Abstract
Pulmonary arterial hypertension (PAH) can be caused by pathogenic variants in the gene bone morphogenetic protein receptor 2 (BMPR2). While BMPR2 protein expression levels are known to be reduced in the lung tissue of heritable PAH (HPAH) patients, a systematic study evaluating expression in more easily accessible blood samples and its clinical relevance is lacking. Thus, we analyzed the BMPR2 mRNA expression in idiopathic/HPAH patients and healthy controls in blood by quantitative polymerase chain reaction and protein expression by enzyme-linked immunosorbent assay. Clinical parameters included right heart catherization, echocardiography, six-minute walking test and laboratory tests. BMPR2 variant-carriers (n = 23) showed significantly lower BMPR2 mRNA expression in comparison to non-carriers (n = 56) and healthy controls (n = 30; p < 0.0001). No difference in BMPR2 protein expression was detected. Lower BMPR2 mRNA expression correlated significantly with greater systolic pulmonary artery pressure and pulmonary vascular resistance. Higher BMPR2 mRNA expression correlated with greater glomerular filtration rate, cardiac index and six-minute walking distance. We demonstrated the feasibility to assess BMPR2 expression in blood and, for the first time, that BMPR2 mRNA expression levels are significantly reduced in variant carriers and correlated with clinical parameters. Further studies may evaluate the usefulness of BMPR2 mRNA expression in blood as a new marker for disease severity.
1. Introduction
Pulmonary arterial hypertension (PAH) is characterized by a progressive pulmonary vascular remodeling which leads to increased pulmonary vascular resistance, right ventricular hypertrophy and, ultimately, right heart failure [1]. As of today, different pathogenic germline variants in 18 genes have been described in heritable PAH (HPAH), of which new ones are constantly being discovered [2]. Most of these pathogenic variants affect the bone morphogenetic protein receptor type II (BMPR2) gene and are identified in 50–80% of HPAH patients and in about 15–20% of idiopathic PAH (IPAH) patients [3,4]. These disease-causing variants are inherited in an autosomal dominant manner, but with reduced penetrance. Thus, not all family members carrying a pathogenic variant develop manifest PAH. Reasons for this reduced penetrance may be the higher expression of the wildtype variant in a heterozygous carrier or the need for additional disease triggers, such as another pathogenic variant as a “second hit” in affected patients [5].
In healthy lung tissue, BMPR2 is mainly expressed in endothelial cells [6]. In PAH patients, BMPR2 expression could also be detected in the endothelium and the myofibroblasts of plexiform lesions [7]. However, the BMPR2 protein expression was greatly reduced in pulmonary endothelial cells of BMPR2 variant carriers [6]. A less pronounced but still highly significant reduction in expression was identified in non-variant carriers in comparison to healthy controls [6].
In line with BMPR2 variants disrupting important molecular pathways, disease severity has been shown to be significantly worse in variant carriers [4,8]. HPAH patients with pathogenic variants in the BMPR2 gene were characterized by an earlier age at diagnosis, worse hemodynamics and higher rate of lung transplantation as well as all-cause mortality compared to non-variant carriers [4,8]. Hence, it is important to regularly screen apparently healthy BMPR2 variant carriers clinically to identify possible disease onset as early as possible [9,10]. An early detection allows to start treatment at an early stage and may lead to a better outcome [9]. Since lung biopsies are contraindicated in PAH patients due to a high risk of morbidity and mortality [1], our objective was, firstly, to clarify whether BMPR2 mRNA and protein blood expression levels showed differences in affected BMPR2 variant carriers in comparison to non-variant carriers and to healthy controls; and secondly, we aimed to correlate BMPR2 mRNA and protein blood expression levels with clinical and laboratory parameters.
