vitreoretinal phenotypes and linkage to COL 2A1. Eye 1994, 8 Pt 6, 609–614. [Google Scholar] [CrossRef]
- Richards, A.J.; Yates, J.R.; Williams, R.; Payne, S.J.; Pope, F.M.; Scott, J.D.; Snead, M.P. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Hum. Mol. Genet. 1996, 5, 1339–1343. [Google Scholar] [CrossRef]
- Snead, M.P.; Yates, J.R.; Williams, R.; Payne, S.J.; Pope, F.M.; Scott, J.D. Stickler syndrome type 2 and linkage to the COL11A1 gene. Ann. N. Y. Acad. Sci. 1996, 785, 331–332. [Google Scholar] [CrossRef]
- Brunner, H.G.; van Beersum, S.E.; Warman, M.L.; Olsen, B.R.; Ropers, H.H.; Mariman, E.C. A Stickler syndrome gene is linked to chromosome 6 near the COL11A2 gene. Hum. Mol. Genet. 1994, 3, 1561–1564. [Google Scholar] [CrossRef]
- Sirko-Osadsa, D.A.; Murray, M.A.; Scott, J.A.; Lavery, M.A.; Warman, M.L.; Robin, N.H. Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha2(XI) chain of type XI collagen. J. Pediatr. 1998, 132, 368–371. [Google Scholar] [CrossRef]
- Snead, M.P.; McNinch, A.M.; Poulson, A.V.; Bearcroft, P.; Silverman, B.; Gomersall, P.; Parfect, V.; Richards, A.J. Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye 2011, 25, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Snead, M.P.; Richards, A.J.; McNinch, A.M.; Alexander, P.; Martin, H.; Nixon, T.R.W.; Bale, P.; Shenker, N.; Brown, S.; Blackwell, A.M.; et al. Stickler syndrome-lessons from a national cohort. Eye 2021, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ihanamäki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog; Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C. Loeys-Dietz Syndrome. In GeneReviews(®); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Washington, DC, USA, 1993. [Google Scholar]
- Van Laer, L.; Dietz, H.; Loeys, B. Loeys-Dietz syndrome. Adv. Exp. Med. Biol. 2014, 802, 95–105. [Google Scholar] [CrossRef]
- Kloeckener-Gruissem, B.; Amstutz, C. VCAN-Related Vitreoretinopathy. In GeneReviews(®); University of Washington: Washington, DC, USA, 2016. [Google Scholar]
- Meredith, S.P.; Richards, A.J.; Flanagan, D.W.; Scott, J.D.; Poulson, A.V.; Snead, M.P. Clinical characterisation and molecular analysis of Wagner syndrome. Br. J. Ophthalmol. 2007, 91, 655–659. [Google Scholar] [CrossRef]
- Hoornaert, K.P.; Marik, I.; Kozlowski, K.; Cole, T.; Le Merrer, M.; Leroy, J.G.; Coucke, P.J.; Sillence, D.; Mortier, G.R. Czech dysplasia metatarsal type: Another type II collagen disorder. Eur. J. Hum. Genet. 2007, 15, 1269–1275. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Sun, L.; Yan, W.; Huang, L.; Zhang, Z.; Zhang, T.; Ding, X. Knobloch Syndrome Associated with Novel COL18A1 Variants in Chinese Population. Genes 2021, 12, 1512. [Google Scholar] [CrossRef]
- Richards, A.J.; Meredith, S.; Poulson, A.; Bearcroft, P.; Crossland, G.; Baguley, D.M.; Scott, J.D.; Snead, M.P. A novel mutation of COL2A1 resulting in dominantly inherited rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci. 2005, 46, 663–668. [Google Scholar] [CrossRef]
- Spickett, C.; Hysi, P.; Hammond, C.J.; Prescott, A.; Fincham, G.S.; Poulson, A.V.; McNinch, A.M.; Richards, A.J.; Snead, M.P. Deep Intronic Sequence Variants in COL2A1 Affect the Alternative Splicing Efficiency of Exon 2, and May Confer a Risk for Rhegmatogenous Retinal Detachment. Hum. Mutat. 2016, 37, 1085–1096. [Google Scholar] [CrossRef][Green Version]
