Abstract
Subpolar and polar ecotypes of Deschampsia sukatschewii (Popl.) Roshev, D. cespitosa (L.) P. Beauv, and D. antarctica E. Desv. are well adapted to stressful environmental conditions, which make them useful model plants for genetic research and breeding. For the first time, the comparative repeatome analyses of subpolar and polar D. sukatschewii, D. cespitosa, and D. antarctica was performed using RepeatExplorer/TAREAN pipelines and FISH-based chromosomal mapping of the identified satellite DNA families (satDNAs). In the studied species, mobile genetic elements of class 1 made up the majority of their repetitive DNA; interspecific variations in the total amount of Ty3/Gypsy and Ty1/Copia retroelements, DNA transposons, ribosomal, and satellite DNA were revealed; 12–18 high confident and 7–9 low confident putative satDNAs were identified. According to BLAST, most D. sukatschewii satDNAs demonstrated sequence similarity with satDNAs of D. antarctica and D. cespitosa indicating their common origin. Chromosomal mapping of 45S rDNA, 5S rDNA, and satDNAs of D. sukatschewii allowed us to construct the species karyograms and detect new molecular chromosome markers important for Deschampsia species. Our findings confirmed that genomes of D. sukatschewii and D. cespitosa were more closely related compared to D. antarctica according to repeatome composition and patterns of satDNA chromosomal distribution.
1. Introduction
Several species of the cosmopolitan grass genus Deschampsia P. Beauv. (Poaceae) are well adapted to stressful environmental conditions including extreme polar habitats [1,2,3]. In particular, polar and subpolar ecotypes of D. sukatschewii (Popl.) Roshev and D. cespitosa (L.) P. Beauv. are widespread in the Arctic and sub-Arctic regions of Canada, Europe, Siberia, Chukotka Peninsula, and the Altai mountains [1,4,5,6]. D. antarctica E. Desv. is one of two native angiosperms adapted to extreme Antarctic environments, which can be found in diverse Antarctic habitats, including the west coast of the Antarctic Peninsula, the Maritime Antarctic, sub-Antarctic Islands, and northern Patagonia [3,7,8,9]. Such native cold-hardy ecotypes of the Deschampsia species are resources of genes associated with environmental stress tolerance and can also serve as models in crop breeding strategies [10,11].
Plant responses to environmental stresses might include some genetic changes (e.g., alternation in metabolic pathways and transcriptional regulation of genes) and cytological alterations [12]. Currently, genome diversity and comparative chromosomal phylogeny of cold-hardy ecotypes of Deschampsia are being intensively studied. Transcriptome sequencing of D. antarctica has been performed under various abiotic stress conditions and its expression profile has been examined [10]. For D. antarctica populations from the Maritime Antarctic, retrotransposon-based genetic polymorphism was reported, which could be related to the environmentally induced mobilization of random transposable elements as well as unique reproductive features of this species [13]. For polar and subpolar ecotypes of Deschampsia, structural chromosomal variations, including chromosome rearrangements, aneusomaty, mixo- and aneuploidy were revealed [14,15,16], indicating importance of molecular cytogenetic characterization of such plants. A comparative molecular cytogenetic analysis of several Deschampsia species, including subpolar ecotypes of D. antarctica, D. cespitosa, and D. sukatschewii, was performed using fluorescence in situ hybridization (FISH) with 45S/5S rDNA and sequential rapid genomic in situ hybridization with genomic DNAs of closely related and distant species of this genus. As a result, some intra- and interspecific differences in their karyotypes were revealed [16].
Repetitive DNAs (including mobile genetic elements and tandem repetitive DNA (satellite DNA)) are the major and fast-evolving part of genomes of vascular plants, which could contribute to speciation processes [13,17,18,19,20,21]; and comparative repeatome analyses make it possible to identify genomic differences both in closely related and distant plant taxa. Several satellite DNA families (satDNAs), including CON/COM repeats, are shared between many taxa of the Aveneae/Poeae tribe, indicating that they can be used as effective molecular and chromosomal markers for characterizing the plant genome, for assessing intra- and interspecific variability of genomes, and also in phylogenetic studies [22,23,24]. Recently, CON/COM satDNAs were identified and characterized in genomes of several species of Deschampsia and related genera, which made it possible to clarify some phylogenetic relationships between these species [25].
One of the effective modern approaches for characterization of repetitive DNA in one or several plant species includes genome-wide bioinformatic analyses by RepeatExplorer/TAREAN (Tandem Repeat Analyzer) pipelines, which use graph-based clustering and analyze next-generation sequencing data [26,27,28]. Having many advantages (e.g., it does not require a reference genome for contigs assembling, offers an easy-to-use interface, a rather fast analysis with detailed results), these pipelines are frequently used to create repeat databases and also to identify satDNAs suitable as FISH probes for further molecular cytogenetics [29,30,31,32,33]. Recently, a number of satDNAs were identified in genomes of South American accessions of D. antarctica and D. cespitosa, and also chromosomal distribution of some satDNAs were analyzed in several Deschampsia species growing in the same region [34,35]. However, for a thorough understanding of the relationship between species within the genus Deschampsia, further studies of the genomic diversity are needed. In particular, intra- and interspecific variability in the composition and genomic organization of transposable elements, as well as satDNA should be explored in different Deschampsia species and accessions from other growing areas.
The present work performed a comparative characterization of repeatomes of subpolar and polar accessions of D. sukatschewii, D. cespitosa, and D. antarctica, including genome-wide bioinformatic analyses of low-coverage high-throughput DNA sequencing data using RepeatExplorer and TAREAN pipelines and the Basic Local Alignment Search Tool (BLAST). Also, FISH-based chromosomal mapping of the identified specific satDNAs and a search for new molecular chromosome markers were carried out to provide information about the changes that might occur in their genomes during speciation.
2. Materials and Methods
2.1. Plant Material
Seeds of D. antarctica (KEW-0661919, Falkland Is., UK) were obtained from the collection of Weddle Seed Conservation Department, Royal Botanic Gardens, Kew, UK. Seeds of D. cespitosa (688, Vologda region, Russia) and D. sukatschewii (78, Altai Mountains, Russia) were provided by the laboratory of genetic resources of fodder plants, Federal Williams Research Center of Forage Production and Agroecology (FWRC FPA), Moscow, Russia. Seeds of the studied accessions were germinated in Petri dishes on the moist filter paper for 3–5 days. Then the plants were grown in a greenhouse at 15 °C.
2.2. Genomic DNA Extraction and Sequencing
Genomic DNA of D. sukatschewii and D. cespitosa were isolated from young leaves of the studied accessions using the GeneJet Plant Genomic DNA Purification Kit (Thermo Fisher Scientific, Vilnius, Lithuania). The quality of the DNA samples was checked with the Implen Nano Photometer N50 (Implen, Munich, Germany). The concentration and purification of the extracted DNAs were assessed with the Qubit 4.0 fluorometer and Qubit 1X dsDNA HS Assay Kit (Thermo Fisher Scientific, Eugene, OR, USA).
For D. sukatschewii and D. cespitosa, whole genome sequencing with low coverage was performed at the Beijing Genomics Institute (BGISeq platform) (Shenzhen, Guangdong, China) according to the NGS protocol for generating 5–10 million of paired-end reads of 150 bp in length, which provided at least 0.147–0.350× of the coverage of the D. cespitosa genome (1C = 4283.64–5105.16 Mbp, Eurasian region) [36,37]. The raw sequencing data for D. cespitosa (SAMN26938767) and D. sukatschewii (SAMN26938768) were uploaded to the National Center for Biotechnology Information (NCBI) BioProject database under accession number PRJNA819861 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA819861, accessed on 25 March 2022).
