DLK2 Acts as a Potential Prognostic Biomarker for Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Pan-Cancer Analysis
2.2. TNMplot Analysis
2.3. UALCAN Analysis
2.4. Kaplan–Meier Plotter (KM Plotter) Analysis
2.5. LinkedOmics Database Analysis
2.6. TIMER Database Analysis
2.7. Statistical Analysis
3. Results
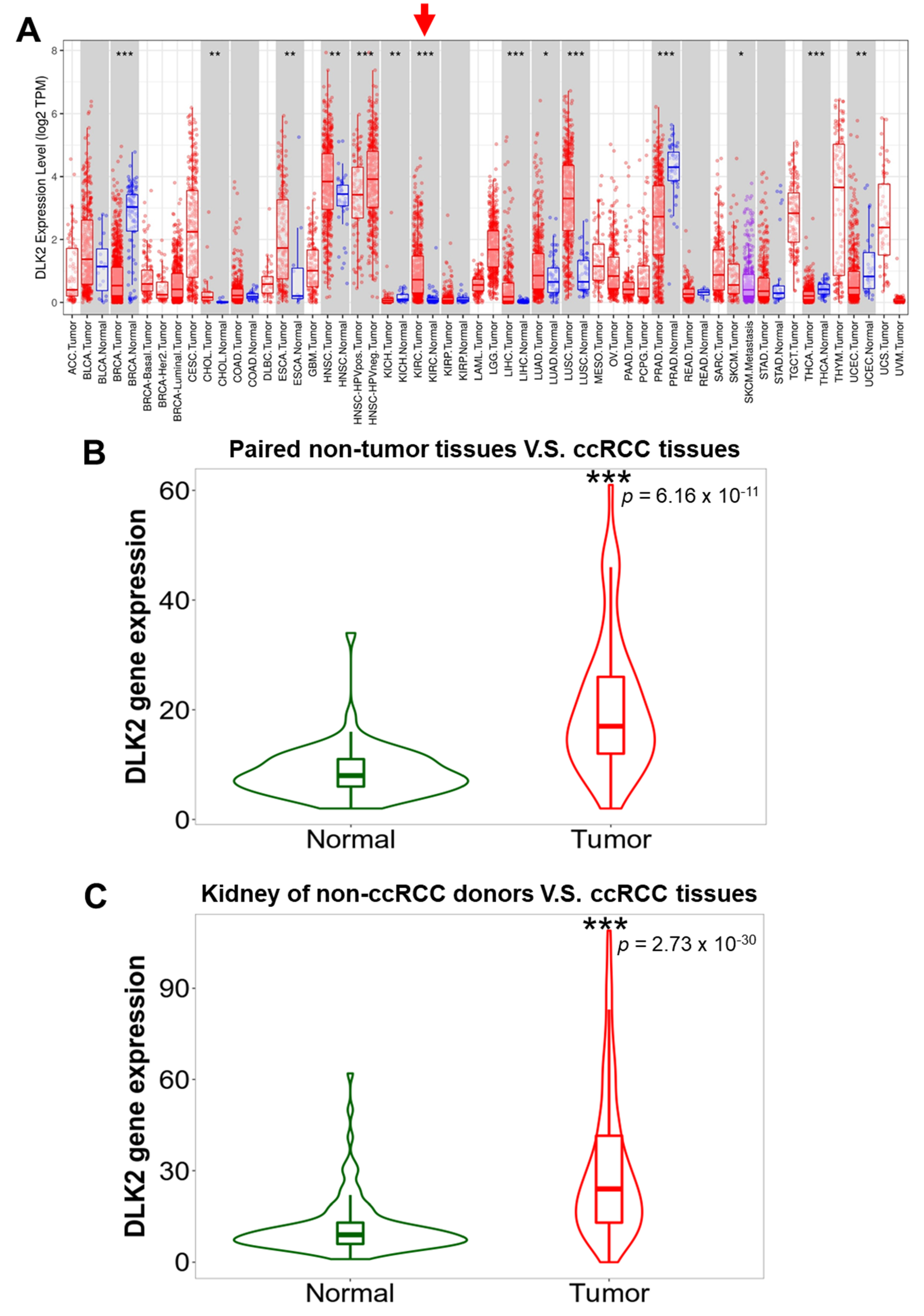
3.1. DLK2 Was Upregulated in the Tumor Tissues of ccRCC Compared with Normal Kidney Tissues
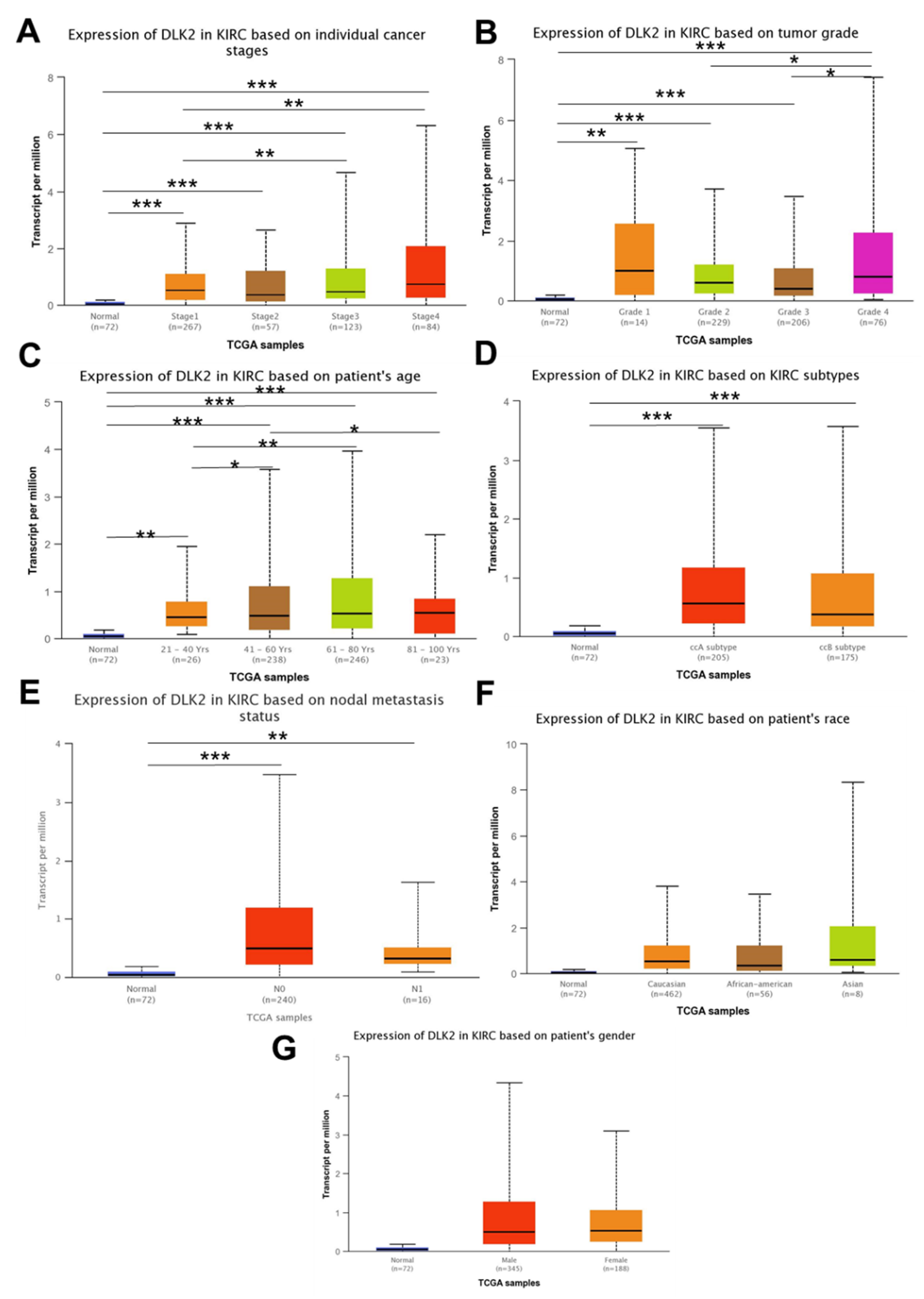
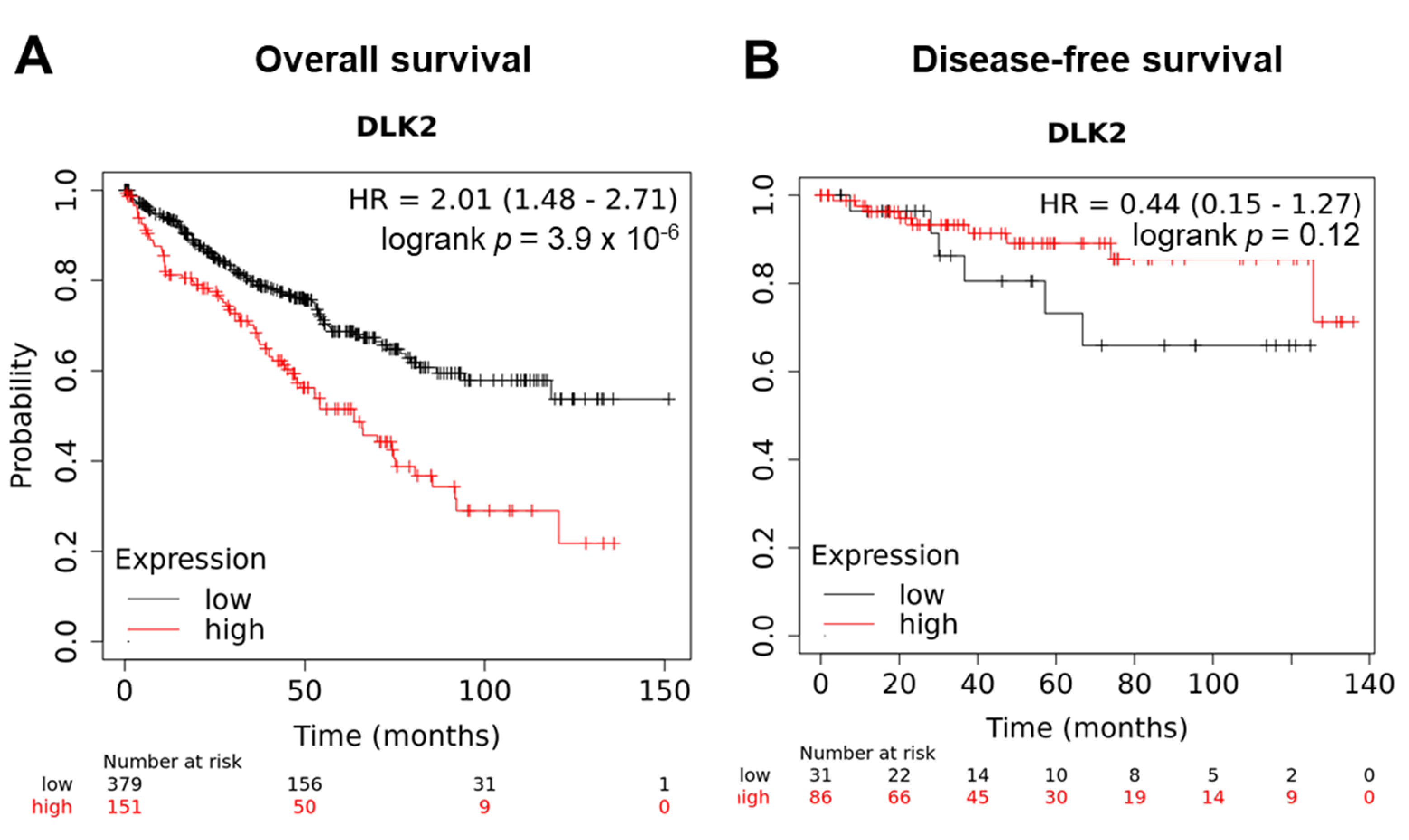
3.2. The DLK2 Expression Was Associated with Advanced Tumor Stages/Grades and Worse Overall Survival in ccRCC Patients
3.3. The Gene Clusters Positively and Negatively Correlated with DLK2 Expression Were Identified in ccRCC
3.4. DLK2-Associated Functional Enrichment Items in ccRCC Were Identified Using the LinkedOmics Tool
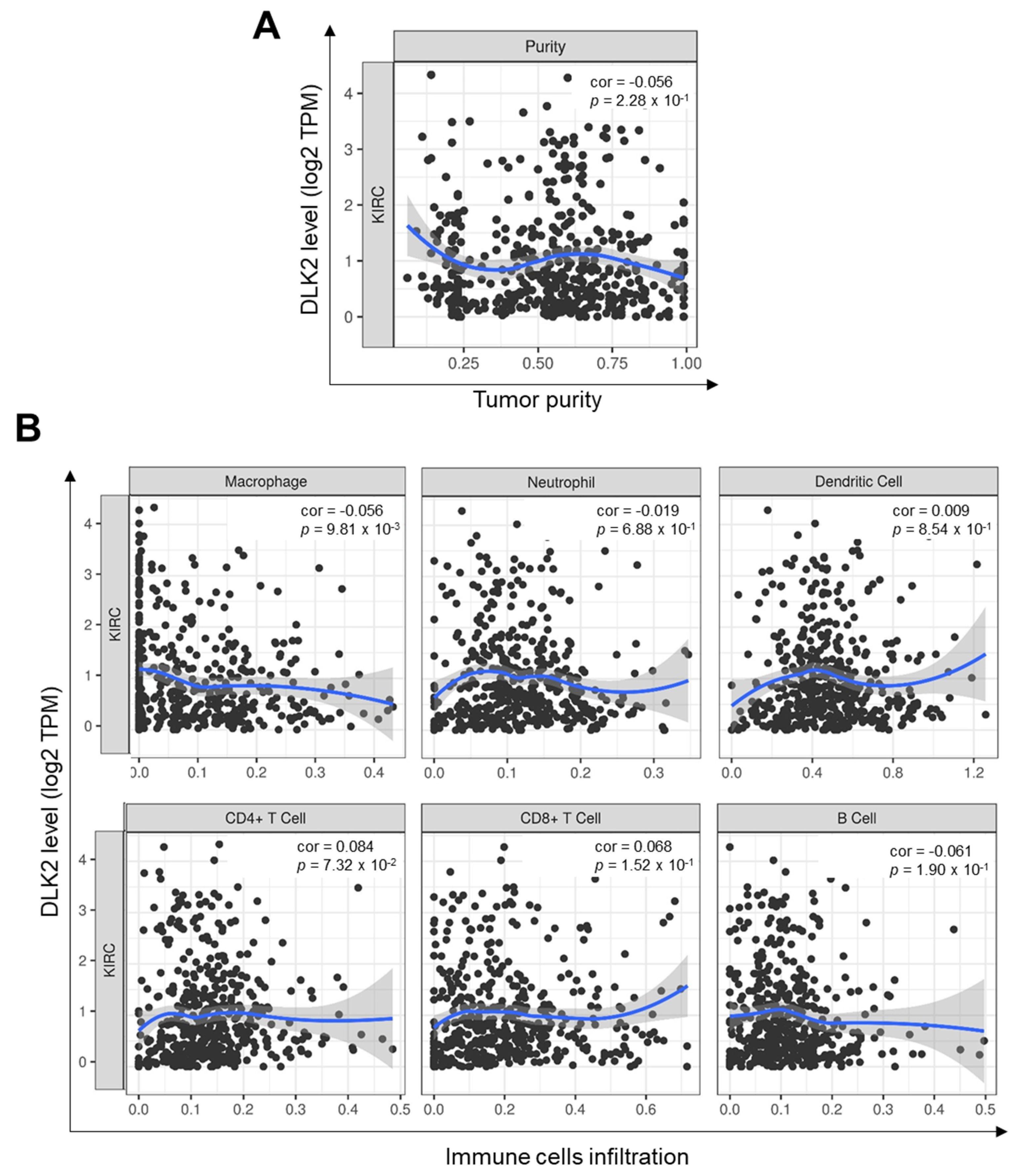
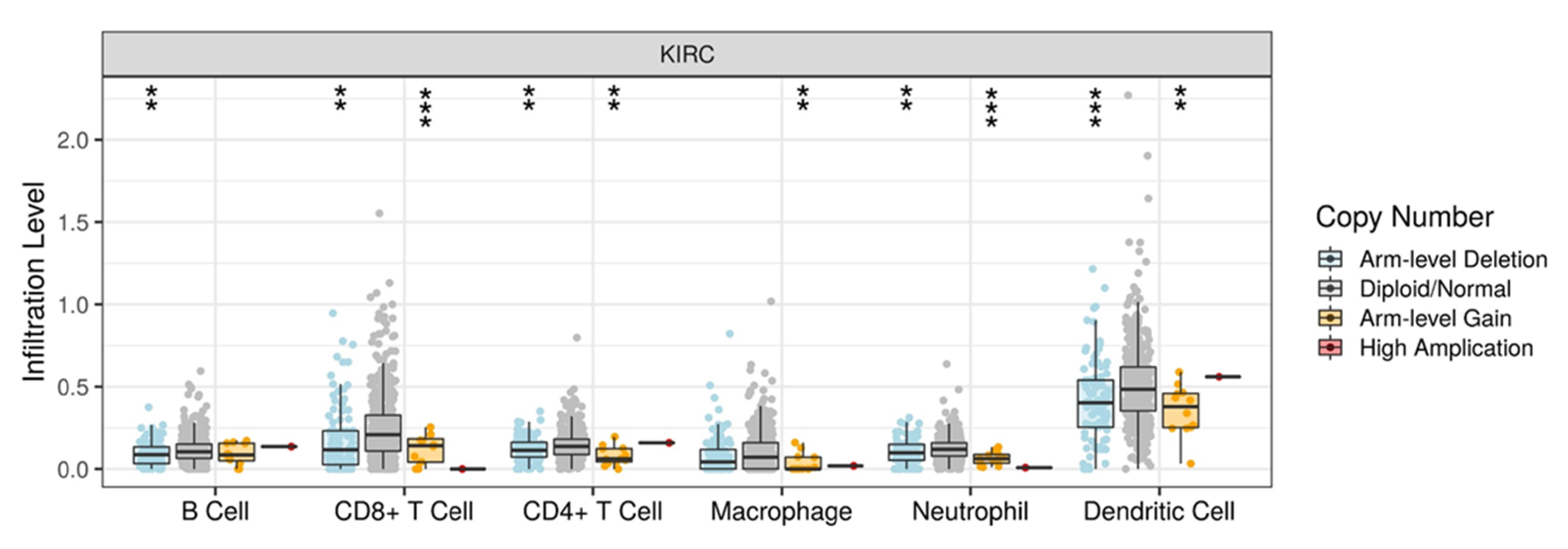
3.5. DLK2 Expression Was Negatively Correlated with the Macrophages Infiltrations and Positively Correated with the M1 to M2 Polarization of Macrophages in ccRCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.H.; Xu, L.Y.; Wu, Y.P.; Ke, Z.B.; Huang, P.; Lin, F.; Li, X.D.; Xue, X.Y.; Wei, Y.; Zheng, Q.S.; et al. Tumor volume: A new prognostic factor of oncological outcome of localized clear cell renal cell carcinoma. BMC Cancer 2021, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Breau, R.H.; Allmer, C.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; Blute, M.L. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: Indications and recommendations for surgical templates. Eur. Urol. 2011, 59, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Ricketts, C.J.; Sourbier, C.; Linehan, W.M. New strategies in renal cell carcinoma: Targeting the genetic and metabolic basis of disease. Clin. Cancer Res. 2015, 21, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; Lopez, J.I.; Watkins, T.B.K.; Nicol, D.; et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell 2018, 173, 595–610.e511. [Google Scholar] [CrossRef]
- Casuscelli, J.; Vano, Y.A.; Fridman, W.H.; Hsieh, J.J. Molecular Classification of Renal Cell Carcinoma and Its Implication in Future Clinical Practice. Kidney Cancer 2017, 1, 3–13. [Google Scholar] [CrossRef]
- Singh, P.; Agarwal, N.; Pal, S.K. Sequencing systemic therapies for metastatic kidney cancer. Curr. Treat. Options Oncol. 2015, 16, 316. [Google Scholar] [CrossRef]