2. Materials and Methods
2.1. Study Design
This was a prospective, explorative, cross-sectional study investigating the RNA and protein expression level of BMPR2 in the blood of PAH patients and healthy controls. The majority of patients was diagnosed and treated at the Centre for Pulmonary Hypertension, Thoraxklinik Heidelberg gGmbH at Heidelberg University Hospital, Germany. Three of the BMPR2 variant carriers were treated at the Carl Gustav Carus University Hospital Dresden, Germany and four at the University Hospital Leipzig, Germany. Age- and gender-matched healthy controls were invited to participate if a routine blood draw was performed.
2.2. Clinical Investigations
Clinical parameters for patient characterization were obtained from clinical records at the time of study enrollment and included demographic data, comorbidities, vital signs, hemodynamics measured by echocardiography and right heart catheterization, spirometry, blood gas analysis, lung function, 6 min walking distance, as well as the laboratory parameters for the assessment of renal function (creatinine, urea, and glomerular filtration rate) and other routine parameters, such as N-terminal pro-brain natriuretic peptide (NT-proBNP). If no echocardiography, right heart catheterization, spirometry or lung function was performed during the inclusion visit, the data from the most recent examination were used.
2.3. Genetic Investigations
DNA was extracted from 3–10 mL of EDTA-blood samples using an automated procedure (Autopure or QIAsymphony, Qiagen, Hilden, Germany). Genetic diagnostic analysis was carried out with our patented (EP3507380) PAH-specific gene panel, including the following PAH diagnostic genes: ACVRL1, AQP1, ATP13A3, BMPR1B, BMPR2, CAV1, EIF2AK4, ENG, GDF2, KCNA5, KCNK3, KLF2, SMAD4, SMAD9, SOX17 and TBX4 (customized SureSelect QXT kit, Agilent, Germany). The procedure was carried out as previously described [2,11].
Multiplex ligation-dependent probe amplification (MLPA) was performed in addition to identify exon deletions and duplications in the genes ACVRL1, BMPR2 and ENG (P093-C2, MRC-Holland, Amsterdam, The Netherlands). Familial variants were sought by Sanger sequencing (ABI Genetic Analyzer 3130xl, Applied Biosystems, MA, USA) or MLPA. Variants were characterized following the American College of Medical Genetics and Genomics guidelines [12].
2.4. Expression Level Analyses
For each patient, whole blood was collected in PAXgene Blood RNA tubes (bd, Heidelberg, Germany) and serum using serum gel tubes (Sarstedt, Nümbrecht, Germany). RNA was extracted with a PAXgene Blood RNA kit (Qiagen, Hilden, Germany) by a semi-automated procedure following the manufacturer’s protocol (QIAcube, Qiagen, Germany). The extracted RNA was stored at −80 °C before the production of complementary DNA (cDNA) with Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Quantitative polymerase chain reaction (qPCR) was performed in accordance with MIQE-guidelines (minimum information for publication of quantitative real-time PCR experiments) using a LightCycler® 480 Real-Time PCR instrument (software version 1.5, Roche, Basel, Switzerland) [13]. Gene-specific primers and probes (Universal ProbeLibrary, Roche, Basel, Switzerland) were used in combination with qPCR Probe-MasterMix. To calculate the difference of the cycle threshold (∆CT) values of BMPR2, the CT value of the two housekeeping genes ESD (esterase D) and RPS18 (40S ribosomal protein S18) were considered, following the 2nd derivative maximum method. The detailed procedure is described elsewhere [13]. To better illustrate the values determined, 1/∆CT of the relative BMPR2 mRNA expression was used in the statistical analysis.
Serum samples were used for enzyme-linked immunosorbent assay (ELISA). Briefly, serum collected from whole blood was frozen at −80 °C to enable a joined analysis of samples and minimize batch effects. Serum from each proband was pipetted into a 96-well plate and treated with ELISA reagents according to the manufacturer’s instructions (human BMPR2 ELISA kit, Elabscience, Wuhan, China). Results were quantified at 450 nm using a microplate reader (Sunrise, Tecan, Männedorf, Switzerland). For each patient, three technical replicates were measured in ELISA and qPCR.