2.3. Sequence Analysis and Identification of DNA Repeats
For genome-wide comparative analyses, genome sequences of D. sukatschewii and D. cespitosa and also the publicly available D. antarctica sequencing data (https://www.ebi.ac.uk/ena/browser/view/PRJNA237267?show=reads, (accessed on 28 January 2018) PRJNA237267 gDNA-Seq for Antarctic hairgrass, Korea Polar Research Institute), were used. Interspecific comparisons, reconstruction, and quantification of major repeat families were performed with the use of RepeatExplorer 2 and TAREAN pipelines [27,38]. For each studied species, the genomic reads were filtered by quality, and 1 million high-quality reads were randomly selected for further analyses, which corresponds to 0.0147–0.0350× of a coverage of the D. cespitosa genome (1C = 4283.64–5105.16 Mbp, Eurasian region) [36,37], and is within the limits recommended by the developers of these programs (genome coverage of 0.01–0.50× is recommended) [38]. RepeatExplorer/TAREAN was launched with the preset settings based on Galaxy platform (https://repeatexplorer-elixir.cerit-sc.cz/galaxy/, 25 March 2022). Initially, the preprocessing of the genomic reads was performed. The reads were filtered in terms of quality using a cut-off of 10, trimmed, and filtered by size to obtain high-quality reads. Default threshold was explicitly set to 90% sequence similarity spanning at least 55% of the read length (in the case of reads differing in length it applies to the longer one). The sequence homology of the identified satDNAs of D. sukatschewii with repeats of D. cespitosa and D. antarctica was estimated by BLAST (NCBI, Bethesda, MD, USA). Based on twelve abundant satDNAs of D. sukatschewii, oligonucleotide FISH probes Ds 52, Ds 56, Ds 65, Ds 81, Ds 83, Ds 88, Ds 124, Ds 138, Ds 144, Ds 146, Ds 179, and Ds 226 (Table 1) were generated by the Primer3-Plus software [39].

Table 1.
List of the oligonucleotide FISH probes.
2.4. Chromosome Spread Preparation
Root tips (0.5–1 cm long) were kept in ice water for 24 h for accumulation of mitotic divisions and then fixed in the ethanol and glacial acetic acid fixative (3:1) for 2 days at room temperature. The fixed roots were incubated in 1% acetocarmine solution (in 45% acetic acid) for 30–40 min. Then, the root meristem was cut from the tip cap, macerated in 45% acetic acid, and a squashed preparation was made with the use of a cover slip. After freezing in liquid nitrogen, the cover slip was removed; the obtained preparation was dehydrated in 96% ethanol for 3 min and air dried for 15 min.
2.5. Fluorescence In Situ Hybridization
In FISH assays, we used two wheat DNA probes: pTa71 enclosing 18S-5.8S-26S (45S) rDNA [40] and pTa794 containing 5S rDNA [41]. These DNA probes were labeled directly with fluorochromes Aqua 431 dUTP, Red 580 dUTP, or Green 496 dUTP (ENZO Life Sciences, Farmingdale, NY, USA) by nick translation according to manufacturers’ protocols. Moreover, oligonucleotide probes Ds 52, Ds 56, Ds 65, Ds 81, Ds 83, Ds 88, Ds 124, Ds 138, Ds 144, Ds 146, Ds 179, and Ds 226 were used. These probes were produced and labeled directly with 6-FAM- or Cy3-dUTP in Evrogen JSC (Moscow, Russia).
Several sequential FISH procedures were performed with various combinations of these labeled DNA probes as described previously [6,42]. Before the first FISH procedure, chromosome slides were pretreated with 1 mg/mL RNase A (Roche Diagnostics, Mannheim, Germany) in 2 × SSC at 37 °C for 1 h. Then, the slides were washed three times for 10 min in 2 × SSC, dehydrated through a graded ethanol series (70%, 85%, and 96%) for 3 min each and air dried for 15 min. A total of 15 µL of hybridization mixture containing 40 ng of each labeled probe was added to each slide. The slides with DNA probes were covered with coverslips, sealed with rubber cement, denatured at 74 °C for 5 min, chilled on ice and placed in a moisture chamber at 37 °C. After overnight hybridization, the slides were washed in 0.1 × SSC (10 min, 44 °C), twice in 2 × SSC for 10 min at 44 °C, followed by a 5-min wash in 2 × SSC and three 3-min washes in PBS at room temperature. Then, the slides were dehydrated through the graded ethanol series for 3 min each, air dried for 15 min, and stained with DAPI (4′,6-diamidino-2-phenylindole) dissolved (0.1 μg/mL) in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). After documenting FISH results, the chromosome slides were washed twice in 2 × SSC for 10 min. Then, sequential FISH procedures were conducted on the same slides.
2.6. Chromosome Analysis
The chromosome slides were inspected using the epifluorescence Olympus BX61 microscope with the standard narrow band pass filter set and UPlanSApo 100×/1.40 oil UIS2 objective (Olympus, Tokyo, Japan). Chromosome images were captured with a monochrome CCD (charge-coupled device) camera (Snap, Roper Scientific, Tucson, AZ, USA) in grayscale channels, pseudo-colored, and processed with Adobe Photoshop 10.0 (Adobe Systems, Birmingham, AL, USA) and VideoTesT-FISH 2.1 (IstaVideoTesT, St. Petersburg, Russia) software. At least five plants and 15 metaphase plates were examined in each sample. Chromosome pairs in karyotypes were identified according to the chromosome size and morphology, localization of chromosome markers, and also the cytological nomenclature proposed previously [16].
3. Results
3.1. Comparative Analyses of the Repetitive DNA Sequences
The comparative repeatome analysis of D. antarctica, D. cespitosa, and D. sukatschewii showed that mobile genetic elements made up the majority of their repetitive DNAs (Table 2). Retrotransposon elements, including Ty3-Gypsy and Ty1-Copia superfamilies (transposable elements of Class I), were highly abundant and represented 41.21–43.41% of their genomes. Within the Ty1-Copia superfamily, SIRE and Angela were most abundant, and Ty3-Gypsy retroelements were dominated by the Tat-Retand and Athila non-chromoviruses and chromovirus Tekay. In D. cespitosa and D. sukatschewii, Ty3-Gypsy elements significantly exceeded Ty1-Copia retrotransposons. In D. antarctica, however, Ty1-Copia retroelements were roughly twice abundant than Ty3-Gypsy elements. The genome of D. antarctica contained the largest proportion of unclassified LTR retroelements (14.03%) if compared with D. cespitosa (1.31%) and D. sukatschewii (4.27%). DNA transposons (Class II) were found in lower amount (2.48–2.89%) compared to retrotransposons, and the least quantity was revealed in D. sukatschewii. The total amount of satellite DNA ranged from 1.61% (D. sukatschewii) to 2.85% (D. cespitosa). The content of ribosomal DNA was notably less in D. antarctica (0.06%) if compared with D. sukatschewii (0.29%) and D. cespitosa (0.6%). In the studied accessions, 12–18 high confident and 7–9 low confident putative satellites were revealed by TAREAN (Figure 1, Table 2).

Table 2.
Proportion of major repetitive DNA sequences identified in genomes of the studied Deschampsia species.
