Prevalence of Pathogenic Germline BRCA1/2 Variants and Their Association with Clinical Characteristics in Patients with Epithelial Ovarian Cancer in a Rural Area of Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Description
2.2. Clinicopathological Data
2.3. gBRCA1/2 Analysis
2.4. Statistical Analysis
3. Results
3.1. Description of the Study Population
3.2. gBRCA1/2 PV Detected in OC Cases
3.3. Association between gBRCA1/2 PV and Clinicopathological Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.-E.; Fabbro, M.; Theillet, C.; Bibeau, F.; Rouanet, P.; Ray-Coquard, I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Calò, V.; Bruno, L.; Rizzo, S.; Bazan, V.; Di Fede, G. Hereditary ovarian cancer. Crit. Rev. Oncol. Hematol. 2009, 69, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Kanchi, K.L.; Johnson, K.J.; Lu, C.; McLellan, M.D.; Leiserson, M.D.M.; Wendl, M.C.; Zhang, Q.; Koboldt, D.C.; Xie, M.; Kandoth, C.; et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat. Commun. 2014, 5, 3156. [Google Scholar] [CrossRef]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Hirasawa, A.; Imoto, I.; Naruto, T.; Akahane, T.; Yamagami, W.; Nomura, H.; Masuda, K.; Susumu, N.; Tsuda, H.; Aoki, D. Prevalence of pathogenic germline variants detected by multigene sequencing in unselected Japanese patients with ovarian cancer. Oncotarget 2017, 8, 112258–112267. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, T.; Aoki, D.; Hattori, K.; Jinushi, M.; Kigawa, J.; Takeshima, N.; Tsuda, H.; Watanabe, Y.; Yoshihara, K.; Sugiyama, T. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: Characterizing the cross-sectional approach to ovarian cancer genetic testing of BRCA (CHARLOTTE). Int. J. Gynecol. Cancer 2019, 29, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, S.; Kang, E.; Park, S.; Kim, Z.; Lee, M.H.; Korean Breast Cancer Society. Influence of the Angelina Jolie announcement and insurance reimbursement on practice patterns for hereditary breast cancer. J. Breast Cancer 2017, 20, 203–207. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. (Eds.) WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; IARC: Lyon, France, 2014. [Google Scholar]
- Okamoto, N.; Naruto, T.; Kohmoto, T.; Komori, T.; Imoto, I. A novel PTCH1 mutation in a patient with Gorlin syndrome. Hum. Genome Var. 2014, 1, 14022. [Google Scholar] [CrossRef]