- Niinivirta, M.; Enblad, G.; Lindskog, C.; Ponten, F.; Dragomir, A.; Ullenhag, G.J. Tumoral Pyruvate Kinase L/R as a Predictive Marker for the Treatment of Renal Cancer Patients with Sunitinib and Sorafenib. J. Cancer 2019, 10, 3224–3231. [Google Scholar] [CrossRef]
- Cheng, G.; Li, M.; Ma, X.; Nan, F.; Zhang, L.; Yan, Z.; Li, H.; Zhang, G.; Han, Y.; Xie, L.; et al. Systematic Analysis of microRNA Biomarkers for Diagnosis, Prognosis, and Therapy in Patients with Clear Cell Renal Cell Carcinoma. Front. Oncol. 2020, 10, 543817. [Google Scholar] [CrossRef]
- Petersen, E.V.; Chudakova, D.A.; Skorova, E.Y.; Anikin, V.; Reshetov, I.V.; Mynbaev, O.A. The Extracellular Matrix-Derived Biomarkers for Diagnosis, Prognosis, and Personalized Therapy of Malignant Tumors. Front. Oncol. 2020, 10, 575569. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.; Kota, V.; Rojiani, M.V.; Rojiani, A.M.; Kolhe, R. Prognostic and therapeutic implications of extracellular matrix associated gene signature in renal clear cell carcinoma. Sci. Rep. 2021, 11, 7561. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Liu, M.; Xu, Y.; Wu, G. Glycolysis-Related Genes Serve as Potential Prognostic Biomarkers in Clear Cell Renal Cell Carcinoma. Oxid. Med. Cell Longev. 2021, 2021, 6699808. [Google Scholar] [CrossRef]
- Xing, Q.; Zeng, T.; Liu, S.; Cheng, H.; Ma, L.; Wang, Y. A novel 10 glycolysis-related genes signature could predict overall survival for clear cell renal cell carcinoma. BMC Cancer 2021, 21, 381. [Google Scholar] [CrossRef]
- Ellinger, J.; Gromes, A.; Poss, M.; Bruggemann, M.; Schmidt, D.; Ellinger, N.; Tolkach, Y.; Dietrich, D.; Kristiansen, G.; Muller, S.C. Systematic expression analysis of the mitochondrial complex III subunits identifies UQCRC1 as biomarker in clear cell renal cell carcinoma. Oncotarget 2016, 7, 86490–86499. [Google Scholar] [CrossRef]
- Knoll, M.; Macher-Goeppinger, S.; Kopitz, J.; Duensing, S.; Pahernik, S.; Hohenfellner, M.; Schirmacher, P.; Roth, W. The ribosomal protein S6 in renal cell carcinoma: Functional relevance and potential as biomarker. Oncotarget 2016, 7, 418–432. [Google Scholar] [CrossRef]
- Chen, S.; Yu, M.; Ju, L.; Wang, G.; Qian, K.; Xiao, Y.; Wang, X. The immune-related biomarker TEK inhibits the development of clear cell renal cell carcinoma (ccRCC) by regulating AKT phosphorylation. Cancer Cell Int. 2021, 21, 119. [Google Scholar] [CrossRef]
- Reese, B.; Silwal, A.; Daugherity, E.; Daugherity, M.; Arabi, M.; Daly, P.; Paterson, Y.; Woolford, L.; Christie, A.; Elias, R.; et al. Complement as Prognostic Biomarker and Potential Therapeutic Target in Renal Cell Carcinoma. J. Immunol. 2020, 205, 3218–3229. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, J.; Sun, Z.; Cao, P.; Zheng, X.; Gao, Z.; Cao, H.; Zhang, F.; Wang, W. Interferon-inducible protein 16 may be a biomarker and prognostic factor in renal cell carcinoma by bioinformatics analysis. Medicine 2021, 100, e24257. [Google Scholar] [CrossRef]