2.5. Statistical Methods
Data including patient characteristics, clinical parameters and laboratory data were evaluated by two professional statisticians (N.B. and S.L.), using descriptive statistics with mean ± standard deviation and frequency tables. Frequency data were presented as n and % and were analyzed using the chi-square test. Clinical characteristics and laboratory data of BMPR2 variant carriers, non-carriers and healthy controls were compared with Mann–Whitney U test for independent samples. Differences between variant carriers and non-carriers were analyzed by the Mann–Whitney U test and students’ t-test. Association between clinical characteristics and laboratory data was tested by Pearson correlation. Parameters with more than 30% missing values were not considered for correlation analysis. p values < 0.05 were considered statistically significant. All analyses were performed with SPSS V 25.0 (IBM Corp., Armonk, NY, USA) or SAS 9.4 for Windows (SAS Corp., Charlotte, NC, USA).
3. Results
3.1. Baseline Characteristics
Between May 2019 and January 2020, 110 PAH patients and healthy volunteers were screened for this study. One participant was excluded with the differential diagnosis of pulmonary veno-occlusive disease (PVOD). Thus, 79 I/HPAH patients and 30 healthy controls were included (Table 1, Figure 1). Genetic testing revealed 23 of the 79 PAH patients (29.1%) to be BMPR2 variant-carriers (Figure 1). In the remaining 56 PAH patients (70.9%), no (likely) pathogenic BMPR2 variant was identified. Of note, two IPAH patients without a disease-causing BMPR2 variant had a pathogenic missense variant in the gene SMAD9 and GDF2, respectively. The 56 patients were classified as BMPR2 non-carriers (Figure 1). For all patients and the healthy controls, a BMPR2 gene mRNA and protein expression analysis was performed in addition to the routine clinical examinations. Out of 79 PAH patients, 58 were female (73%, Table 1). Mean age was 51.4 ± 16 years.

Table 1.
Baseline characteristics of PAH patients.
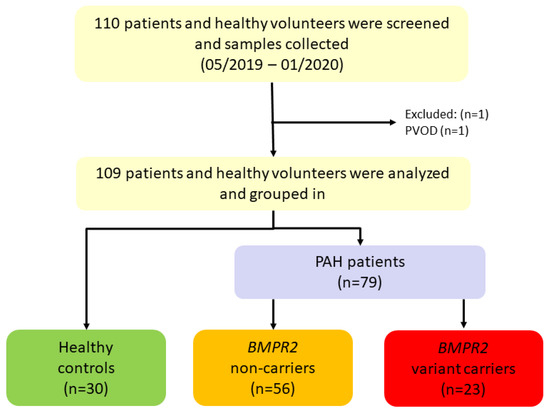
Figure 1.
Recruitment of study population from May 2019 to January 2020. 110 participants were screened, and samples were collected of which 109 were analyzed and included. BMPR2: bone morphogenetic protein receptor type II, PAH: pulmonary arterial hypertension, PVOD: pulmonary veno-occlusive disease.
Table 1 shows baseline characteristics of the whole study cohort and of BMPR2 variant carriers and non-carriers. Right heart catheterization revealed on average severely increased pulmonary pressures with high pulmonary vascular resistance. Most patients showed an enlarged right heart with mild impairment of right ventricular systolic function. On average, patients displayed a normal renal function. One patient, who was a positive vasoresponder, received only calcium channel blocker therapy. Most patients received double or triple combination treatment (Table 1). Healthy controls were age and gender matched to non-variant carriers. Out of 30 healthy controls, 22 were female (73%). The mean age was 46.8 ± 13.9 years (vs. 73% female in the patient cohort with an age of 51.4 ± 16 years).
3.2. Clinical Differences between BMPR2 Variant Carriers and Non-Carriers
Patients with a pathogenic BMPR2 variant suffered from more severe PAH than non-carriers (Table 1). They showed a significantly higher systolic and mean pulmonary arterial pressure (p = 0.001 and 0.029, respectively), a significantly higher pulmonary vascular resistance (p < 0.001), lower cardiac output (p = 0.003) and lower cardiac index (p = 0.005) measured by right heart catheterization (Table 1).