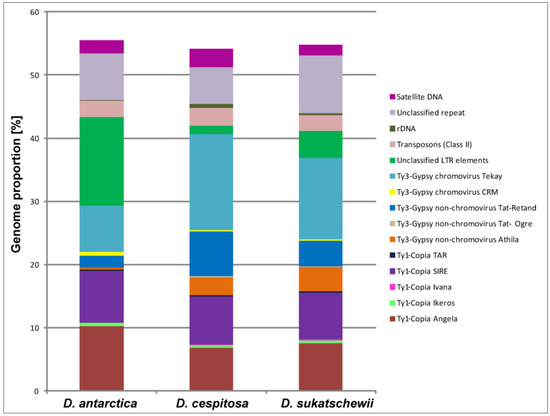
Figure 1.
Genome proportion of most abundant repetitive DNA sequences identified in the studied Deschampsia species. The genome proportion of individual repeat types was obtained as a ratio of reads specific to individual repeat types to all reads used for clustering analyses by the RepeatExplorer pipelines.
3.2. BLAST Analysis
According to BLAST, most of the satDNAs identified in the genome of D. sukatschewii (Ds 52, Ds 56, Ds 81, Ds 83, Ds 88, Ds 124, Ds 138, Ds 142, Ds 166, Ds 179, Ds 182, and Ds 226) demonstrated sequence similarity with the satDNAs of D. antarctica and/or D. cespitosa, and also the species belonged to other genera including Festuca, Helictotrichon, Leymus, Poa, Secale, Setaria, Tripidium, and Triticum (Table 3). Four Ds satDNAs (Ds 65, Ds 144, Ds 158, and Ds 211) demonstrated sequence homology only with the satDNAs of D. antarctica (Da satDNAs) and/or D. cespitosa (Dc satDNAs). For Ds 146 satDNA, homology with tandem repeats of other species was not revealed within available NCBI database (Table 3).

Table 3.
Comparison of satDNAs identified in Deschampsia sukatschewii with our results on D. cespitosa and D. antarctica and also available data.
3.3. Chromosomal Structural Variations
The performed karyotype analyses showed that the studied Deschampsia accessions presented diploid karyotype with 2n = 2x = 26 chromosomes (Figure 2, Figure 3, Figure 4 and Figure 5).
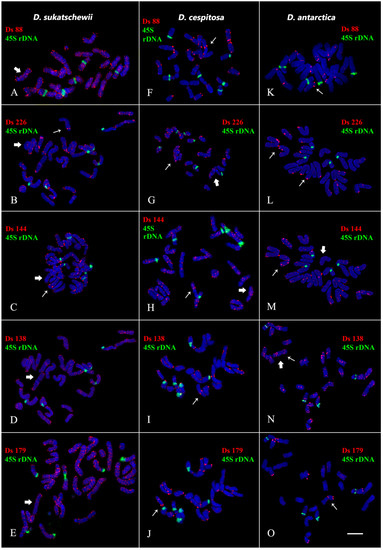
Figure 2.
Localization of Ds 88, Ds 138, Ds 144, Ds 179, and Ds 226 satDNA probes on chromosomes of the studied Deschampsia species. Merged fluorescent images of D. sukatschewii, D. cespitosa, and D. antarctica after FISH with 45S rDNA (green) and the Ds satDNA probes (red). Chromosome DAPI-staining (grey). (A,B,D,E,G,H,N)—mixed clustered and dispersed localization of Ds satDNAs on chromosomes; (C,F,I–M,O)—clustered localization of Ds satDNAs on chromosomes. Thick and thin arrows indicate dispersed and clustered hybridization signals, respectively. Scale bar—5 μm.
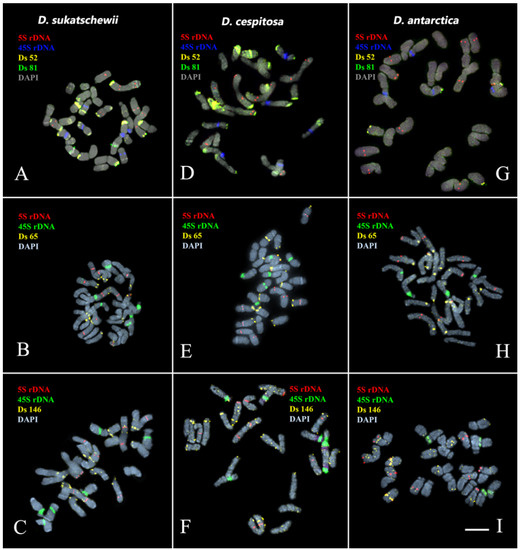
Figure 3.
Localization of Ds 52, Ds 65, Ds 81, and Ds 146 satDNA probes on chromosomes of the studied Deschampsia species. Merged fluorescent images of D. sukatschewii, D. cespitosa, and D. antarctica after multicolor FISH with 5S rDNA (red), 45S rDNA (blue), Ds 52 (yellow), and Ds 81 (green)—(A,D,G); 5S rDNA (red), 45S rDNA (green), and Ds 65 (yellow)—(B,E,H); and 5S rDNA (red), 45S rDNA (green), and Ds 146 (yellow)—(C,F,I). Chromosome DAPI-staining—grey. Scale bar—5 μm.
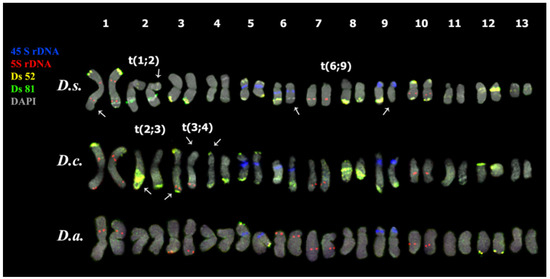
Figure 4.
Karyotypes of the studied Deschampsia species. Karyograms of D. sukatschewii (D.s.), D. cespitosa (D.c.) and D. antarctica (D.a.) after multicolor FISH with 45S rDNA (blue), 5S rDNA (red), Ds 52 (yellow), and Ds 81 (green) (the same metaphase plates as in Figure 3A,D,G). Chromosome DAPI-staining—grey. Arrows point to chromosomal rearrangements.

Figure 5.
Karyotypes of the studied Deschampsia species. Karyograms of D. sukatschewii (D.s.), D. cespitosa (D.c.) and D. antarctica (D.a.) after multicolor FISH with (A) 45S rDNA (green), 5S rDNA (red) and Ds 65 (yellow) (the same metaphase plates as in Figure 3B,E,H); and (B) 45S rDNA (green), 5S rDNA (red) and Ds 146 (yellow) (the same metaphase plates as in Figure 3C,F,I). Chromosome DAPI-staining—grey. Arrows point to chromosomal rearrangements.
In karyotypes of D. sukatschewii and D. cespitosa, similar patterns of chromosome distribution of 45S and 5S rDNA clusters were observed. Six bright 45S rDNA signals were detected in the short arms of chromosome pairs 5, 6, and 9 with satellites of different sizes and secondary constrictions. Ten hybridization signals of 5S rDNA were observed on chromosome pairs 1 (in the proximal regions of both arms), 3 (in the terminal regions of the long arms), and also in the proximal regions of the long arms of chromosome pairs 7 and 10 (Figure 4 and Figure 5).
In the karyotype of D. antarctica, four hybridization signals of 45S rDNA were revealed in the short arms of two chromosome pairs 5 and 9 with satellites of different sizes and secondary constrictions (Figure 4 and Figure 5). Ten loci of 5S rDNA were localized on chromosome pairs 1 (in the proximal regions of the short arm), 3 (in the terminal regions of the long arms), 6 (in the distal regions of the short arms), and also in the proximal regions of the long arms of chromosome pairs 7 and 10 (Figure 4 and Figure 5).