- Quan, J.; Bai, Y.; Yang, Y.; Han, E.L.; Bai, H.; Zhang, Q.; Zhang, D. Bioinformatics analysis of C3 and CXCR4 demonstrates their potential as prognostic biomarkers in clear cell renal cell carcinoma (ccRCC). BMC Cancer 2021, 21, 814. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, E.; Long, J.; Hu, Z.; Peng, J.; Liu, L.; Tang, F.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Wu, K.; Zheng, X.; Yao, Z.; Zheng, Z.; Huang, W.; Mu, X.; Sun, F.; Liu, Z.; Zheng, J. Accumulation of CD45RO+CD8+ T cells is a diagnostic and prognostic biomarker for clear cell renal cell carcinoma. Aging 2021, 13, 14304–14321. [Google Scholar] [CrossRef] [PubMed]
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar] [PubMed]
- Lin, J.; Yu, M.; Xu, X.; Wang, Y.; Xing, H.; An, J.; Yang, J.; Tang, C.; Sun, D.; Zhu, Y. Identification of biomarkers related to CD8(+) T cell infiltration with gene co-expression network in clear cell renal cell carcinoma. Aging 2020, 12, 3694–3712. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, J.; Chen, S.; Ma, X.; Ying, Y.; Li, J.; Wang, W.; Wang, X.; Xie, L. Prognostic Value of Tumor-Associated Macrophages in Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 657318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, L.; Liu, X.; Wang, G.; Zhu, Y.; Qian, K.; Xiao, Y.; Wang, X. Bioinformatics and functional analyses of key genes and pathways in human clear cell renal cell carcinoma. Oncol. Lett. 2018, 15, 9133–9141. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, L.; Huang, C.; Dong, Y.; Fan, L.; Tao, L.; Peng, Z.; Xiang, R. Identification of Hub Genes Associated With Clear Cell Renal Cell Carcinoma by Integrated Bioinformatics Analysis. Front. Oncol. 2021, 11, 726655. [Google Scholar] [CrossRef]
- Peng, R.; Wang, Y.; Mao, L.; Fang, F.; Guan, H. Identification of Core Genes Involved in the Metastasis of Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2020, 12, 13437–13449. [Google Scholar] [CrossRef]
- Garcia-Gallastegi, P.; Ruiz-Garcia, A.; Ibarretxe, G.; Rivero-Hinojosa, S.; Gonzalez-Siccha, A.D.; Laborda, J.; Crende, O.; Unda, F.; Garcia-Ramirez, J.J. Similarities and differences in tissue distribution of DLK1 and DLK2 during E16.5 mouse embryogenesis. Histochem. Cell Biol. 2019, 152, 47–60. [Google Scholar] [CrossRef]