3.3. BMPR2 Characterization and Expression
The pathogenic variants in the BMPR2 gene in the 23 variant carriers were characterized as base pair deletions (n = 7) or duplications (n = 2) leading to a frameshift, whole exon deletions (n = 2), whole exon duplications (n = 1), splice site variants (n = 6) or nonsense (n = 5) variants.
BMPR2 mRNA expression levels in whole blood measured by qPCR significantly differed between BMPR2 variant carriers, non-carriers and healthy controls (p < 0.0001, Figure 2). BMPR2 variant carriers had the lowest expression levels. Non-carriers showed lower BMPR2 mRNA expression in comparison to healthy controls, which was not statistically significant (p = 0.151, Figure 2). The two IPAH patients with pathogenic variants in the genes GDF2 or SMAD9 had lower BMPR2 mRNA expression levels (0.394 and 0.433, respectively) than average in the BMPR2 non-carrier group (0.445), albeit having higher values than the average of the BMPR2 variant carrier group (0.353).
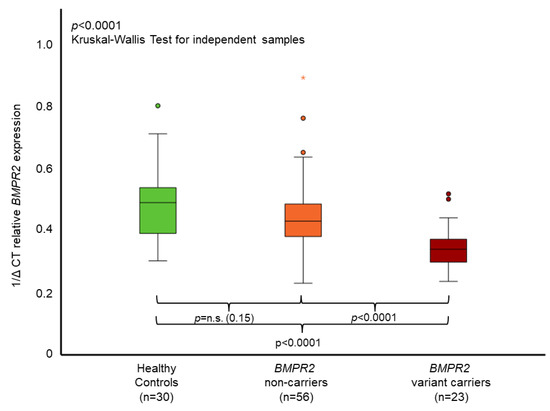
Figure 2.
Mean relative BMPR2 mRNA expression in whole blood. The boxplots provide median values (horizontal lines), interquartile range (box), 1.5× interquartile range (whiskers), outliers (indicated by circles within 1.5 to 3× interquartile range) and extreme outliers (indicated by asterisks > 3× interquartile range). A significant difference of BMPR2 mRNA expression could be identified between healthy controls, BMPR2 non-carriers and BMPR2 variant carriers. With Bonferroni correction, p-values were healthy controls vs. non-carriers p = 0.453, non-carriers vs. variant carriers p = 0.0002, and healthy controls vs. variant carriers p < 0.0001. The 1/delta cycle threshold (1/∆CT) denotes the level of BMPR2 mRNA gene expression measured by qPCR. n.s. = non-significant.
No significant difference could be identified between the groups on BMPR2 protein level measured by enzyme-linked immunosorbent assay (ELISA) in sera (healthy controls: 0.31 ± 0.23 ng/mL, non-carriers 0.48 ± 0.43 ng/mL, BMPR2 variant carriers 0.51 ± 0.49 ng/mL, p = 0.52).
3.4. Correlation of BMPR2 mRNA Expression with Further Laboratory and Clinical Characteristics
Low BMPR2 mRNA expression correlated weakly but significantly with low glomerular filtration rate, high hematocrit and high urea levels (Table 2). Patients with lower BMPR2 mRNA expression had significantly higher systolic pulmonary arterial pressures (sPAP) measured by right heart catheterization and echocardiography, greater right ventricular wall thickness, higher pulmonary vascular resistance and a lower cardiac index and a shorter 6 min walking distance (Table 2).

Table 2.
Correlation of BMPR2 mRNA expression with laboratory and clinical characteristics.
