Chronic Myelogenous Leukemia with Double Philadelphia Chromosome and Coexpression of p210 and p190 Fusion Transcripts
Abstract
1. Introduction
2. Patients and Methods
2.1. Conventional Culture and Cytogenetics
2.2. Fluorescent In Situ Hybridization
2.3. Real-Time Quantitative Reverse Transcription of Polymerase Chain Reaction (qRT-PCR)
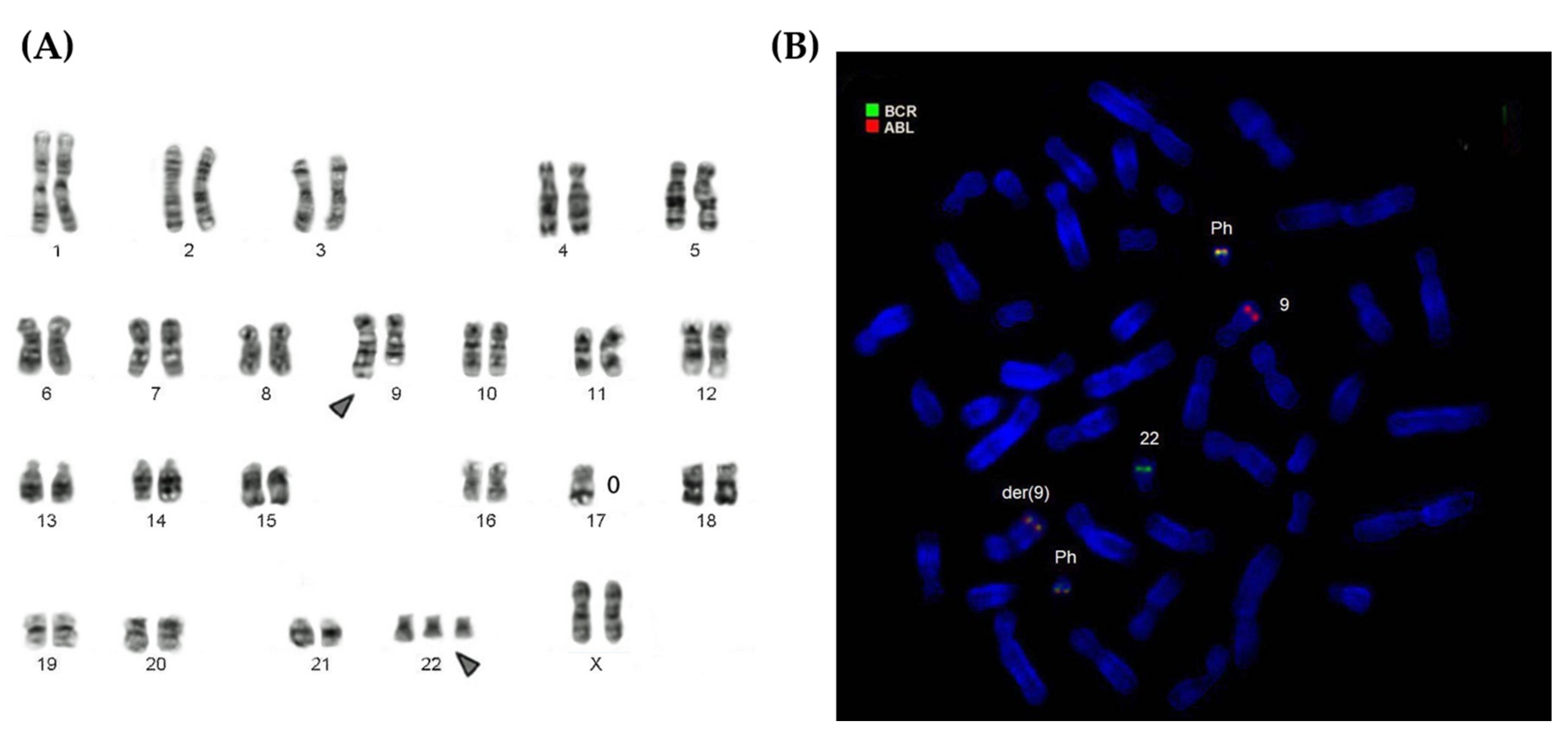
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Abdulmawjood, B.; Costa, B.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Genetic Biomarkers in Chronic Myeloid Leukemia: What Have We Learned So Far? Int. J. Mol. Sci. 2021, 22, 12516. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C.L.; Callahan, W.; Witte, O.N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell 1992, 70, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.V.; Barnes, D.J. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat. Rev. Cancer 2007, 7, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management. Am. J. Hematol. 2014, 89, 547–556. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Gupta, S.K.; Ali, V.; Verma, M. Transport and metabolism of tyrosine kinase inhibitors associated with chronic myeloid leukemia therapy: A review. Mol. Cell. Biochem. 2022, 477, 1261–1279. [Google Scholar] [CrossRef]
- Shteper, P.J.; Ben-Yehuda, D. Molecular evolution of chronic myeloid leukaemia. Semin. Cancer Biol. 2001, 11, 313–323. [Google Scholar] [CrossRef]
- Höglund, M.; Sandin, F.; Simonsson, B. Epidemiology of chronic myeloid leukaemia: An update. Ann. Hematol. 2015, 94 (Suppl. S2), S241–S247. [Google Scholar] [CrossRef]
- Pérez-Jacobo, F.; Tuna-Aguilar, E.; Demichelis-Gómez, R.; Crespo-Solís, E.; Valencia-Rocha, U.; Aguayo, Á.; López-Karpovitch, X. Prognostic Factors, Response to Treatment, and Survival in Patients With Chronic Myeloid Leukemia in Blast Phase: A Single-Institution Survey. Clin. Lymphoma Myeloma Leuk. 2015, 15, 778–784. [Google Scholar] [CrossRef]
- Verma, D.; Kantarjian, H.M.; Jones, D.; Luthra, R.; Borthakur, G.; Verstovsek, S.; Rios, M.B.; Cortes, J. Chronic myeloid leukemia (CML) with p190 BCR-ABL: Analysis of characteristics, outcomes, and prognostic significance. Blood 2009, 114, 2232–2235. [Google Scholar] [CrossRef]
- Tabassum, N.; Saboor, M.; Ghani, R.; Moinuddin, M. Heterogeneity of BCR-ABL rearrangement in patients with chronic myeloid leukemia in Pakistan. Pak. J. Med. Sci. 2014, 30, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kolenova, A.; Maloney, K.W.; Hunger, S.P. Philadelphia Chromosome-positive Acute Lymphoblastic Leukemia or Chronic Myeloid Leukemia in Lymphoid Blast Crisis. J. Pediatr. Hematol. Oncol. 2016, 38, e193–e195. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ding, W.J.; Jiang, F.; Chen, Z.X.; Cen, J.N.; Qi, X.F.; Liang, J.Y.; Liu, D.D.; Pan, J.L.; Chen, S.N. Coexistence of p210BCR-ABL and CBFβ-MYH11 fusion genes in myeloid leukemia: A report of 4 cases. Oncol. Lett. 2017, 14, 5171–5178. [Google Scholar] [CrossRef]