- Pittaway, J.F.H.; Lipsos, C.; Mariniello, K.; Guasti, L. The role of delta-like non-canonical Notch ligand 1 (DLK1) in cancer. Endocr. Relat. Cancer 2021, 28, R271–R287. [Google Scholar] [CrossRef]
- Garces, C.; Ruiz-Hidalgo, M.J.; Bonvini, E.; Goldstein, J.; Laborda, J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation 1999, 64, 103–114. [Google Scholar] [CrossRef]
- Baladron, V.; Ruiz-Hidalgo, M.J.; Nueda, M.L.; Diaz-Guerra, M.J.; Garcia-Ramirez, J.J.; Bonvini, E.; Gubina, E.; Laborda, J. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 2005, 303, 343–359. [Google Scholar] [CrossRef]
- Nueda, M.L.; Baladron, V.; Sanchez-Solana, B.; Ballesteros, M.A.; Laborda, J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 2007, 367, 1281–1293. [Google Scholar] [CrossRef]
- Huang, C.C.; Kuo, H.M.; Wu, P.C.; Cheng, S.H.; Chang, T.T.; Chang, Y.C.; Kung, M.L.; Wu, D.C.; Chuang, J.H.; Tai, M.H. Soluble delta-like 1 homolog (DLK1) stimulates angiogenesis through Notch1/Akt/eNOS signaling in endothelial cells. Angiogenesis 2018, 21, 299–312. [Google Scholar] [CrossRef]
- Huang, C.C.; Cheng, S.H.; Wu, C.H.; Li, W.Y.; Wang, J.S.; Kung, M.L.; Chu, T.H.; Huang, S.T.; Feng, C.T.; Huang, S.C.; et al. Delta-like 1 homologue promotes tumorigenesis and epithelial-mesenchymal transition of ovarian high-grade serous carcinoma through activation of Notch signaling. Oncogene 2019, 38, 3201–3215. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Zhang, Y.; Han, N.; Di, X.; Xiao, T.; Cheng, S.; Gao, Y.; Liu, Y. DLK1 promotes lung cancer cell invasion through upregulation of MMP9 expression depending on Notch signaling. PLoS ONE 2014, 9, e91509. [Google Scholar] [CrossRef]
- Nueda, M.L.; Naranjo, A.I.; Baladron, V.; Laborda, J. The proteins DLK1 and DLK2 modulate NOTCH1-dependent proliferation and oncogenic potential of human SK-MEL-2 melanoma cells. Biochim. Biophys. Acta 2014, 1843, 2674–2684. [Google Scholar] [CrossRef]
- Marquez-Exposito, L.; Rodrigues-Diez, R.R.; Rayego-Mateos, S.; Fierro-Fernandez, M.; Rodrigues-Diez, R.; Orejudo, M.; Santos-Sanchez, L.; Blanco, E.M.; Laborda, J.; Mezzano, S.; et al. Deletion of delta-like 1 homologue accelerates renal inflammation by modulating the Th17 immune response. FASEB J. 2021, 35, e21213. [Google Scholar] [CrossRef]
- Fabian, K.P.; Chi-Sabins, N.; Taylor, J.L.; Fecek, R.; Weinstein, A.; Storkus, W.J. Therapeutic efficacy of combined vaccination against tumor pericyte-associated antigens DLK1 and DLK2 in mice. Oncoimmunology 2017, 6, e1290035. [Google Scholar] [CrossRef]