4. Discussion
This study showed that BMPR2 mRNA levels measured in peripheral blood were significantly reduced in PAH patients with a (likely) pathogenic BMPR2 variant compared to healthy controls. Non-variant carriers showed a trend of reduced BMPR2 mRNA expression levels. Furthermore, the study revealed for the first time a significant correlation between lower BMPR2 mRNA expression levels measured in blood cells and worse values for hemodynamic and laboratory parameters. In particular, the significant correlation of low BMPR2 mRNA expression with worse hemodynamics and lower exercise capacity suggests that this analysis could be used as a marker for disease severity reflecting underlying pathomechanisms.
4.1. Correlation of BMPR2 mRNA Expression with Further Laboratory and Clinical Characteristics
Disease-causing BMPR2 germline variants seem to reduce mRNA expression not only in pulmonary endothelial cells [6], but also in peripheral blood cells. The two patients with pathogenic variants in GDF2, which encodes the BMPR2 ligand BMP9, and SMAD9, which encodes the downstream pathway signaling protein SMAD8, had intermediate BMPR2 mRNA expression levels compared to the group averages. Thus, also pathogenic variants in other members of the BMPR2 pathway may be able to reduce BMPR2 mRNA expression possibly via impaired positive feedback loops.
In our study, a reduced BMPR2 mRNA expression was also observed in trend in BMPR2 non-carriers suffering from PAH in comparison to healthy controls, suggesting that there could be different pathomechanisms reducing BMPR2 mRNA expression apart from pathogenic BMPR2 variants [6]. These findings could be important for better understanding of the development of PAH not only in variant carriers, but also in PAH patients without BMPR2 variants. However, a study with larger group sizes is required to draw definite conclusions, as no clear significant differences could be identified in this study, providing only first preliminary data. In a small cohort of 23 PAH patients with mixed etiologies, a reduced BMPR2 mRNA expression in mononuclear blood cells was previously reported by Spiekerkoetter et al. 2017 in comparison to 13 healthy controls. The same phase IIa study showed a dose-dependent, albeit non-significant, increase in BMPR2 mRNA expression by the immunomodulator FK506 (tacrolimus), highlighting BMPR2 as a potential therapeutic target [14].
A recent phase II study demonstrated the TGF-β superfamily ligand trap sotatercept to be a therapeutic option to rebalance growth-promoting and growth-inhibiting signaling in PAH patients [15]. Sotatercept additionally given to a background therapy could significantly decrease pulmonary vascular resistance [15]. The effect of sotatercept on BMPR2 mRNA expression was not reported. An assessment of BMPR2 mRNA expression levels in peripheral blood may also prove to be a valuable marker for therapy response in PAH patients.
4.2. BMPR2 mRNA Expression and Correlation to Other Parameters
To our knowledge, this is the first study that found a correlation between clinical and laboratory parameters and the BMPR2 mRNA expression measured in blood cells. The negative correlation to hemodynamic parameters, such as sPAP, pulmonary vascular resistance or right ventricular wall thickness, and the positive correlation with the cardiac index might highlight BMPR2 mRNA expression to be a potential prognostic factor or indicator of hemodynamic status. Similarly, the positive correlation with 6 min walking distance could highlight an important relationship with exercise capacity. However, future trials are needed to verify these results and to assess if BMPR2 mRNA expression levels may be a useful marker for follow-up in affected patients and/or for screening in asymptomatic BMPR2 variant carriers to possibly monitor disease onset. Moreover, further studies are needed to investigate the consistency of BMPR2 measurements in the same individual or its alterations, e.g., due to clinical deterioration. Similarly, the response in particular to novel, upcoming treatment options addressing the BMPR2 pathway, such as sotatercept, could be investigated by measuring the effect on BMPR2 mRNA expression in blood.
Apart from the BMPR2 gene and its pathway, various other genes have shown to be involved in the pathogenesis of PAH, such as the potassium channel gene KCNK3 or the transcription factors SOX17 and TBX4 [16]. On a molecular level, an array of different pathways contributes to PAH development, such as pathways involved in inflammation, vascular stiffness, platelet-derived growth factor signaling, iron homeostasis or estrogen signaling [17]. Hence, it is also important to consider other factors in future studies and view PAH as a consequence of a multitude of molecular changes.