- Vinhas, R.; Lourenço, A.; Santos, S.; Ribeiro, P.; Silva, M.; de Sousa, A.B.; Baptista, P.V.; Fernandes, A.R. A double Philadelphia chromosome-positive chronic myeloid leukemia patient, co-expressing P210BCR-ABL1 and P195BCR-ABL1 isoforms. Haematologica 2018, 103, e549–e552. [Google Scholar] [CrossRef] [PubMed]
- Adnan-Awad, S.; Kim, D.; Hohtari, H.; Javarappa, K.; Brandstoetter, T.; Mayer, I.; Potdar, S.; Heckman, C.; Kytölä, S.; Porkka, K.; et al. Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets. Leukemia 2021, 35, 1964–1975. [Google Scholar] [CrossRef]
- Fabarius, A.; Leitner, A.; Hochhaus, A.; Müller, M.C.; Hanfstein, B.; Haferlach, C.; Göhring, G.; Schlegelberger, B.; Jotterand, M.; Reiter, A.; et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: Long-term observation of 1151 patients from the randomized CML Study IV. Blood 2011, 118, 6760–6768. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Ornellas, M.H.; Dobbin, J.; De Souza Fernandez, T. Double Philadelphia-chromosome: A resistance factor on the imatinib mesylate therapy for chronic myeloid leukemia. Int. J. Lab. Hematol. 2008, 30, 346–348. [Google Scholar] [CrossRef]
- Ikeda, K.; Harada-Shirado, K.; Matsumoto, H.; Noji, H.; Ogawa, K.; Takeishi, Y. Molecular Response of e19a2 BCR-ABL1 Chronic Myeloid Leukemia With Double Philadelphia Chromosome to Dasatinib. J. Clin. Oncol. 2016, 34, e130-3. [Google Scholar] [CrossRef]
- Scheres, J.M. Identification of two Robertsonian translocations with a Giemsa banding technique. Humangenetik 1972, 15, 253–256. [Google Scholar] [CrossRef]
- McGowan-Jordan, J.; Hastings, R.; Moore, S. ISCN. An International System for Human Cytogenomic Nomenclature; Karger AG: Basel, Switzerland, 2020. [Google Scholar]
- Hopman, A.H.; Ramaekers, F.C.; Raap, A.K.; Beck, J.L.; Devilee, P.; van der Ploeg, M.; Vooijs, G.P. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry 1988, 89, 307–316. [Google Scholar]