We observed different patterns of chromosome distribution of twelve Ds satDNAs (Ds 52, Ds 56, Ds 65, Ds 81, Ds 83, Ds 88, Ds 124, Ds 138, Ds 144, Ds 146, Ds 179, and Ds 226) in karyotypes of D. sukatschewii, D. cespitosa, and D. antarctica, which exhibited interspecific differences in their clustered and/or dispersed localization (detailed in Supplement Table S1, Figure 2, Figure 3, Figure 4 and Figure 5).
4. Discussion
Most eukaryotic genomes contain large numbers of repetitive DNA sequences [43,44]. Transposable elements (TEs) as well as tandem repeats (satellite DNA) are highly abundant and diverse parts of genomes [45,46]. In plants, TEs can constitute up to 90% of their genomes [47,48,49]. Due to the fact that TEs are capable of changing their location and/or copy numbers, they can influence the genome organization and evolution [50,51]. Currently, TEs are separated into two major classes, class I (retrotransposons, including LTR retrotransposons) and class II (DNA transposons), based on TEs structural characteristics and mode of replication [50,52]. In plant genomes, LTR retrotransposons include the Ty1-Copia and Ty3-Gypsy superfamilies, which are further divided into a number of families mostly specific to a single or a group of closely related species [53]. In plant genomes, LTR retrotransposons are highly abundant, making up to 75% of nuclear DNA [54,55]. In our study, a comparative repeatome analysis of D. sukatschewii, D. cespitosa, and D. antarctica also showed that LTR retrotransposons made up the majority of their genomes. LTR retrotransposons are considered to be main contributors to the variations of nuclear genomes within angiosperms [33,56,57,58,59]. These retroelements are able to replicate using the copy and paste mechanism and, thus, generate new copies of the elements and increase the size of the genome [45]. However, the LTR copies can also be efficiently eliminated from the genome, through both solo LTR formation and accumulation of deletions, which reduces the genome size [54]. Genome size is often treated as an intrinsic property of a species, and intra- and interspecific variations in genome size might reflect different evolutionary processes during speciation [60].
D. cespitosa is a variable and widespread species with many subspecies and closely related species (including D. sukatschewii) [61], and the genome size of D. cespitosa accessions highly depends on their geographical location and habitat [37]. Nevertheless, the average genome size of diploid D. cespitosa (1C = 4.38–5.22 pg, Eurasian region) roughly corresponds to that of diploid D. antarctica (1C = 4.98–5.31 pg) [36,37,62,63]. These data are consistent with our results showing about the same content of retrotransposons in genomes of the studied Deschampsia species, which constituted an essential portion of their repeatomes (41–43%). At the same time, we revealed interspecific differences in content of Ty3-Gypsy and Ty1-Copia and also in genome proportions of SIRE, Angela, non-chromovirus Retand, and chromovirus Tekay. For instance, a ratio of Ty3-Gypsy/Ty1-Copia retrotransposons revealed in genomes of closely related D. cespitosa and D. sukatschewii differed greatly from that detected in D. antarctica. In genomes of D. cespitosa and D. sukatschewii, the Ty3-Gypsy elements were about 1.5 times more abundant than Ty1-Copia. More content of Ty3-Gypsy retroelements in the genome compared to Ty1-Copia is typical for many taxa of Poaceae. For example, in Avena genomes, Ty3-Gypsy elements were nearly three times more abundant than Ty1-Copia [64]; in genomes of Lolium and Festuca species, Ty3-Cypsy retrotransposons were four times more abundant compared to Ty1-Copia elements [33]. Moreover, among the studied species, some interspecific variations in the total amount of DNA transposons were detected. The observed interspecific differences might be related to the processes occurred in genomes of these Deschampsia species during speciation, which is supported by some previous research. In particular, it was shown that some evolutionary changes in genomes of diploid species of Melampodium correlated with differences in the abundance of the SIRE (Ty1-Copia), Athila (Ty3-Gypsy), and CACTA (DNA transposon) lineages [58].
We also found that in D. antarctica, the genome proportion of unclassified LTR retroelements significantly exceeded that revealed in the other two Deschampsia species, which highlights the need for more research on these TEs in D. antarctica. These differences could be related to specific attributes of the D. antarctica genome or environmentally induced genetic peculiarities of the studied accessions. Environmentally induced retrotransposon-based genetic diversity was previously described in populations of D. antarctica from the Maritime Antarctic [13]. Intense stress might induce rapid changes in the structure, organization, and function of plant genomes especially in populations with low genetic diversity [65], which is typical for D. antarctica [66,67,68]. Moreover, in many plant species, which grew under various abiotic and biotic stresses, transcriptional activation of TEs was revealed [69,70,71], and it was regarded as a mechanism responsible for genome plasticity under changing environmental conditions [72].
It was reported for different Poaceae species that satDNAs sequences can vary in a number of features, including nucleotide composition, abundance, and distribution in genomes [73,74]. The comparative analysis of the studied accessions detected interspecific variations in the content of ribosomal DNA, which was notably lower in D. antarctica compared to D. cespitosa and D. sukatschewii. These data are consistent with the different number of satellite chromosomes bearing nucleolar organizer regions (NORs) identified in karyotypes of D. antarctica (two pairs) and the other two species (three pairs) [15,16] since it is known that NORs contain tandemly repeated rDNA sequences [75]. Moreover, our results showed that genomes of the studied Deschampsia accessions contained substantial portions of satellite DNA sequences, and interspecific variations in their abundance were also revealed. D. cespitosa has the highest amount of satellite DNA among the studied species, which is consistent with earlier reported data [34]. Tandem repeats, such as rDNA and other satDNAs, are generally found to be a fast-evolving fraction of the repeatome, showing divergence in both copy number and sequence between closely related species [60]. SatDNAs are known to have a variable length of the repeat unit (monomer) and usually form tandem arrays up to 100 Mb [20,76]. Although they are considered to be non-coding sequences, the satellite monomers mostly exhibit lengths of 160 to 180 bp or 320 to 370 bp though other lengths are also found in plants [77], which correspond to the length of mono- and dinucleosomes [78,79]. The sequences of satellite monomers evolve concertedly via the process of molecular drive; and mutations are homogenized in a genome and become fixed in the populations [80]. The sequence identity inside an array evolves according to the process called ‘concerted evolution’, which results to the maintenance of homogeneity of satDNA monomers within a species during evolution [81]. The abundance of satDNA can vary within the plant genomes even between generations resulting in high polymorphism in the length of satellite arrays [80]. At the same time, some satDNA sequences demonstrate sequence conservation for long evolutionary periods [82]. Since many satellite DNAs exist in a genome, the evolution of species-specific satDNA might be the result of copy number changes within a library of satellite sequences common for a group of species [79,80,82].
The high-throughput DNA sequencing and subsequent genome-wide bioinformatic analysis provide important data on the structural diversity of satDNA [21,83,84]. In the studied accessions of D. antarctica, D. cespitosa, and D. sukatschewii, more satDNA families (20, 27, and 21, correspondingly) were identified by genomic analyses with TAREAN if compared with reported earlier data on South American accessions of D. antarctica and D. cespitosa (34 satDNAs in total) [34], which indicated a high level of satDNA diversity in Deschampsia genomes. Moreover, a relatively large number of the satDNAs were identified in Deschampsia genomes compared to several other Poaceae species including Festuca pratensis (eight satDNAs), Agropyron cristatum (fourteen satDNAs), and Poa species (four satDNAs) [31,32,85], which might be related to some features of Deschampsia genomes.