4.3. Limitations
This study did not enroll asymptomatic BMPR2 variant carriers due to the restricted time frame. Therefore, we will evaluate the differences in BMPR2 expression in PAH variant carriers and not affected carriers in a subsequent, larger, prospective study. Furthermore, the sample size was not large enough to investigate expression level differences between patients with different types of pathogenetic variants, such as nonsense or frameshift variants. Protein BMPR2 expression showed no significantly different levels in the serum of our patients. This could also be due to the nature of BMPR2 being a transmembrane protein, which should be present at lower levels in serum. Finally, the study only included a single time point of measurement. Further studies are required for longitudinal measurements not only to provide a range for intra-patient measurements in stable patients, but also to identify BMPR2 mRNA expression changes upon treatment changes or clinical worsening.
5. Conclusions
This study showed that BMPR2 mRNA expression levels could be quantified in peripheral blood and were significantly reduced in BMPR2 variant carriers. Non-carriers showed, in trend, reduced expression rates. Reduced BMPR2 mRNA expression levels were significantly associated with disease severity. Further studies are needed to evaluate whether BMPR2 mRNA expression in peripheral blood is useful as a new marker for pathogenesis and disease severity. This study underlines the central role of BMPR2 signaling in PAH and provides impetus for future research.
Author Contributions
Conceptualization, C.A.E., V.T. and E.G.; methodology, C.A.E., N.B., V.T. and E.G.; validation, V.T. and N.B.; formal analysis, N.B. and V.T.; investigation, V.T., H.-J.S., M.H., M.A.S., S.R., K.H., P.X., B.E., R.S., M.M.H. and D.J.; resources, K.H., E.G., C.A.E.; data curation, V.T., C.A.E. and N.B.; writing—original draft preparation, V.T., C.A.E., N.B. and E.G.; writing—review and editing, H.-J.S., M.H., M.A.S., S.R., K.H., P.X., B.E., R.S., M.M.H. and D.J.; visualization, N.B., V.T. and C.A.E.; supervision, C.A.E. and E.G.; project administration, C.A.E.; funding acquisition, C.A.E. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Center for Lung Research (DZL), grant number 82DZLB44A2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of Heidelberg University Hospital, Germany (protocol code S-226/2019, 25 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
This work is the doctoral thesis of VT. We would like to thank Silke Lange for her statistical support (58454 Witten, Germany).
Conflicts of Interest
E.G., C.A.E. and K.H. report an issued patent “Gene panel specific for pulmonary hypertension and its uses” European Patent ID: EP3507380; V.T., M.A.S., S.R., R.S., D.J. declare that they have no competing interest related to this study; N.B. received speaker fees from Actelion pharmaceuticals/Janssen Medical, Bayer HealthCare and MSD, outside the submitted work; H.J.S. reports consulting fees from Actelion/Janssen and speaker fees for Actelion, Bayer, GSK, Janssen and MSD outside the submitted work; M.H. has received personal fees for consultations and lectures from Acceleron, Actelion, AstraZeneca, Bayer, BerlinChemie, GSK, Janssen-Cilag, MSD, and Novartis, outside the publication; P.X. has received personal fees from MSD and OMT outside the submitted work; B.E. received travel fees, consulting fees, speaking fees, and/or honoraria from Actelion, MSD, Bayer and OMT, outside the submitted work; M.M.H. has received fees for consultations and/or lecturers from Acceleron, Actelion, Bayer, GSK, Janssen, MSD and Pfizer, all outside the submitted work; E.G. has received grants and personal fees from Actelion, Bayer AG, and MSD; grants from GSK, Novartis, and United Therapeutics and personal fees from SCOPE, OrPha Swiss GmbH, and Zurich Heart House, outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [PubMed]