- Robinson, M.K.; Coe, K.; Bradshaw, W.T. A Case Report of Donnai-Barrow Syndrome. Adv. Neonatal. Care 2021, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, S.; Al-Gazali, L.; Hill, R.S.; Donnai, D.; Black, G.C.; Bieth, E.; Chassaing, N.; Lacombe, D.; Devriendt, K.; Teebi, A.; et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet. 2007, 39, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Grosel, J.M. A review of Ehlers-Danlos syndrome. Jaapa 2020, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.J.; McNinch, A.; Martin, H.; Oakhill, K.; Rai, H.; Waller, S.; Treacy, B.; Whittaker, J.; Meredith, S.; Poulson, A.; et al. Stickler syndrome and the vitreous phenotype: Mutations in COL2A1 and COL11A1. Hum. Mutat. 2010, 31, E1461–E1471. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.J.; Schmid, T.M.; Eyre, D.R. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur. J. Biochem. 2003, 270, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.J.; Laidlaw, M.; Whittaker, J.; Treacy, B.; Rai, H.; Bearcroft, P.; Baguley, D.M.; Poulson, A.; Ang, A.; Scott, J.D.; et al. High efficiency of mutation detection in type 1 stickler syndrome using a two-stage approach: Vitreoretinal assessment coupled with exon sequencing for screening COL2A1. Hum. Mutat. 2006, 27, 696–704. [Google Scholar] [CrossRef]
- Richards, A.J.; Laidlaw, M.; Meredith, S.P.; Shankar, P.; Poulson, A.V.; Scott, J.D.; Snead, M.P. Missense and silent mutations in COL2A1 result in Stickler syndrome but via different molecular mechanisms. Hum. Mutat. 2007, 28, 639. [Google Scholar] [CrossRef]
- Richards, A.J.; Snead, M.P. The influence of pre-mRNA splicing on phenotypic modification in Stickler’s syndrome and other type II collagenopathies. Eye 2008, 22, 1243–1250. [Google Scholar] [CrossRef][Green Version]
- Richards, A.J.; Baguley, D.M.; Yates, J.R.; Lane, C.; Nicol, M.; Harper, P.S.; Scott, J.D.; Snead, M.P. Variation in the vitreous phenotype of Stickler syndrome can be caused by different amino acid substitutions in the X position of the type II collagen Gly-X-Y triple helix. Am. J. Hum. Genet. 2000, 67, 1083–1094. [Google Scholar] [CrossRef][Green Version]
- Knutsson, J.; Bagger-Sjöbäck, D.; von Unge, M. Collagen type distribution in the healthy human tympanic membrane. Otol. Neurotol. 2009, 30, 1225–1229. [Google Scholar] [CrossRef]
- Hall, J.G.; Herrod, H. The Stickler syndrome presenting as a dominantly inherited cleft palate and blindness. J. Med. Genet. 1975, 12, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Ars, B.; Dirckx, J. Eustachian Tube Function. Otolaryngol. Clin. N. Am. 2016, 49, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.J.; Martin, S.; Yates, J.R.; Scott, J.D.; Baguley, D.M.; Pope, F.M.; Snead, M.P. COL2A1 exon 2 mutations: Relevance to the Stickler and Wagner syndromes. Br. J. Ophthalmol. 2000, 84, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Acke, F.R.; Malfait, F.; Vanakker, O.M.; Steyaert, W.; De Leeneer, K.; Mortier, G.; Dhooge, I.; De Paepe, A.; De Leenheer, E.M.; Coucke, P.J. Novel pathogenic COL11A1/COL11A2 variants in Stickler syndrome detected by targeted NGS and exome sequencing. Mol. Genet. Metab. 2014, 113, 230–235. [Google Scholar] [CrossRef]
- Richards, A.J.; Fincham, G.S.; McNinch, A.; Hill, D.; Poulson, A.V.; Castle, B.; Lees, M.M.; Moore, A.T.; Scott, J.D.; Snead, M.P. Alternative splicing modifies the effect of mutations in COL11A1 and results in recessive type 2 Stickler syndrome with profound hearing loss. J. Med. Genet. 2013, 50, 765–771. [Google Scholar] [CrossRef]
- Luo, Y.; Karsdal, M. Type XI Collagen. In Biochemistry of Collagens, Laminins and Elastin; Academic Press: Cambridge, MA, USA, 2016; pp. 77–80. [Google Scholar]
- Acke, F.R.; Dhooge, I.J.; Malfait, F.; De Leenheer, E.M. Hearing impairment in Stickler syndrome: A systematic review. Orphanet. J. Rare Dis. 2012, 7, 84. [Google Scholar] [CrossRef]
- Shpargel, K.B.; Makishima, T.; Griffith, A.J. Col11a1 and Col11a2 mRNA expression in the developing mouse cochlea: Implications for the correlation of hearing loss phenotype with mutant type XI collagen genotype. Acta Otolaryngol. 2004, 124, 242–248. [Google Scholar] [CrossRef]
- Alexander, P.; Gomersall, P.; Stancel-Lewis, J.; Fincham, G.S.; Poulson, A.; Richards, A.; McNinch, A.; Baguley, D.M.; Snead, M. Auditory dysfunction in type 2 Stickler Syndrome. Eur. Arch. Otorhinolaryngol. 2021, 278, 2261–2268. [Google Scholar] [CrossRef]