Despite satDNAs are considered to be fast-evolving genome fractions, some of them remain preserved for long evolutionary periods and have a highly conserved monomer sequence, which might be related to their interaction with specific proteins necessary for heterochromatin formation and also to their putative regulatory role in gene expression [80,86]. SatDNAs are known to contribute to the essential processes of formation of crucial chromosome structures, e.g., DNA packaging and chromatin condensation [19,79,87,88]. In the present study, three Ds repeats (Ds 56, Ds 83, and Ds 124) showed high sequence similarity with CON1, CON2, and COM2 sequences. CON/COM satDNAs were originally isolated from the Helictotrichon genome [22,89] and then revealed in several taxa of the Aveneae/Poeae tribe complex including Deschampsia [23,25,90]. In different taxa, the nucleotide sequences in monomers of CON/COM satDNAs demonstrated a high degree of identity, which suggested their ancient origin, though they could change slightly and independently in different species of Deschampsia and related genera [22,25,89]. Moreover, BLAST detected regions of local similarity between sequences of several other Ds satDNAs (Ds 52, Ds 81, Ds 88, Ds 138, Ds 142, Ds 166, Ds 179, Ds 182, and Ds 226) and corresponding satDNAs identified in other Deschampsia species and/or the species belonged to the related genera, which indicated that those plants might also share a common evolutionary ancestor. Several Ds satDNAs (Ds 65, Ds 144, Ds 158, and Ds 211) had high sequence similarity only with satDNAs of D. cespitosa and/or D. antarctica confirming their close relationship.
SatDNAs are often associated with heterochromatin regions and are localized in the certain chromosome regions (centromeric, terminal, and/or intercalary), which allow them to be explored with cytogenetic techniques, including FISH. The patterns of chromosomal distribution of satDNAs facilitate the recognition of homologous chromosome pairs and recombination as well as differences between lineages and species [19,20]. High sequence homology of certain satDNAs allowed us to use the oligonucleotide FISH probes, developed based on the most abundant Ds satDNAs, in the comparative karyotype analysis of the studied Deschampsia species. However, despite the large number of common repeats, different patterns of chromosomal distribution of these Ds were observed, and depending on the species, localization of most examined Ds satDNAs could be clustered and/or dispersed, which was probably related to different amount and organization of these homologous repeats in genomes of the related species. Large Ds clusters were predominantly localized in the pericentromeric and/or terminal regions of chromosomes of the studied species. Moreover, other patterns of Ds chromosomal distribution were observed including bright clusters combined with dispersed signals or small satDNA clusters in the intercalary chromosome regions, which is typical for plants [19,20,21]. Several Ds satDNAs exhibited only specific clustered localization on chromosomes of all studied species, which allowed us to explore interspecific variations in their distribution on chromosomes. These results were consistent with earlier reported data on patterns of chromosomal distribution of CON/COM and Da satDNAs in several Deschampsia species [25,34,35].
According to BLAST, any satDNAs, which would be homologous to Ds 81/Dc 135, were not identified in the D. antarctica genome. Moreover, BLAST did not detect any satDNAs homologous to Ds 146 within Deschampsia or other taxa. However, the performed FISH-based chromosome mapping of both Ds 81 and Ds 146 revealed bright hybridization signals in karyotypes of all studied Deschampsia species. This could be related to some peculiarities of the used sequencing technique, subsequent bioinformatic processing, and also the satDNA abundancy in the genome. Thus, our results demonstrate that the cytogenetic studies can increase the possibilities for satellite DNA analysis as they provide valuable additional data on genomic relationships among related species.
Among the examined Ds tandem repeats, four satDNAs (Ds 52, Ds 81, Ds 65, and Ds 146) demonstrated species-specific patterns of their chromosomal distribution in all studied Deschampsia species, which is important for comparative karyotype studies and also analyze the genome differentiation within Deschampsia. Specifically, hybridization signals of Ds 52 and also Ds 81 partially overlapped with sites of CON1 satDNA studied previously [25]. Both Ds 65 and Ds 146 demonstrated unique clustered species-specific patterns of chromosomal distribution indicating that they could be used as new promising chromosomal markers for Deschampsia species.
SatDNA repeats was shown to represent recombination ‘‘hotspots’’ of genome reorganization, and the occurrence of satDNA in interstitial and telomeric heterochromatin reduces genetic recombination in the adjacent regions [91]. In our study, the comparison of patterns of chromosomal distribution of Ds 65 and Ds 146 made it possible to identify different chromosomal rearrangements in some karyotypes of D. sukatschewii, D. cespitosa, and D. antarctica and detect the breakpoints on chromosomes.
The comparison of patterns of chromosomal distribution of Ds 52, Ds 81, Ds 65, and Ds 146 indicated predominant similarity between karyotypes of D. sukatschewii and D. cespitosa compared to D. antarctica, which was consistent with our previously reported data on other chromosomal markers [16,25]. Notably, the chromosomes bearing 45S and 5S rDNA clusters had the most similar patterns in all three species indicating that structures of these chromosomes were rather conserved. Satellite DNA-based chromosomal markers are particularly useful for chromosome identification, the analysis of chromosome rearrangements, as well as evolution of genomes within Poaceae [24,64,92]. This is especially important for Deschampsia due to the lack of effective molecular cytogenetic markers suitable for karyotype analyses within this genus [15]. At the same time, comprehensive genomic studies to assess the variability of satDNA arrays are still required to provide valuable data for investigating the functional and structural features of Deschampsia genomes, and also the paths of chromosomal reorganization of genomes during speciation.
5. Conclusions
For the first time, the comparative repeatome analyses among valuable subpolar and polar accessions of D. sukatschewii, D. cespitosa, and D. antarctica was performed with the use of the modern effective approach (combining high-throughput DNA sequencing, genome-wide bioinformatic analyses, and FISH-based chromosome mapping of the identified specific satDNAs). Analyses of chromosome patterns of distribution of twelve abundant D. sukatschewii satDNAs allowed us to detect four new effective molecular chromosome markers. Due to the shortage of such markers in Deschampsia, this is especially important for comparative karyotypic studies within the genus to analyze the changes occurring in their genomes during speciation. For the first time, the unique species karyograms were constructed, which made it possible to compare the localization of these markers on homologous chromosomes of the studied species. Our results confirmed that genomes of the subarctic D. sukatschewii and D. cespitosa accessions were more closely related if compared with the D. antarctica accession according to repeatome composition and patterns of satDNA chromosomal distribution. Our findings demonstrated that cytogenetic studies might expand the possibilities of repeatome analyses as they provide important additional data on genomic relationships within Deschampsia as well as increase knowledge on genome organization in these species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13050762/s1, Table S1: title ‘FISH chromosome mapping of the D. sukatschewii satDNA probes in the studied Deschampsia species’.
Author Contributions
Conceptualization, A.V.A. and O.V.M.; methodology, O.V.M. and A.V.A.; software, A.V.A., O.Y.Y. and O.V.M.; validation, O.V.M.; formal analysis, A.V.A., S.A.Z., O.Y.Y., N.L.B. and T.E.S.; investigation, A.V.A., S.A.Z., O.Y.Y., N.L.B., T.E.S. and O.V.M.; resources, N.L.B.; data curation, A.V.A., O.Y.Y. and O.V.M.; supervision, O.V.M.; visualization, A.V.A., O.Y.Y., T.E.S. and S.A.Z.; writing—original draft preparation, A.V.A., O.Y.Y., S.A.Z., T.E.S. and O.V.M.; writing—review and editing, A.V.A. and O.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Assignment of Ministry of Science and Higher Education of the Russian Federation (project No. 121052000140-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article and Supplementary Materials. The datasets of D. cespitosa (SAMN26938767) and D. sukatschewii (SAMN26938768), generated during this study, can be found here: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA819861 (accessed on 25 March 2022), BioProject accession number PRJNA819861.