- Torii, Y.; Nanjo, K.; Toubai, T.; Hosokawa, M.; Sato, R.; Yamada, A.; Aizawa, K.; Himuro, M.; Ito, S.; Yamamoto, M.; et al. A unique three-way Philadelphia chromosome variant t(4;9;22)(q21;q34;q11.2) in a newly diagnosed patient with chronic phase chronic myeloid leukemia: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R.; Voskanyan, A.; Lauseker, M.; Pfirrmann, M.; Kalmanti, L.; Rinaldetti, S.; Kohlbrenner, K.; Haferlach, C.; Schlegelberger, B.; Fabarius, A.; et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia 2020, 34, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Pickl, W.F.; Van’t Veer, M.; Morilla, R.; Swansbury, J.; Strobl, H.; Attarbaschi, A.; Hopfinger, G.; Ashley, S.; Bene, M.C.; et al. Mixed-phenotype acute leukemia: Clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood 2011, 117, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Kohla, S.A.; Sabbagh, A.A.; Omri, H.E.; Ibrahim, F.A.; Otazu, I.B.; Alhajri, H.; Yassin, M.A. Mixed Phenotype Acute Leukemia with Two Immunophenotypically Distinct B and T Blasts Populations, Double Ph+ Chromosome and Complex Karyotype: Report of an Unusual Case. Clin. Med. Insights Blood Disord. 2015, 8, 25–31. [Google Scholar] [CrossRef]
- Peterson, L.F.; Mitrikeska, E.; Giannola, D.; Lui, Y.; Sun, H.; Bixby, D.; Malek, S.N.; Donato, N.J.; Wang, S.; Talpaz, M. p53 stabilization induces apoptosis in chronic myeloid leukemia blast crisis cells. Leukemia 2011, 25, 761–769. [Google Scholar] [CrossRef]
- Fu, S.; Hu, Y.; Fu, Y.; Chen, F.; Liu, X.; Zhang, M.; Wang, X.; Tu, S.; Zhang, J. Novel BCR-ABL1 fusion and leukemic mutations of SETBP1, PAX5, and TP53 detected by next generation sequencing in chronic myeloid leukemia. Cancer Biol. Ther. 2016, 17, 1003–1009. [Google Scholar] [CrossRef][Green Version]
- Druker, B.J. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008, 112, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Sweet, K.; Hazlehurst, L.; Sahakian, E.; Powers, J.; Nodzon, L.; Kayali, F.; Hyland, K.; Nelson, A.; Pinilla-Ibarz, J. A phase I clinical trial of ruxolitinib in combination with nilotinib in chronic myeloid leukemia patients with molecular evidence of disease. Leuk. Res. 2018, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Sawyers, C.; Hochhaus, A.; Guilhot, F.; Schiffer, C.; Gambacorti-Passerini, C.; Niederwieser, D.; Resta, D.; Capdeville, R.; Zoellner, U.; et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 2002, 346, 645–652. [Google Scholar] [CrossRef]
- Vainstein, V.; Eide, C.A.; O’Hare, T.; Shukron, O.; Druker, B.J. Integrating in vitro sensitivity and dose-response slope is predictive of clinical response to ABL kinase inhibitors in chronic myeloid leukemia. Blood 2013, 122, 3331–3334. [Google Scholar] [CrossRef][Green Version]
- Junmei, Z.; Fengkuan, Y.; Yongping, S.; Baijun, F.; Yuzhang, L.; Lina, L.; Qinglan, Z. Coexistence of P190 and P210 BCR/ABL transcripts in chronic myeloid leukemia blast crisis resistant to imatinib. Springerplus 2015, 4, 170. [Google Scholar] [CrossRef][Green Version]