- Jia, D.; Li, S.; Li, D.; Xue, H.; Yang, D.; Liu, Y. Mining TCGA database for genes of prognostic value in glioblastoma microenvironment. Aging 2018, 10, 592–605. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol.Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Lanczky, A.; Gyorffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Zhang, Q.; Xia, J.; Wang, Z. Extent and characteristics of immune infiltration in clear cell renal cell carcinoma and the prognostic value. Transl. Androl. Urol. 2019, 8, 609–618. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef]
- Ka, M.B.; Daumas, A.; Textoris, J.; Mege, J.L. Phenotypic diversity and emerging new tools to study macrophage activation in bacterial infectious diseases. Front. Immunol. 2014, 5, 500. [Google Scholar] [CrossRef]
- Sjolund, J.; Bostrom, A.K.; Lindgren, D.; Manna, S.; Moustakas, A.; Ljungberg, B.; Johansson, M.; Fredlund, E.; Axelson, H. The notch and TGF-beta signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS ONE 2011, 6, e23057. [Google Scholar] [CrossRef]
- Slaninova, V.; Krafcikova, M.; Perez-Gomez, R.; Steffal, P.; Trantirek, L.; Bray, S.J.; Krejci, A. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol. 2016, 6, 150155. [Google Scholar] [CrossRef]
- Nam, H.; Kundu, A.; Karki, S.; Brinkley, G.J.; Chandrashekar, D.S.; Kirkman, R.L.; Liu, J.; Liberti, M.V.; Locasale, J.W.; Mitchell, T.; et al. The TGF-beta/HDAC7 axis suppresses TCA cycle metabolism in renal cancer. JCI Insight 2021, 6, e148438. [Google Scholar] [CrossRef]
- Boyer-Di Ponio, J.; Wright-Crosnier, C.; Groyer-Picard, M.T.; Driancourt, C.; Beau, I.; Hadchouel, M.; Meunier-Rotival, M. Biological function of mutant forms of JAGGED1 proteins in Alagille syndrome: Inhibitory effect on Notch signaling. Hum. Mol. Genet. 2007, 16, 2683–2692. [Google Scholar] [CrossRef]
- Roberts, A.B.; McCune, B.K.; Sporn, M.B. TGF-beta: Regulation of extracellular matrix. Kidney Int. 1992, 41, 557–559. [Google Scholar] [CrossRef]
- Boguslawska, J.; Kedzierska, H.; Poplawski, P.; Rybicka, B.; Tanski, Z.; Piekielko-Witkowska, A. Expression of Genes Involved in Cellular Adhesion and Extracellular Matrix Remodeling Correlates with Poor Survival of Patients with Renal Cancer. J. Urol. 2016, 195, 1892–1902. [Google Scholar] [CrossRef]
- Fan, L.; Li, P.; Yin, Z.; Fu, G.; Liao, D.J.; Liu, Y.; Zhu, J.; Zhang, Y.; Wang, L.; Yan, Q.; et al. Ribosomal s6 protein kinase 4: A prognostic factor for renal cell carcinoma. Br. J. Cancer 2013, 109, 1137–1146. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.; Zou, Z.; Wang, X.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Lu, Y. Knockdown of ribosomal protein S15A inhibits human kidney cancer cell growth in vitro and in vivo. Mol. Med. Rep. 2019, 19, 1117–1127. [Google Scholar] [CrossRef]