- Song, J.; Eichstaedt, C.A.; Rodríguez Viales, R.; Benjamin, N.; Harutyunova, S.; Fischer, C.; Grünig, E.; Hinderhofer, K. Identification of genetic defects in pulmonary arterial hypertension by a new gene panel diagnostic tool. Clin. Sci. 2016, 130, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.D.; Eickelberg, O.; Elliott, C.G.; Geraci, M.W.; Hanaoka, M.; Loyd, J.E.; Newman, J.H.; Phillips, J.A., 3rd; Soubrier, F.; Trembath, R.C.; et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54, S32–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfarr, N.; Szamalek-Hoegel, J.; Fischer, C.; Hinderhofer, K.; Nagel, C.; Ehlken, N.; Tiede, H.; Olschewski, H.; Reichenberger, F.; Ghofrani, A.H.; et al. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respir. Res. 2011, 12, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichstaedt, C.A.; Song, J.; Benjamin, N.; Harutyunova, S.; Fischer, C.; Grünig, E.; Hinderhofer, K. EIF2AK4 mutation as “second hit” in hereditary pulmonary arterial hypertension. Respir. Res. 2016, 17, 141. [Google Scholar] [PubMed] [Green Version]
- Atkinson, C.; Stewart, S.; Upton, P.D.; Machado, R.; Thomson, J.R.; Trembath, R.C.; Morrell, N.W. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002, 105, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Jonigk, D.; Golpon, H.; Bockmeyer, C.L.; Maegel, L.; Hoeper, M.M.; Gottlieb, J.; Nickel, N.; Hussein, K.; Maus, U.; Lehmann, U.; et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am. J. Pathol. 2011, 179, 167–179. [Google Scholar] [CrossRef]
- Evans, J.D.W.; Girerd, B.; Montani, D.; Wang, X.-J.; Galiè, N.; Austin, E.D.; Elliott, G.; Asano, K.; Grünig, E.; Yan, Y.; et al. BMPR2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir. Med. 2016, 4, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Hinderhofer, K.; Fischer, C.; Pfarr, N.; Szamalek-Hoegel, J.; Lichtblau, M.; Nagel, C.; Egenlauf, B.; Ehlken, N.; Grünig, E. Identification of a new intronic BMPR2-mutation and early diagnosis of heritable pulmonary arterial hypertension in a large family with mean clinical follow-up of 12 years. PLoS ONE 2014, 9, e91374. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Girerd, B.; Jaïs, X.; Laveneziana, P.; Lau, E.M.T.; Bouchachi, A.; Hascoët, S.; Günther, S.; Godinas, L.; Parent, F.; et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur. Respir. J. 2021, 58, 2004229. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, C.A.; Verweyen, J.; Halank, M.; Benjamin, N.; Fischer, C.; Mayer, E.; Guth, S.; Wiedenroth, C.B.; Egenlauf, B.; Harutyunova, S.; et al. Myeloproliferative Diseases as Possible Risk Factor for Development of Chronic Thromboembolic Pulmonary Hypertension-A Genetic Study. Int. J. Mol. Sci. 2020, 21, 3339. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.A.; Granzow, M.; Warth, A.; Schnabel, P.A.; Thomas, M.; Herth, F.J.; Dienemann, H.; Muley, T.; Meister, M. Glycodelin: A New Biomarker with Immunomodulatory Functions in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 3529–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiekerkoetter, E.; Sung, Y.K.; Sudheendra, D.; Scott, V.; Del Rosario, P.; Bill, M.; Haddad, F.; Long-Boyle, J.; Hedlin, H.; Zamanian, R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1602449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, C.A.; Sassmannshausen, Z.; Shaukat, M.; Cao, D.; Xanthouli, P.; Gall, H.; Sommer, N.; Ghofrani, H.A.; Seyfarth, H.J.; Lerche, M.; et al. Gene panel diagnostics reveals new pathogenic variants in pulmonary arterial hypertension. Respir. Res. 2022, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Humbert, M. Pathobiology of pulmonary arterial hypertension: Understanding the roads less travelled. Eur. Respir. Rev. 2017, 26, 170093. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