Acknowledgments
The authors acknowledge N.N. Kozlov and V.L. Korovina (Laboratory of genetic resources of fodder plants, FWRC of Forage Production and Agroecology, Lobnya, Moscow region, Russia) for valuable plant materials used for experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tzvelev, N.N. Arctic Flora of the USSR; Nauka: Leningrad, USSR, 1964; Volume 2. [Google Scholar]
- Hulten, E. Flora of Alaska and Neighboring Territories; Stanford University Press: Stanford, UK, 1968. [Google Scholar]
- Alberdi, M.; Bravo, L.A.; Gutierrez, A.; Gidekel, M.; Corcuera, L.J. Ecophysiology of Antarctic vascular plants. Physiol. Plant 2002, 115, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Löve, A.; Löve, D. Chromosome numbers of central and northwest European plant species. In “Opera Botanica” a Societate Botanica Lundensi, 5; Almqvist & Wiksell: Stockholm, Sweden, 1961. [Google Scholar]
- Hulten, E.; Fries, M. Atlas of North European Vascular Plants. North of the Tropic of Cancer; Koeltz Scientific Books: Konigstein, Germany, 1986. [Google Scholar]
- Amosova, A.V.; Zoshchuk, S.A.; Rodionov, A.V.; Ghukasyan, L.; Samatadze, T.E.; Punina, E.O.; Loskutov, I.G.; Yurkevich, O.Y.; Muravenko, O.V. Molecular cytogenetics of valuable Arctic and sub-Arctic pasture grass species from the Aveneae/Poeae tribe complex (Poaceae). BMC Genet. 2019, 20, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.M. Chromosome numbers of Falkland Islands angiosperms. BAS Bull. 1967, 14, 69–82. [Google Scholar]
- Moore, D.M. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv. II. Taxonomy, distribution and relationships. BAS Bull. 1970, 23, 63–80. [Google Scholar]
- Chiapella, J.; Zuloaga, F.O. A Revision of Deschampsia, Avenella, and Vahlodea (Poaceae, Poeae, Airinae) in South America. Ann. Mo. Bot. Gard. 2010, 97, 141–162. [Google Scholar] [CrossRef]
- Lee, J.; Noh, E.K.; Choi, H.S.; Shin, S.C.; Park, H.; Lee, H. Transcriptome sequencing of the Antarctic vascular plant Deschampsia antarctica Desv. under abiotic stress. Planta 2013, 237, 823–836. [Google Scholar] [CrossRef]
- Byun, M.Y.; Lee, J.; Cui, L.H.; Kang, Y.; Oh, T.K.; Park, H.; Lee, H.; Kim, W.T. Constitutive expression of DaCBF7, an Antarctic vascular plant Deschampsia antarctica CBF homolog, resulted in improved cold tolerance in transgenic rice plants. Plant Sci. 2015, 236, 61–74. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—From gene to biotechnology. AoB PLANTS 2017, 9, plx025. [Google Scholar] [CrossRef] [Green Version]
- Androsiuk, P.; Chwedorzewska, K.J.; Dulska, J.; Milarska, S.; Giełwanowska, I. Retrotransposon based genetic diversity of Deschampsia antarctica Desv. from King George Island (Maritime Antarctic). Ecol. Evol. 2021, 11, 648–663. [Google Scholar] [CrossRef]
- Cardone, S.; Sawatani, P.; Rush, P.; García, A.M.; Poggio, L.; Schrauf, G. Karyological studies in Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009, 32, 427–433. [Google Scholar] [CrossRef]
- Amosova, A.V.; Bolsheva, N.L.; Samatadze, T.E.; Twardovska, M.O.; Zoshchuk, S.A.; Andreev, I.O.; Badaeva, E.D.; Kunakh, V.A.; Muravenko, O.V. Molecular cytogenetic analysis of Deschampsia antarctica Desv. (Poaceae), Maritime Antarctic. PLoS ONE 2015, 10, e0138878. [Google Scholar] [CrossRef]
- Amosova, A.V.; Bolsheva, N.L.; Zoshchuk, S.A.; Twardovska, M.O.; Yurkevich, O.Y.; Andreev, I.O.; Samatadze, T.E.; Badaeva, E.D.; Kunakh, V.A.; Muravenko, O.V. Comparative molecular cytogenetic characterization of seven Deschampsia (Poaceae) species. PLoS ONE 2017, 12, e0175760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flavell, R.B.; O’Dell, M.; Hutchinson, J. Nucleotide sequence organization in plant chromosomes and evidence for sequence translocation during evolution. Cold Spring Harb. Symp. Quant. Biol. 1981, 45, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.B. Repetitive DNA and chromosome evolution in plants. Philos. Trans. R. Soc. B Biol. Sci. 1986, 312, 227–242. [Google Scholar]
- Schmidt, T.; Heslop-Harrison, J.S. Genomes, genes and junk: The large-scale organization of plant chromosomes. Trends Plant Sci. 1998, 3, 195–199. [Google Scholar] [CrossRef]
- Mehrotra, S.; Goyal, V. Repetitive sequences in plant nuclear DNA: Types, distribution, evolution and function. Genom. Proteom. Bioinform. 2014, 12, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Ramos, M.A. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef]
- Grebenstein, B.; Grebenstein, O.; Sauer, W.; Hemleben, V. Characterization of a highly repeated DNA component of perennial oats (Helictotrichon, Poaceae) with sequence similarity to a A-genome-specific satellite DNA of rice (Oryza). Theor. Appl. Genet. 1995, 90, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Winterfeld, G.; Röser, M. Chromosomal localization and evolution of satellite DNAs and heterochromatin in grasses (Poaceae), especially tribe Aveneae. Plant Syst. Evol. 2007, 264, 75–100. [Google Scholar] [CrossRef]
- Röser, M.; Winterfeld, G.; Doring, E.; Schneider, J. Chromosome evolution in grass tribes Aveneae/Poeae (Poaceae): In-sights from karyotype structure and molecular phylogeny. Schlechtendalia 2014, 28, 1–21. [Google Scholar]
- Amosova, A.; Ghukasyan, L.; Yurkevich, O.; Bolsheva, N.; Samatadze, T.; Zoshchuk, S.; Muravenko, O. Cytogenomics of Deschampsia P. Beauv (Poaceae) species based on sequence analyses and FISH mapping of CON/COM satellite DNA families. Plants 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Neumann, P.; Macas, J. Graph-based clustering and characterization of repetitive sequences in next-generation sequencing data. BMC Bioinform. 2010, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novák, P.; Neumann, P.; Pech, J.; Steinhaisl, J.; Macas, J. RepeatExplorer: A galaxybased web server for genome-wide characterization of eukaryotic repetitive elements from next-generation sequence reads. Bioinformatics 2013, 29, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novák, P.; Neumann, P.; Macas, J. Global analysis of repetitive DNA from unassembled sequence reads using RepeatExplorer2. Nat. Protoc. 2020, 15, 3745–3776. [Google Scholar] [CrossRef]
- Macas, J.; Kejnovský, E.; Neumann, P.; Novák, P.; Koblížková, A.; Vyskot, B. Next generation sequencing-based analysis of repetitive DNA in the model dioecious plant Silene latifolia. PLoS ONE 2011, 6, e27335. [Google Scholar] [CrossRef]
- Renny-Byfield, S.; Kovařík, A.; Chester, M.; Nichols, R.A.; Macas, J.; Novák, P.; Leitch, A.R. Independent, rapid and targeted loss of highly repetitive DNA in natural and synthetic allopolyploids of Nicotiana tabacum. PLoS ONE 2012, 7, e36963. [Google Scholar] [CrossRef] [Green Version]