- Martins, T.; Eusebio, N.; Correia, A.; Marinho, J.; Casares, F.; Pereira, P.S. TGFbeta/Activin signalling is required for ribosome biogenesis and cell growth in Drosophila salivary glands. Open Biol. 2017, 7, 160258. [Google Scholar] [CrossRef]
- Benelli, D.; Cialfi, S.; Pinzaglia, M.; Talora, C.; Londei, P. The translation factor eIF6 is a Notch-dependent regulator of cell migration and invasion. PLoS ONE 2012, 7, e32047. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Tao, S.; Chen, Q.; Lin, C.; Dong, H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 191. [Google Scholar] [CrossRef]
- Klose, R.; Adam, M.G.; Weis, E.M.; Moll, I.; Wustehube-Lausch, J.; Tetzlaff, F.; Oka, C.; Ehrmann, M.; Fischer, A. Inactivation of the serine protease HTRA1 inhibits tumor growth by deregulating angiogenesis. Oncogene 2018, 37, 4260–4272. [Google Scholar] [CrossRef]
- Nakken, S.; Eikrem, O.; Marti, H.P.; Beisland, C.; Bostad, L.; Scherer, A.; Flatberg, A.; Beisvag, V.; Skandalou, E.; Furriol, J.; et al. AGAP2-AS1 as a prognostic biomarker in low-risk clear cell renal cell carcinoma patients with progressing disease. Cancer Cell Int. 2021, 21, 690. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, A.; Wang, X. Upregulation of lncRNA AGAP2-AS1 is an independent predictor of poor survival in patients with clear cell renal carcinoma. Oncol. Lett. 2020, 19, 3993–4001. [Google Scholar] [CrossRef]
- Bauerschmitt, H.; Funes, S.; Herrmann, J.M. The membrane-bound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. J. Biol. Chem. 2008, 283, 17139–17146. [Google Scholar] [CrossRef]
- Schiavon, C.R.; Turn, R.E.; Newman, L.E.; Kahn, R.A. ELMOD2 regulates mitochondrial fusion in a mitofusin-dependent manner, downstream of ARL2. Mol. Biol. Cell 2019, 30, 1198–1213. [Google Scholar] [CrossRef]
- Matsudaira, T.; Mukai, K.; Noguchi, T.; Hasegawa, J.; Hatta, T.; Iemura, S.I.; Natsume, T.; Miyamura, N.; Nishina, H.; Nakayama, J.; et al. Endosomal phosphatidylserine is critical for the YAP signalling pathway in proliferating cells. Nat. Commun. 2017, 8, 1246. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, T.C.; Chen, W.Q.; Zhou, L.J.; Wu, Y.; Zeng, L.; Pei, H.P. Screening for differentially expressed genes between left- and right-sided colon carcinoma by microarray analysis. Oncol. Lett. 2013, 6, 353–358. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Chen, Z.; Fu, F.; Huang, X.; Huang, Z. Pseudogene HSPB1P1 contributes to renal cell carcinoma proliferation and metastasis by targeting miR-296-5p to regulate HMGA1 expression. Cell Biol. Int. 2021, 45, 2479–2489. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, R.; Jiang, L.; Zhang, C.; Zheng, Q.; Jia, J.; Chen, Y.; Huang, J. miR-331-3p Inhibits Proliferation and Promotes Apoptosis of Nasopharyngeal Carcinoma Cells by Targeting elf4B-PI3K-AKT Pathway. Technol. Cancer Res. Treat. 2020, 19, 1533033819892251. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Yang, Q.; Ding, M.; Zhong, J.; Zhang, C.Y.; Ge, J.; Wang, J.; Zhang, C. miR-28-5p acts as a tumor suppressor in renal cell carcinoma for multiple antitumor effects by targeting RAP1B. Oncotarget 2016, 7, 73888–73902. [Google Scholar] [CrossRef]
- Wang, H.; Yan, B.; Zhang, P.; Liu, S.; Li, Q.; Yang, J.; Yang, F.; Chen, E. MiR-496 promotes migration and epithelial-mesenchymal transition by targeting RASSF6 in colorectal cancer. J. Cell. Physiol. 2020, 235, 1469–1479. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Wang, J. MicroRNA373 promotes tumorigenesis of renal cell carcinoma in vitro and in vivo. Mol. Med. Rep. 2017, 16, 7048–7055. [Google Scholar] [CrossRef][Green Version]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers 2017, 9, 153. [Google Scholar] [CrossRef]
- Tian, X.; Sun, D.; Zhang, Y.; Zhao, S.; Xiong, H.; Fang, J. Zinc finger protein 278, a potential oncogene in human colorectal cancer. Acta Biochim. Biophys. Sin. 2008, 40, 289–296. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhang, F.; Liao, S.; He, X.; Zhuo, D.; Huang, H.; Wu, Y. Vitamin D receptor suppresses proliferation and metastasis in renal cell carcinoma cell lines via regulating the expression of the epithelial Ca2+ channel TRPV5. PLoS ONE 2018, 13, e0195844. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fan, Y.; Wan, F. LncRNA IGBP1-AS1/miR-24-1/ZIC3 loop regulates the proliferation and invasion ability in breast cancer. Cancer Cell Int. 2020, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Gobe, G.; Johnson, D.W.; Healy, H. The emerging role of nuclear factor kappa B in renal cell carcinoma. Int. J. Biochem. Cell Biol. 2011, 43, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grunwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Sharma, R.; Kadife, E.; Myers, M.; Kannourakis, G.; Prithviraj, P.; Ahmed, N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 186. [Google Scholar] [CrossRef]
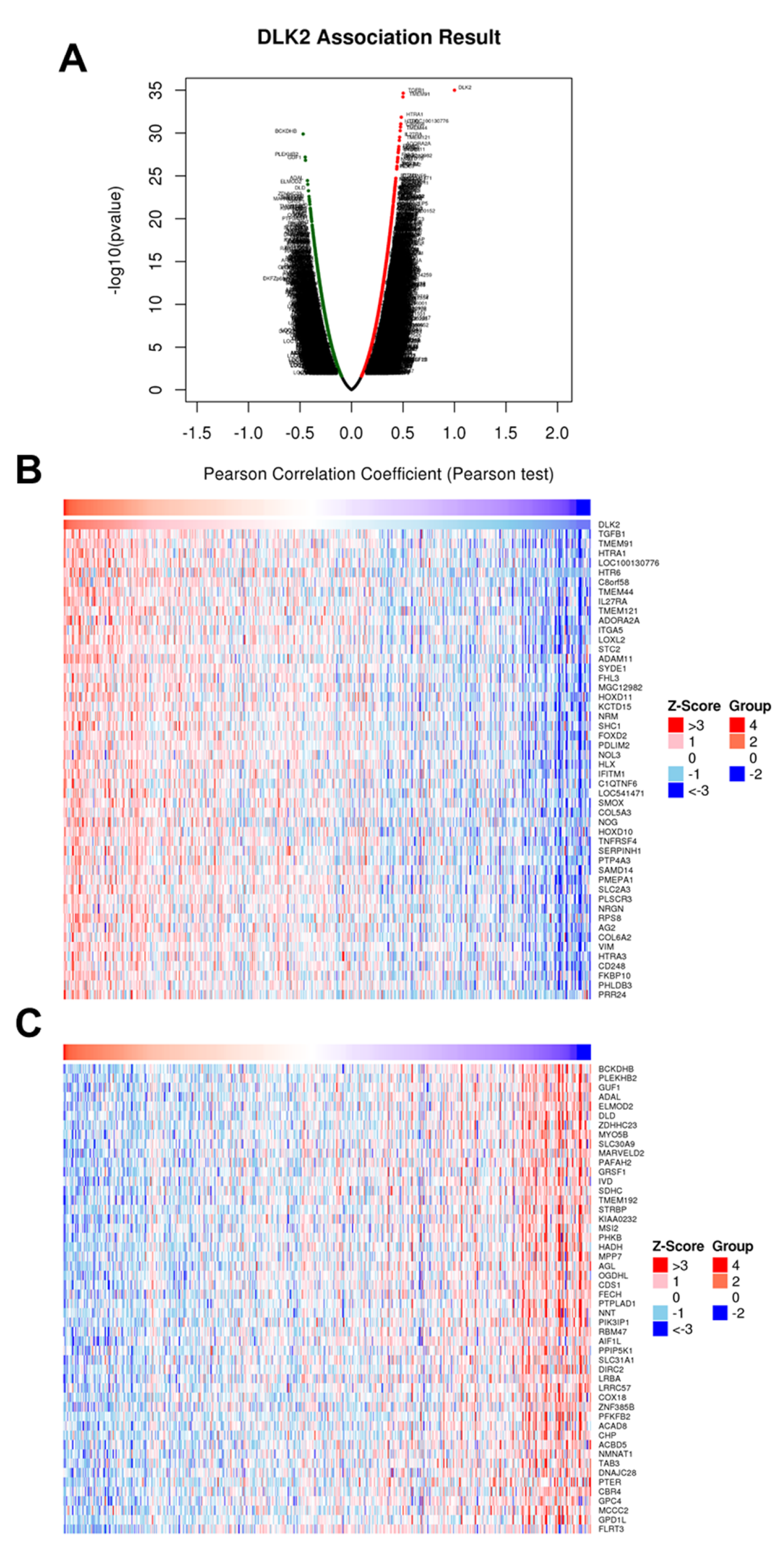
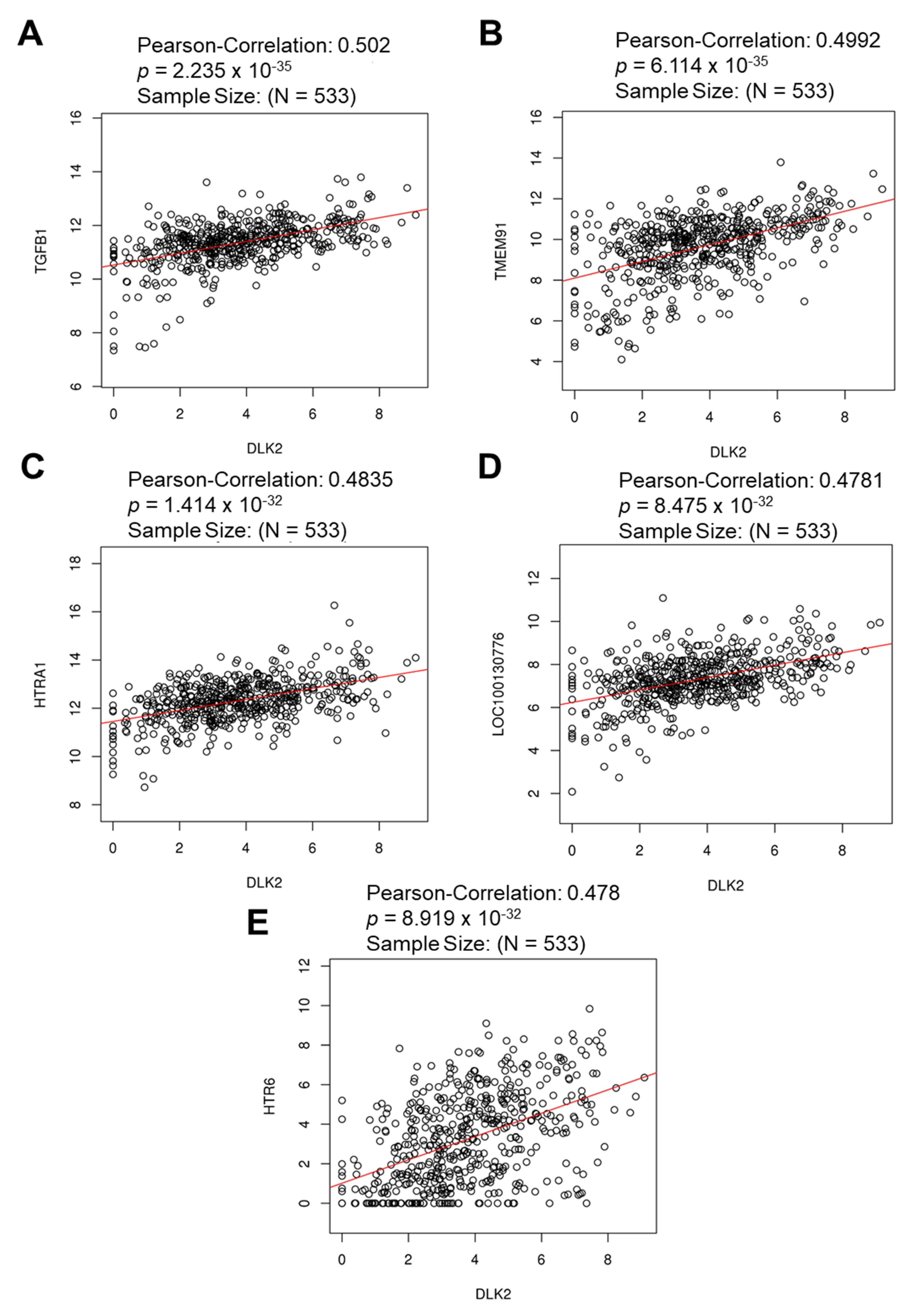