- Zeng, D.F.; Chang, C.; Li, J.P.; Kong, P.Y.; Zhang, X.; Gao, L. Extramedullary T-lymphoblastic blast crisis in chronic myelogenous leukemia: A case report of successful diagnosis and treatment. Exp. Ther. Med. 2015, 9, 850–852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nimer, S. Is it important to decipher the heterogeneity of “normal karyotype AML”? Best Pract. Res. Clin. Haematol. 2008, 21, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Rahul, E.; Gupta, I.; Chopra, A.; Ranjan, A.; Gupta, A.K.; Meena, J.P.; Viswanathan, G.K.; Bakhshi, S.; Misra, A.; et al. Molecular and genomic landscapes in secondary & therapy related acute myeloid leukemia. Am. J. Blood Res. 2021, 11, 472–497. [Google Scholar] [PubMed]
- Molica, M.; Zacheo, I.; Diverio, D.; Alimena, G.; Breccia, M. Long-term outcome of chronic myeloid leukaemia patients with p210 and p190 co-expression at baseline. Br. J. Haematol. 2015, 169, 148–150. [Google Scholar] [CrossRef]
- Ayatollahi, H.; Keramati, M.R.; Shirdel, A.; Kooshyar, M.M.; Raiszadeh, M.; Shakeri, S.; Sadeghian, M.H. BCR-ABL fusion genes and laboratory findings in patients with chronic myeloid leukemia in northeast Iran. Caspian J. Intern. Med. 2018, 9, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rabian, F.; Lengline, E.; Rea, D. Towards a Personalized Treatment of Patients with Chronic Myeloid Leukemia. Curr Hematol Malig. Rep. 2019, 14, 492–500. [Google Scholar] [CrossRef]
- Van Rhee, F.; Hochaaus, A.; Lin, F.; Melo, J.; Goldman, J.M.; Cross, N.C. P190 BCR-ABL mRNA is expressed at low levels in p210 positive chronic myeloid leukemia and acute lymphoblastic leukemias. Blood 1996, 87, 5213–5217. [Google Scholar] [CrossRef] [PubMed]
- Hur, M.; Song, E.Y.; Kang, S.H.; Shin, D.H.; Kim, J.Y.; Park, S.S.; Cho, H.I. Lymphoid preponderance and the absence of basophilia and splenomegaly are frequent in m-bcr-positive chronic myelogenous leukemia. Ann. Hematol. 2002, 81, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Andrikovics, H.; Nhajevszky, S.; Szilvasi, A.; Bors, A.; Adam, E.; Kozma, A.; Kajtar, B.; Barta, A.; Poros, A.; Tordai, A. First and second line imatinib treatment in chronic myelogenous leukemia patients expressing rare e1a2 or e19a2 BCR-ABL transcripts. Hematol. Oncol. 