- Křivánková, A.; Kopecký, D.; Stočes, Š.; Doležel, J.; Hřibová, E. Repetitive DNA: A versatile tool for karyotyping in Festuca pratensis Huds. Cytogenet. Genome Res. 2017, 151, 96–105. [Google Scholar] [CrossRef]
- Said, M.; Hřibová, E.; Danilova, T.V.; Karafiátová, M.; Čížková, J.; Friebe, B.; Doležel, J.; Gill, B.S.; Vrána, J. The Agropyron cristatum karyotype, chromosome structure and cross-genome homoeology as revealed by fluorescence in situ hybridization with tandem repeats and wheat single-gene probes. Theor. Appl. Genet. 2018, 131, 2213–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwyrtková, J.; Němečková, A.; Čížková, J.; Holušová, K.; Kapustová, V.; Svačina, R.; Kopecký, D.; Till, B.J.; Doležel, J.; Hřibová, E. Comparative analyses of DNA repeats and identification of a novel Fesreba centromeric element in fescues and ryegrasses. BMC Plant Biol. 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- González, M.L.; Chiapella, J.O.; Urdampilleta, J.D. Genomic differentiation of Deschampsia antarctica and D. cespitosa (Poaceae) based on satellite DNA. Bot. J. Linn. Soc. 2020, 194, 326–341. [Google Scholar] [CrossRef]
- González, M.L.; Chiapella, J.O.; Urdampilleta, J.D. Chromosomal differentiation of Deschampsia (Poaceae) based on four satellite DNA families. Front. Genet. 2021, 12, 728664. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.G.; De Lange, P.J.; Ferguson, A.R. Nuclear DNA variation, chromosome numbers and polyploidy in the endemic and indigenous grass flora of New Zealand. Ann. Bot. 2005, 96, 1293–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greimler, J.; Temsch, E.M.; Xue, Z.; Weiss-Schneeweiss, H.; Volkova, P.; Peintinger, M.; Wasowicz, P.; Shang, H.; Schanzer, I.; Chiapella, J.O. Genome size variation in Deschampsia cespitosa sensu lato (Poaceae) in Eurasia. Plant Syst. Evol. 2022, 308, 9. [Google Scholar] [CrossRef]
- Novak, P.; Robledillo, L.A.; Koblizkova, A.; Vrbova, I.; Neumann, P.; Macas, J. TAREAN: A computational tool for identification and characterization of satellite DNA from unassembled short reads. Nucleic Acid Res. 2017, 45, e111. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [Green Version]
- Gerlach, W.L.; Bedbrook, J.R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979, 7, 1869–1885. [Google Scholar] [CrossRef]
- Gerlach, W.L.; Dyer, T.A. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980, 8, 4851–4855. [Google Scholar] [CrossRef] [Green Version]
- Muravenko, O.V.; Yurkevich, O.Y.; Bolsheva, N.L.; Samatadze, T.E.; Nosova, I.V.; Zelenina, D.A.; Volkov, A.; Popov, K.V.; Zelenin, A.V. Comparison of genomes of eight species of sections Linum and Adenolinum from the genus Linum based on chromosome banding, molecular markers and RAPD analysis. Genetica 2009, 135, 245–255. [Google Scholar] [CrossRef]
- Waring, M.; Britten, R.J. Nucleotide sequence repetition—A rapidly reassociating fraction of mouse DNA. Science 1966, 154, 791–794. [Google Scholar] [CrossRef]
- Britten, R.J.; Kohne, D.E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science 1968, 161, 529–540. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, N.; Mravinac, B.; Pavlek, M.; Vojvoda-Zeljko, T.; Šatović, E.; Plohl, M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res. 2015, 23, 583–596. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, P.; Tikhonov, A.; Jin, Y.-K.; Motchoulskaia, N.; Zakharov, D.; Melake-Berhan, A.; Springer, P.S.; Edwards, K.J.; Lee, M.; Avramova, Z.; et al. Nested retrotransposons in the inter-genic regions of the maize genome. Science 1996, 274, 765–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SanMiguel, P.; Bennetzen, J.L. Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotranposons. Ann. Bot. 1998, 82, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Makalowski, W. The human genome structure and organization. Acta Biochim. Pol. 2001, 48, 587–598. [Google Scholar] [CrossRef]
- Finnegan, D.J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989, 5, 103–107. [Google Scholar] [CrossRef]
- Makałowski, W.; Gotea, V.; Pande, A.; Makałowska, I. Transposable elements: Classification, identification, and their use as a tool for comparative genomics. In Evolutionary Genomics. Methods in Molecular Biology; Anisimova, M., Ed.; Humana: New York, NY, USA, 2019; Volume 1910, pp. 170–270. [Google Scholar] [CrossRef] [Green Version]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Neumann, P.; Novák, P.; Hoštáková, N.; Macas, J. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mob. DNA 2019, 10, 1. [Google Scholar] [CrossRef]
- Vitte, C.; Panaud, O. LTR retrotransposons and flowering plant genome size: Emergence of the increase/decrease model. Cytogenet. Genome Res. 2005, 110, 91–107. [Google Scholar] [CrossRef]
- Baucom, R.; Estill, J.; Chaparro, C.; Upshaw, N.; Jogi, A.; Deragon, J.-M.; Westerman, R.P.; SanMiguel, P.J.; Bennetzen, J.L. Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. PLoS Genet. 2009, 5, e1000732. [Google Scholar] [CrossRef]
- Macas, J.; Novák, P.; Pellicer, J.; Čížková, J.; Koblížková, A.; Neumann, P.; Fuková, I.; Doležel, J.; Kelly, L.J.; Leitch, I.J. In depth characterization of repetitive DNA in 23 plant genomes reveals sources of genome size variation in the vegume vribe Fabeae. PLoS ONE 2015, 10, e0143424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-J.; Gao, L.-I. Rapid and recent evolution of LTR retrotransposons drives rice genome evolution during the speciation of AA-genome Oryza species. G3 2017, 7, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, J.; Macas, J.; Novák, P.; Stuessy, T.F.; Villasenor, J.L.; Weiss-Schneweiss, H. Differential genome size and repetitive DNA evolution in diploid species of Melampodium sect Melampodium (Asteraceae). Front. Plant Sci. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zheng, Z.; Li, Y.; Hu, H.; Wang, Z.; Du, X. Which factors contribute most to genome size variation within angiosperms? Ecol. Evol. 2021, 11, 2660–2668. [Google Scholar] [CrossRef]
- Becher, H.; Powell, R.F.; Brown, M.R.; Metherell, C.; Pellicer, J.; Leitch, I.J.; Twyford, A.D. The nature of intraspecific and interspecific genome size variation in taxonomically complex eyebrights. Ann. Bot. 2021, 128, 639–651. [Google Scholar] [CrossRef]
- Kawano, S. Cytogeography and evolution of the Deschampsia caespitosa complex. Can. J. Bot. 1963, 41, 719–742. [Google Scholar] [CrossRef]