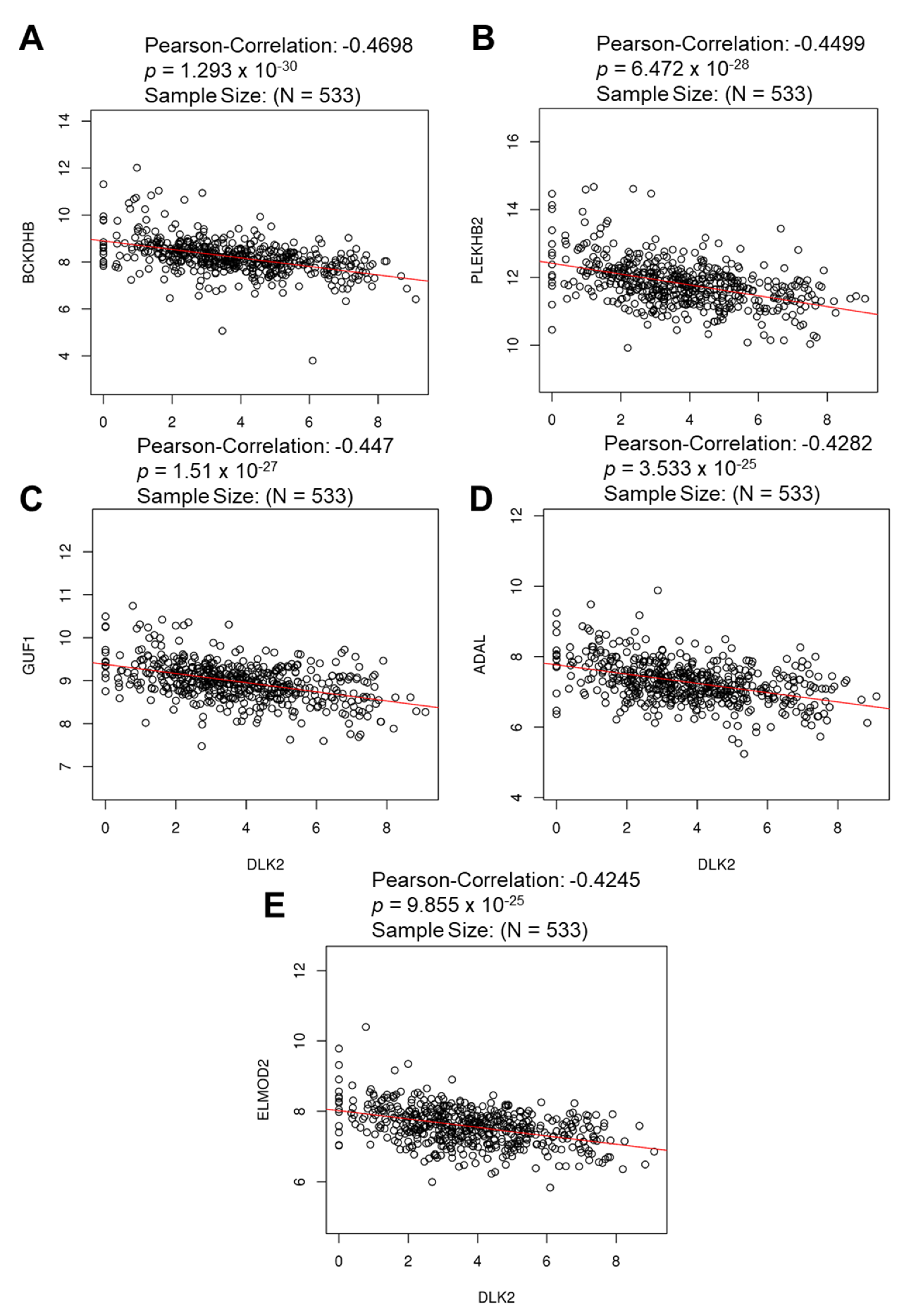
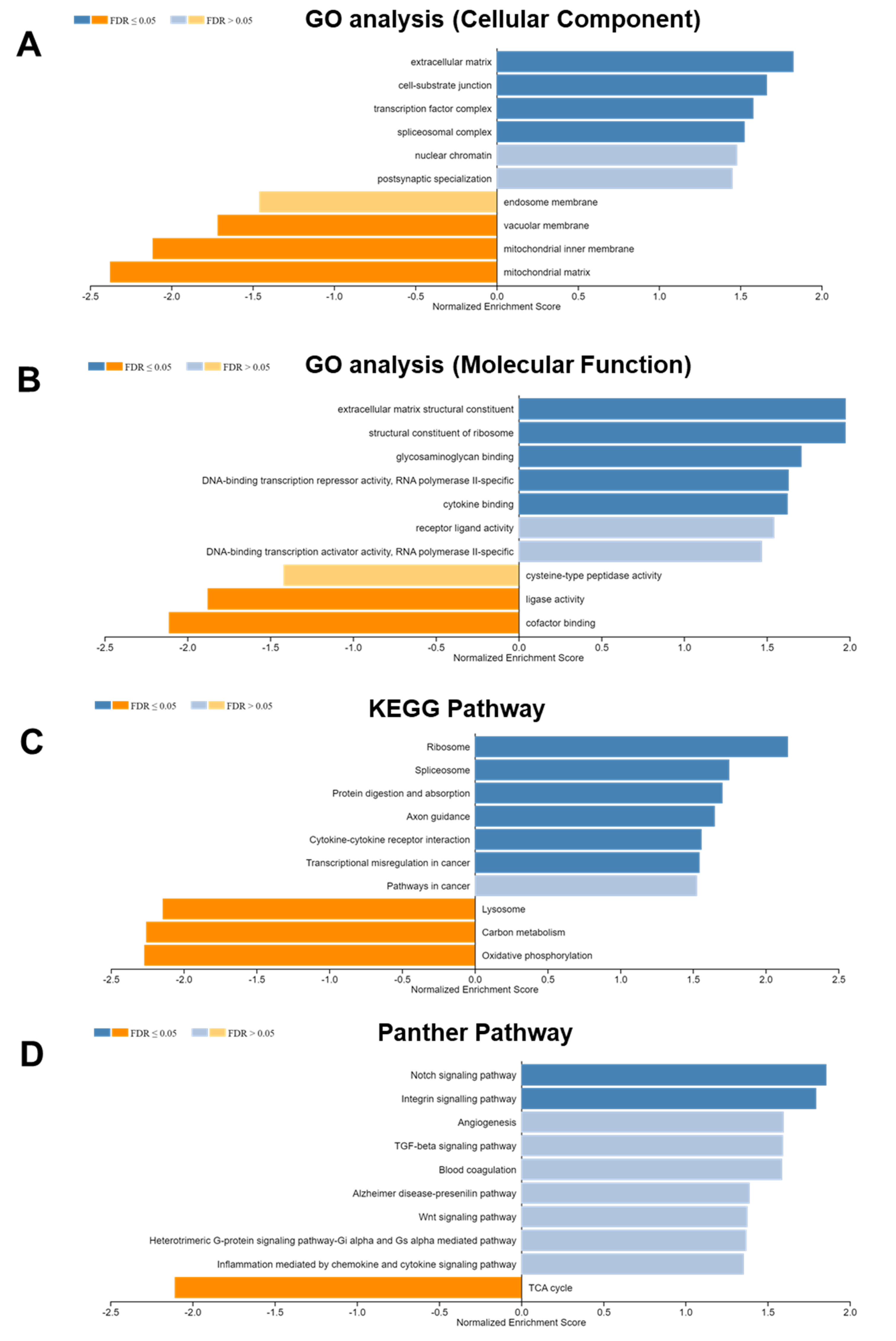


| Enriched Category | Gene Set | Normalized Enrichment Score | FDR | Leading Edge Number | p Value |
|---|---|---|---|---|---|
| miRNA Target | GGGGCCC, miR-296 | 1.865499 | 0.002889 | 25 | 0 |
| CCAGGGG, miR-331 | 1.643038 | 0.038283 | 29 | 0 | |
| AGCTCCT, miR-28 | 1.555229 | 0.119423 | 27 | 0.002294 | |
| CATGTAA, miR-496 | −1.258861 | 0.475076 | 32 | 0.032258 | |
| TTTTGAG, miR-373 | −1.157875 | 0.485488 | 31 | 0.045455 | |
| Transcription Factor Target | V$LFA1_Q6 | 1.723831 | 0.003247 | 73 | 0 |
| V$MAZR_01 | 1.724924 | 0.003788 | 60 | 0 | |
| V$VDR_Q3 | 1.748140 | 0.004132 | 79 | 0 | |
| V$ZIC3_01 | 1.727236 | 0.004546 | 94 | 0 | |
| GGGNNTTTCC_V$NFKB_Q6_01 | 1.751883 | 0.005510 | 50 | 0 |
| Variable | Coefficient | HR | 95% CI | p Value |
|---|---|---|---|---|
| Macrophage | −2.774 | 0.062 | 0.006–0.647 | * 0.020 |
| Neutrophil | 3.211 | 24.809 | 0.389–1582.755 | 0.130 |
| Dendritic cell | 1.119 | 3.062 | 0.517–18.131 | 0.217 |
| CD4+ T cell | −0.524 | 0.592 | 0.039–8.902 | 0.705 |
| CD8+ T cell | −1.741 | 0.175 | 0.037–0.837 | * 0.029 |
| B cell | −0.600 | 0.549 | 0.022–13.757 | 0.714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-G.; Lee, Y.-K.; Huang, S.-C.; Chang, C.-L.; Ko, C.-Y.; Lee, W.-C.; Chen, T.-Y.; Tzou, S.-J.; Huang, C.-Y.; Tai, M.-H.; et al. DLK2 Acts as a Potential Prognostic Biomarker for Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis. Genes 2022, 13, 629. https://doi.org/10.3390/genes13040629
Lee M-G, Lee Y-K, Huang S-C, Chang C-L, Ko C-Y, Lee W-C, Chen T-Y, Tzou S-J, Huang C-Y, Tai M-H, et al. DLK2 Acts as a Potential Prognostic Biomarker for Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis. Genes. 2022; 13(4):629. https://doi.org/10.3390/genes13040629
Chicago/Turabian StyleLee, Man-Gang, Yung-Kuo Lee, Shih-Chung Huang, Chen-Lin Chang, Chou-Yuan Ko, Wen-Chin Lee, Tung-Yuan Chen, Shiow-Jyu Tzou, Cheng-Yi Huang, Ming-Hong Tai, and et al. 2022. "DLK2 Acts as a Potential Prognostic Biomarker for Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis" Genes 13, no. 4: 629. https://doi.org/10.3390/genes13040629
APA StyleLee, M.-G., Lee, Y.-K., Huang, S.-C., Chang, C.-L., Ko, C.-Y., Lee, W.-C., Chen, T.-Y., Tzou, S.-J., Huang, C.-Y., Tai, M.-H., Lin, Y.-W., Kung, M.-L., Tsai, M.-C., Chen, Y.-L., Chang, Y.-C., Wen, Z.-H., Huang, C.-C., & Chu, T.-H. (2022). DLK2 Acts as a Potential Prognostic Biomarker for Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis. Genes, 13(4), 629. https://doi.org/10.3390/genes13040629