2007, 25, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zagaria, A.; Anelli, L.; Coccaro, N.; Tota, G.; Casieri, P.; Cellamare, A.; Impera, L.; Brunetti, C.; Minervini, A.; Minervini, C.F.; et al. BCR-ABL1 e6a2 transcript in chronic myeloid leukemia: Biological features and molecular monitoring by droplet digital PCR. Virchows Arch. 2015, 467, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Agirre, X.; Román-Gómez, J.; Vázquez, I.; Jiménez-Velasco, A.; Larráyoz, M.J.; Lahortiga, I.; Andreu, E.J.; Márquez, J.; de Heredia, J.M.B.; Odero, M.D.; et al. Coexistence of different clonal populations harboring the b3a2 (p210) and e1a2 (p190) BCR-ABL1 fusion transcripts in chronic myelogenous leukemia resistant to imatinib. Cancer Genet. Cytogenet. 2005, 160, 22–26. [Google Scholar] [CrossRef] [PubMed]
| Patient | Diagnosis | Blast Crisis | |||
|---|---|---|---|---|---|
| Isoforms BCR-ABL/ABL % | Isoforms BCR-ABL/ABL % | Karyotype | |||
| p210 | p190 | p210 | p190 | 20 Metaphases * | |
| 1 | 108.54 | 73.68 | 125.16 | 84.74 | 46, XX, t(9;22), −17, +der(22)t(9;22) |
| 2 | 100.59 | 70.35 | 120.46 | 80.86 | 47, XY, t(9;22), +der(22)t(9;22) |
| Patient | Age | Leukocytes | Blasts | OS * | DFS ** |
|---|---|---|---|---|---|
| Years | (mm3) | (%) | Months | Months | |
| 1 | 44 | 328,720.00 | 85 | 7 | 4 |
| 2 | 45 | 147,720.00 | 55 | 12 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, S.S.d.; Seabra, A.D.; Macambira, L.H.R.; Carneiro, D.M.; Nunes, P.F.; Pontes, T.B.; Mello-Junior, F.A.R.; Leão, L.B.C.; Cordeiro, F.d.N.C.d.S.; Carneiro, T.X.; et al. Chronic Myelogenous Leukemia with Double Philadelphia Chromosome and Coexpression of p210 and p190 Fusion Transcripts. Genes 2022, 13, 580. https://doi.org/10.3390/genes13040580
Cruz SSd, Seabra AD, Macambira LHR, Carneiro DM, Nunes PF, Pontes TB, Mello-Junior FAR, Leão LBC, Cordeiro FdNCdS, Carneiro TX, et al. Chronic Myelogenous Leukemia with Double Philadelphia Chromosome and Coexpression of p210 and p190 Fusion Transcripts. Genes. 2022; 13(4):580. https://doi.org/10.3390/genes13040580
Chicago/Turabian StyleCruz, Samara Silveira da, Aline Damasceno Seabra, Lais Helena Rescinho Macambira, Débora Monteiro Carneiro, Patrícia Ferreira Nunes, Thais Brilhante Pontes, Fernando Augusto Rodrigues Mello-Junior, Lucyana Barbosa Cardoso Leão, Fernanda de Nazaré Cardoso dos Santos Cordeiro, Thiago Xavier Carneiro, and et al. 2022. "Chronic Myelogenous Leukemia with Double Philadelphia Chromosome and Coexpression of p210 and p190 Fusion Transcripts" Genes 13, no. 4: 580. https://doi.org/10.3390/genes13040580
APA StyleCruz, S. S. d., Seabra, A. D., Macambira, L. H. R., Carneiro, D. M., Nunes, P. F., Pontes, T. B., Mello-Junior, F. A. R., Leão, L. B. C., Cordeiro, F. d. N. C. d. S., Carneiro, T. X., Moreira-Nunes, C. A., & Burbano, R. M. R. (2022). Chronic Myelogenous Leukemia with Double Philadelphia Chromosome and Coexpression of p210 and p190 Fusion Transcripts. Genes, 13(4), 580. https://doi.org/10.3390/genes13040580