- Bennett, M.D.; Smith, J.B.; Lewis Smith, R.I. DNA amounts of angiosperms from the Antarctic and South Georgia. Environ. Exp. Bot. 1982, 22, 307–318. [Google Scholar] [CrossRef]
- Pascual-Díaz, J.P.; Serçe, S.; Hradecká, I.; Vanek, M.; Özdemir, B.S.; Sultana, N.; Vural, M.; Vitales, D.; Garcia, S. Genome size constancy in Antarctic populations of Colobanthus quitensis and Deschampsia antarctica. Polar Biol. 2020, 43, 1407–1413. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Zhou, X.; Li, M.; Zhang, F.; Schwarzacher, T.; Heslop-Harrison, J.S. The repetitive DNA landscape in Avena (Poaceae): Chromosome and genome evolution defined by major repeat classes in whole-genome sequence reads. BMC Plant Biol. 2019, 19, 226. [Google Scholar] [CrossRef] [Green Version]
- Stapley, J.; Santure, A.W.; Dennis, S.R. Transposable elements as agents of rapid adaptation may explain the genetic paradox of invasive species. Mol. Ecol. 2015, 24, 2241–2252. [Google Scholar] [CrossRef]
- Holderegger, R.; Stehlic, I.; Lewis, R.I.; Smith Abbott, R.J. Population of Antarctic hairgrass (Deschampsia antarctica) show low genetic diversity. Arct. Antarct. Alp. Res. 2003, 35, 214–217. [Google Scholar] [CrossRef]
- Chwedorzewska, K.J.; Bednarek, P.T. Genetic variability in the Antarctic hairgrass Deschampsia antarctica Desv from Maritime Antarctic and subantarctic sites. Pol. J. Ecol. 2008, 56, 209–216. [Google Scholar]
- Chwedorzewska, K.J.; Bednarek, P.T. Genetic and epigenetic studies on populations of Deschampsia antarctica Desv. from contrasting environments at King George Island (Antarctic). Pol. Polar Res. 2011, 32, 15–26. [Google Scholar] [CrossRef]
- Moreau-Mhiri, C.; Morel, J.B.; Audeon, C.; Ferault, M.; Grandbastien, M.A.; Lucas, H. Regulation of expression of the tobacco Tnt1 retrotransposon in heterologous species following pathogen-related stresses. Plant J. 1996, 9, 409–419. [Google Scholar] [CrossRef]
- Takeda, S.; Sugimoto, K.; Otsuki, H.; Hirochika, H. Transcriptional activation of the tobacco retrotransposon Tto1 by wounding and methyl jasmonate. Plant Mol. Biol. 1998, 36, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Voronova, A.; Jansons, Ā.; Ruņģis, D. Expression of retrotransposon-like sequences in Scots pine (Pinus sylvestris) in response to heat stress. Environ. Exp. Biol. 2011, 9, 121–127. [Google Scholar]
- Schrader, L.; Kim, J.W.; Ence, D.; Zimin, A.; Klein, A.; Wyschetzki, K.; Weichselgartner, T.; Kemena, C.; Stökl, J.; Schultner, E.; et al. Transposable element islands facilitate adaptation to novel environments in an invasive species. Nat. Commun. 2014, 5, 5495. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Cardoso, M.; Jouve, N. Physical organization of simple sequence repeats (SSRs) in Triticeae: Structural, functional and evolutionary implications. Cytogenet. Genome Res. 2008, 120, 210–219. [Google Scholar] [CrossRef]
- Dou, Q.; Liu, R.; Yu, F. Chromosomal organization of repetitive DNAs in Hordeum bogdanii and H. brevisubulatum (Poaceae). Comp. Cytogenet. 2016, 10, 465–481. [Google Scholar] [CrossRef] [Green Version]
- McStay, B. Nucleolar organizer regions: Genomic ‘dark matter’ requiring illumination. Genes Dev. 2016, 30, 1598–1610. [Google Scholar] [CrossRef] [Green Version]
- Kubis, S.; Schmidt, T.; Heslop-Harrison, J.S. Repetitive DNA elements as a major component of plant genomes. Ann. Bot. 1998, 82, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Hemleben, V.; Kovařík, A.; Torres-Ruiz, R.A.; Volkov, R.A.; Beridze, T. Plant highly repeated satellite DNA: Molecular evolution, distribution and use for identification of hybrids. Syst. Biodivers. 2007, 5, 277–289. [Google Scholar] [CrossRef]
- Macas, J.; Mészáros, T.; Nouzová, M. PlantSat: A Specialized Database for Plant Satellite Repeats. Bioinformatics 2002, 18, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Raina, S.N. Organization and evolution of highly repeated satellite DNA sequences in plant chromosomes. Cytogenet. Genome Res. 2005, 109, 15–26. [Google Scholar] [CrossRef]
- Plohl, M.; Meštrovic, N.; Mravinac, B. Satellite DNA evolution. In Repetitive DNA.; Garrido-Ramos, M.A., Ed.; Karger: Granada, Spain, 2012; pp. 126–152. [Google Scholar] [CrossRef]
- Plohl, M.; Petrović, V.; Luchetti, A.; Ricci, A.; Šatović, E.; Passamonti, M.; Mantovani, B. Long-term conservation vs high sequence divergence: The case of an extraordinarily old satellite DNA in bivalve mollusks. Heredity 2010, 104, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Ramos, M.A. Satellite DNA in Plants: More than Just Rubbish. Cytogenet. Genome Res. 2015, 146, 153–170. [Google Scholar] [CrossRef]
- Ruiz-Ruano, F.J.; López-León, M.D.; Cabrero, J.; Camacho, J.P.M. High-throughput analysis of the satellitome illuminates satellite DNA evolution. Sci. Rep. 2016, 6, 28333. [Google Scholar] [CrossRef] [Green Version]
- Lower, S.S.; McGurk, M.P.; Clark, A.G.; Barbash, D.A. Satellite DNA Evolution: Old Ideas, New Approaches. Curr. Opin. Genet. Dev. 2018, 49, 70–78. [Google Scholar] [CrossRef]
- Wei, L.; Liu, B.; Zhang, C.; Yu, Y.; Yang, X.; Dou, Q.; Dong, Q. Identification and characterization of satellite DNAs in Poa, L. Mol. Cytogenet. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Ugarkovic, D. Functional elements residing within satellite DNAs. EMBO Rep. 2005, 6, 1035–1039. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.S. Comparative Genome Organization in Plants: From Sequence and Markers to Chromatin and Chromosomes. Plant Cell 2000, 12, 617–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslop-Harrison, J.S. Planning for remodelling: Nuclear architecture, chromatin and chromosomes. Trends Plant Sci. 2003, 8, 195–197. [Google Scholar] [CrossRef]
- Grebenstein, B.; Grebenstein, O.; Sauer, W.; Hemleben, V. Distribution and complex organization of satellite DNA sequences in Aveneae species. Genome 1996, 39, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- González, M.L.; Chiapella, J.O.; Urdampilleta, J.D. Characterization of some satellite DNA families in Deschampsia antarctica (Poaceae). Polar Biol. 2018, 41, 457–468. [Google Scholar] [CrossRef]
- Miklos, G.L.G.; Gill, A.C. Nucleotide sequences of highly repeated DNAs; compilation and comments. Genet. Res. 1982, 39, 1–30. [Google Scholar] [CrossRef]
- Badaeva, E.D.; Chikida, N.N.; Belousova, M.K.; Ruban, A.S.; Surzhikov, S.A.; Zoshchuk, S.A. A new insight on the evolution of polyploid Aegilops species from the complex Crassa: Molecular-cytogenetic analysis. Plant Syst. Evol. 2021, 307, 3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).