- Watanabe, M.; Hayabuchi, Y.; Ono, A.; Naruto, T.; Horikawa, H.; Kohmoto, T.; Masuda, K.; Nakagawa, R.; Ito, H.; Kagami, S.; et al. Detection of 1p36 deletion by clinical exome-first diagnostic approach. Hum. Genome Var. 2016, 3, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Nakagawa, R.; Naruto, T.; Kohmoto, T.; Suga, K.-I.; Goji, A.; Kagami, S.; Masuda, K.; Imoto, I. A novel missense mutation of COL5A2 in a patient with Ehlers-Danlos syndrome. Hum. Genome Var. 2016, 3, 16030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 2015, 313, 1347–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, A.; Yokoyama, S.; Iwata, H.; Takaiso, N.; Nomizu, T.; Arai, M.; Nakamura, S. Incidence of contralateral and ipsilateral breast cancers and prognosis in BRCA1/2 pathogenic variant carriers based on the Japanese HBOC Consortium registration. J. Hum. Genet. 2021, 66, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chang, T.C.; Lapke, N.; Jung, S.M.; Chi, P.; Chen, C.H.; Yang, L.Y.; Lin, C.T.; Huang, H.J.; Chou, H.H.; et al. Prevalence and clinical significance of BRCA1/2 germline and somatic mutations in Taiwanese patients with ovarian cancer. Oncotarget 2016, 7, 85529–85541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, N.; Huang, G.; Scambia, G.; Chalas, E.; Pignata, S.; Fiorica, J.; Van Le, L.; Ghamande, S.; González-Santiago, S.; Bover, I.; et al. Evaluation of a streamlined oncologist-led BRCA mutation testing and counseling model for patients with ovarian cancer. J. Clin. Oncol. 2018, 36, 1300–1307. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Brady, W.E.; Farley, J.; Armstrong, A.; Uyar, D.S.; Gershenson, D.M. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001). Gynecol. Oncol. 2018, 150, 9–13. [Google Scholar] [CrossRef]
- Sekine, M.; Nishino, K.; Enomoto, T. Differences in ovarian and other cancers risks by population and BRCA mutation location. Genes 2021, 12, 1050. [Google Scholar] [CrossRef]
- Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br. J. Cancer 2000, 83, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momozawa, Y.; Iwasaki, Y.; Parsons, M.T.; Kamatani, Y.; Takahashi, A.; Tamura, C.; Katagiri, T.; Yoshida, T.; Nakamura, S.; Sugano, K.; et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat. Commun. 2018, 9, 4083. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Enomoto, T.; Arai, M.; Yokoyama, S.; Nomura, H.; Nishino, K.; Ikeuchi, T.; Kuriyama, Y.; Nakamura, S.; Nomizu, T.; et al. Correlation between the risk of ovarian cancer and BRCA recurrent pathogenic variants in Japan. J. Hum. Genet 2022, 67, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Enomoto, T.; Aoki, D.; Watanabe, Y.; Kigawa, J.; Takeshima, N.; Inomata, H.; Hattori, K.; Jinushi, M.; Tsuda, H.; et al. Association of gBRCA1/2 mutation locations with ovarian cancer risk in Japanese patients from the CHARLOTTE study. Cancer Sci. 2020, 111, 3350–3358. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Safra, T.; Lai, W.C.; Borgato, L.; Nicoletto, M.O.; Berman, T.; Reich, E.; Alvear, M.; Haviv, I.; Muggia, F.M. BRCA mutations and outcome in epithelial ovarian cancer (EOC): Experience in ethnically diverse groups. Ann. Oncol. 2013, 24 (Suppl S8), viii63–viii68. [Google Scholar] [CrossRef]
- Majdak, E.J.; Debniak, J.; Milczek, T.; Cornelisse, C.J.; Devilee, P.; Emerich, J.; Jassem, J.; De Bock, G.H. Prognostic impact of BRCA1 pathogenic and BRCA1/BRCA2 unclassified variant mutations in patients with ovarian carcinoma. Cancer 2005, 104, 1004–1012. [Google Scholar] [CrossRef]
- Xu, K.; Yang, S.; Zhao, Y. Prognostic significance of BRCA mutations in ovarian cancer: An updated systematic review with meta-analysis. Oncotarget 2017, 8, 285–302. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J. Ovarian Res. 2019, 12, 40. [Google Scholar] [CrossRef]
- Zhong, Q.; Peng, H.-L.; Zhao, X.; Zhang, L.; Hwang, W.-T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: A meta-analysis. Clin. Cancer Res. 2015, 21, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.S.; Rothermundt, C.; Thomas, K.; Bancroft, E.; Eeles, R.; Shanley, S.; Ardern-Jones, A.; Norman, A.; Kaye, S.B.; Gore, M.E. “BRCAness” syndrome in ovarian cancer: A case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J. Clin. Oncol. 2008, 26, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
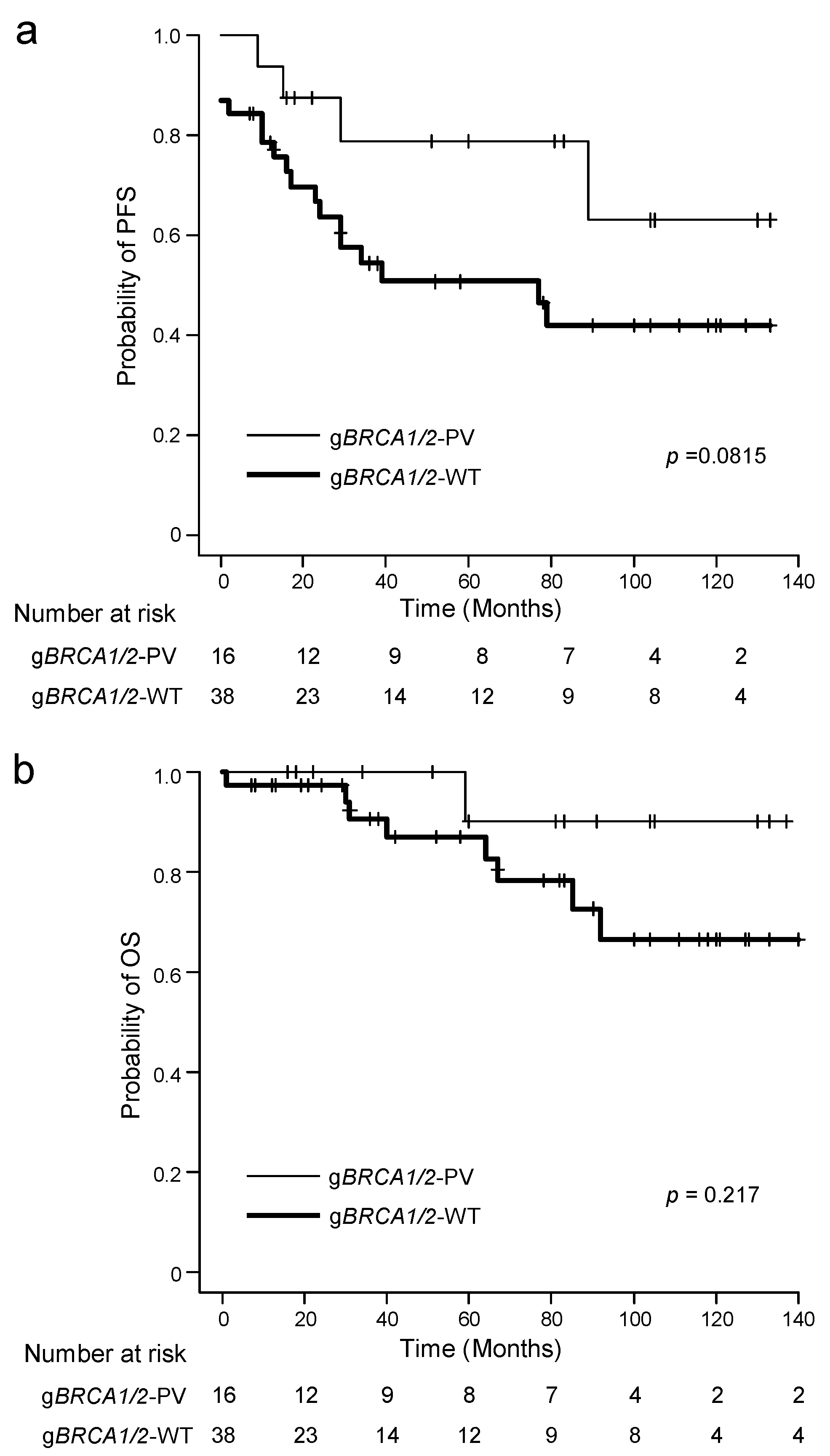
| Characteristic | n | gBRCA1/2 Variant Status | p Value * (gBRCA1/2-PV vs. WT) | |||
|---|---|---|---|---|---|---|
| gBRCA1-PV | gBRCA2-PV | gBRCA1/2-PV | gBRCA1/2-WT | |||
| Total | 123 | 6 (4.9%) | 13 (10.5%) | 19 (15.4%) | 104 (84.6%) | |
| Age at diagnosis, years | <55 vs. ≥55 | |||||
| Median (range) | 54 (28–87) | 56.5 (44–71) | 60 (46–67) | 60 (44–71) | 53 (28–85) | 0.0224 |
| <40 | 15 (12.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 15 (14.4%) | |
| ≥40, <55 | 47 (38.2%) | 3 (50.0%) | 2 (15.4%) | 5 (26.3%) | 42 (40.4%) | |
| ≥55 | 61 (49.6%) | 3 (50.0%) | 11 (84.6%) | 14 (73.7%) | 47 (45.2%) | |
| Menopausal status | Pre vs. Post | |||||
| Pre-menopausal | 46 (37.4%) | 2 (33.3%) | 2 (15.4%) | 4 (21.1%) | 42 (40.4%) | 0.1856 |
| Post-menopausal | 71 (57.7%) | 3 (50.0%) | 10 (76.9%) | 13 (68.4%) | 58 (55.8%) | |
| Unknown | 6 (4.9%) | 1 (16.7%) | 1 (7.7%) | 2 (10.5%) | 4 (3.8%) | |
| Histological type | HGSC vs. non-HGSC | |||||
| HGSC | 47 (38.2%) | 5 (83.3%) | 8 (61.5%) | 13 (68.4%) | 34 (32.7%) | 0.0046 |
| LGSC | 5 (4.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (4.8%) | |