- Iwasa, Y.; Moteki, H.; Hattori, M.; Sato, R.; Nishio, S.Y.; Takumi, Y.; Usami, S. Non-ocular Stickler syndrome with a novel mutation in COL11A2 diagnosed by massively parallel sequencing in Japanese hearing loss patients. Ann. Otol. Rhinol Laryngol 2015, 124 (Suppl. S1), 111s–117s. [Google Scholar] [CrossRef]
- Vuoristo, M.M.; Pappas, J.G.; Jansen, V.; Ala-Kokko, L. A stop codon mutation in COL11A2 induces exon skipping and leads to non-ocular Stickler syndrome. Am. J. Med. Genet. A 2004, 130, 160–164. [Google Scholar] [CrossRef]
- Lawrence, E.A.; Kague, E.; Aggleton, J.A.; Harniman, R.L.; Roddy, K.A.; Hammond, C.L. The mechanical impact of col11a2 loss on joints; col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170335. [Google Scholar] [CrossRef] [PubMed]
- Nixon, T.R.W.; Richards, A.; Towns, L.K.; Fuller, G.; Abbs, S.; Alexander, P.; McNinch, A.; Sandford, R.N.; Snead, M.P. Bone morphogenetic protein 4 (BMP4) loss-of-function variant associated with autosomal dominant Stickler syndrome and renal dysplasia. Eur. J. Hum. Genet. 2019, 27, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.; Tyler, R.C.; Schilter, K.F.; Abdul-Rahman, O.; Innis, J.W.; Kozel, B.A.; Schneider, A.S.; Bardakjian, T.M.; Lose, E.J.; Martin, D.M.; et al. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum. Genet. 2011, 130, 495–504. [Google Scholar] [CrossRef]
- Behesti, H.; Holt, J.K.; Sowden, J.C. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev. Biol. 2006, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeifar, M.; Schlingmann, K.P.; Litwin, M.; Emre, S.; Bakkaloglu, A.; Mehls, O.; Antignac, C.; Schaefer, F.; Weber, S. Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr. Nephrol. 2009, 24, 2361–2368. [Google Scholar] [CrossRef]


| Subtype of AD Stickler Syndrome | Gene | Cytogenetic Location | Distinguishing Features | Phenotype MIM no. |
|---|---|---|---|---|
| Type 1 | COL2A1 | 12q13.11 | Type 1 membranous congenital vitreous anomaly, retinal detachment, congenital megalophthalmos, deafness, arthropathy, cleft palate. High risk of blindness | 108,300 |
| Ocular-only | COL2A1 | 12q13.11 | Type 1 membranous congenital vitreous anomaly, retinal detachment, congenital megalophthalmos. No systemic features. High risk of blindness | 609,508 |
| Type 2 | COL11A1 | 1p21.1 | Beaded type 2 congenital vitreous anomaly, retinal detachment, congenital megalophthalmos, deafness, arthropathy, cleft palate. | 604,841 |
| Type 3 (dominant OSMED) | COL11A2 | 6p21.32 | Non-ocular form of Stickler syndrome. Normal vitreous and ocular phenotype, deafness, arthropathy, cleft palate. | 184,840 |
| Type 7 | BMP4 | 14q22.2 | Hypoplastic vitreous, retinal detachment, deafness, arthropathy, palate abnormality, renal dysplasia. | To be confirmed |
| Product of Wild-Type Allele | Product of Mutated Allele | Collagen Trimer Product (Possibility 1) | Collagen Trimer Product (Possibility 2) | Phenotypic Result of the Pathogenic Variant |
|---|---|---|---|---|
 | No product | 50% of normal amount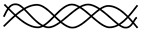 | Haploinsufficiency | |
 |  | 7/8 chance of abnormal trimer  | 1/8 chance of normal trimer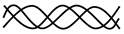 | Dominant negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soh, Z.; Richards, A.J.; McNinch, A.; Alexander, P.; Martin, H.; Snead, M.P. Dominant Stickler Syndrome. Genes 2022, 13, 1089. https://doi.org/10.3390/genes13061089
Soh Z, Richards AJ, McNinch A, Alexander P, Martin H, Snead MP. Dominant Stickler Syndrome. Genes. 2022; 13(6):1089. https://doi.org/10.3390/genes13061089
Chicago/Turabian StyleSoh, Zack, Allan J. Richards, Annie McNinch, Philip Alexander, Howard Martin, and Martin P. Snead. 2022. "Dominant Stickler Syndrome" Genes 13, no. 6: 1089. https://doi.org/10.3390/genes13061089
APA StyleSoh, Z., Richards, A. J., McNinch, A., Alexander, P., Martin, H., & Snead, M. P. (2022). Dominant Stickler Syndrome. Genes, 13(6), 1089. https://doi.org/10.3390/genes13061089