| Endometrioid | 18 (14.6%) | 0 (0%) | 3 (23.1%) | 3 (15.8%) | 15 (14.4%) | |
| Clear cell | 28 (22.8%) | 0 (0%) | 1 (7.7%) | 1 (5.3%) | 27 (26.0%) | |
| Mucinous | 13 (10.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 13 (12.5%) | |
| Others | 12 (9.8%) | 1 (16.7%) | 1 (7.7%) | 2 (10.5%) | 10 (9.6%) | |
| FIGO stage | Stage I + II vs. III + IV | |||||
| I | 53 (43.1%) | 0 (0%) | 2 (15.4%) | 2 (10.5%) | 51 (49.0%) | 0.0002 |
| II | 16 (13.0%) | 0 (0%) | 1 (7.7%) | 1 (5.3%) | 15 (14.4%) | |
| III | 50 (40.7%) | 6 (100%) | 9 (69.2%) | 15 (78.9%) | 35 (33.7%) | |
| IV | 4 (3.3%) | 0 (0%) | 1 (7.7%) | 1 (5.3%) | 3 (2.9%) | |
| Personal history of breast cancer | Presence vs. Absence | |||||
| Presence | 11 (8.9%) | 1 (16.7%) | 2 (15.4%) | 3 (15.8%) | 8 (7.7%) | 0.3733 |
| Absence | 112 (91.1%) | 5 (83.3%) | 11 (8.5%) | 16 (84.2%) | 96 (92.3%) | |
| Family history(including first- and second-degree relatives)of HBOC-associated cancers | Presence vs. Absence | |||||
| Breast cancer | 23 (18.7%) | 3 (50.0%) | 5 (38.5%) | 8 (42.1%) | 15 (14.4%) | 0.0089 |
| Ovarian cancer | 5 (4.1%) | 1 (16.7%) | 1 (7.7%) | 2 (10.5%) | 3 (2.9%) | 0.1701 |
| Prostate cancer | 16 (13.0%) | 1 (16.7%) | 3 (23.1%) | 4 (21.1%) | 12 (11.5%) | 0.2708 |
| Pancreatic cancer | 10 (8.1%) | 0 (0%) | 1 (7.7%) | 1 (5.3%) | 9 (8.7%) | 1.0000 |
| All HBOC-associated cancers | 44 (15.8%) | 4 (66.7%) | 7 (53.8%) | 11 (57.9%) | 33 (31.7%) | 0.0379 |
| No. | Age | Stage | Histological Subtype | Variant Position (OCCR) | Nucleotide Change | Amino Acid Change | Hirasawa et al. [6] (OC) | CHARLOTTE Study [7] (OC) | Yoshimura et al. [16] (BC) | Personal History of BC | Family History of Cancers | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | OC | PanC | PrC | |||||||||||
| BRCA1 (NM_007294.4) | ||||||||||||||
| 1 | 61 | III | HGSC | within | c.2800C>T | p.Gln934* | 3 cases | 4 cases | 28 cases | + | − | − | − | − |
| 2 | 71 | III | HGSC | within | c.3505_3509del | p.Asp1169* | 0 case | 0 case | 2 cases | − | − | − | − | − |
| 3 | 49 | III | HGSC | within | c.3505_3509del | p.Asp1169* | 0 case | 0 case | 2 cases | − | − | + | − | − |
| 4 | 44 | III | HGSC | within | c.3505_3509del | p.Asp1169* | 0 case | 0 case | 2 cases | − | + | − | − | + |
| 5 | 67 | III | HGSC | outside | c.5161C>T | p.Gln1721* | 0 case | 0 case | 3 cases | − | + | − | − | − |
| 6 | 52 | III | Others | outside | c.5161C>T | p.Gln1721* | 0 case | 0 case | 3 cases | − | + | − | − | − |
| BRCA2 (NM_000059.3) | ||||||||||||||
| 7 | 56 | III | Endometrioid | outside | c.805dup | p.Thr269Asnfs*7 | 0 case | 0 case | 1 case | + | − | − | − | + |
| 8 | 46 | III | HGSC | outside | c.2830A>T | p.Lys944* | 0 case | 1 case | 0 case | − | − | − | − | − |
| 9 | 62 | I | HGSC | within | c.3703C>T | p.Gln1235* | 0 case | 0 case | 0 case | − | − | − | − | − |
| 10 | 60 | IV | Clear cell | within | c.5645C>A | p.Ser1882* | 0 case | 0 case | 7 cases | − | − | − | − | − |
| 11 | 72 | III | HGSC | within | c.5645C>A | p.Ser1882* | 0 case | 0 case | 7 cases | − | + | − | − | − |
| 12 | 46 | III | Endometrioid | within | c.6649A>T | p.Lys2217* | 0 case | 0 case | 1 case | + | + | + | − | − |
| 13 | 66 | III | HGSC | within | c.6952C>T | p.Arg2318* | 1 case | 11 cases | 18 cases | − | + | − | − | − |
| 14 | 60 | III | HGSC | within | c.6952C>T | p.Arg2318* | 1 case | 11 cases | 18 cases | − | − | − | − | − |
| 15 | 60 | I | Clear cell | outside | c.7758G>A | p.Trp2586* | 0 case | 0 case | 0 case | − | + | − | + | − |
| 16 | 59 | II | Endometrioid | outside | c.7758G>A | p.Trp2586* | 0 case | 0 case | 0 case | − | − | − | − | − |
| 17 | 67 | III | HGSC | outside | c.7922dup | p.Phe2642Ilefs*3 | 0 case | 0 case | 0 case | − | − | − | − | − |
| 18 | 67 | III | HGSC | outside | c.7922dup | p.Phe2642Ilefs*3 | 0 case | 0 case | 0 case | − | + | − | − | + |
| 19 | 59 | III | HGSC | outside | c.9076C>T | p.Gln3026* | 0 case | 1 case | 10 cases | − | − | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, A.; Imoto, I.; Tange, S.; Nishimura, M.; Iwasa, T. Prevalence of Pathogenic Germline BRCA1/2 Variants and Their Association with Clinical Characteristics in Patients with Epithelial Ovarian Cancer in a Rural Area of Japan. Genes 2022, 13, 1085. https://doi.org/10.3390/genes13061085
Abe A, Imoto I, Tange S, Nishimura M, Iwasa T. Prevalence of Pathogenic Germline BRCA1/2 Variants and Their Association with Clinical Characteristics in Patients with Epithelial Ovarian Cancer in a Rural Area of Japan. Genes. 2022; 13(6):1085. https://doi.org/10.3390/genes13061085
Chicago/Turabian StyleAbe, Akiko, Issei Imoto, Shoichiro Tange, Masato Nishimura, and Takeshi Iwasa. 2022. "Prevalence of Pathogenic Germline BRCA1/2 Variants and Their Association with Clinical Characteristics in Patients with Epithelial Ovarian Cancer in a Rural Area of Japan" Genes 13, no. 6: 1085. https://doi.org/10.3390/genes13061085
APA StyleAbe, A., Imoto, I., Tange, S., Nishimura, M., & Iwasa, T. (2022). Prevalence of Pathogenic Germline BRCA1/2 Variants and Their Association with Clinical Characteristics in Patients with Epithelial Ovarian Cancer in a Rural Area of Japan. Genes, 13(6), 1085. https://doi.org/10.3390/genes13061085






