First Description of Inheritance of a Postzygotic OPA1 Mosaic Variant
Abstract
1. Introduction
2. Materials and Methods
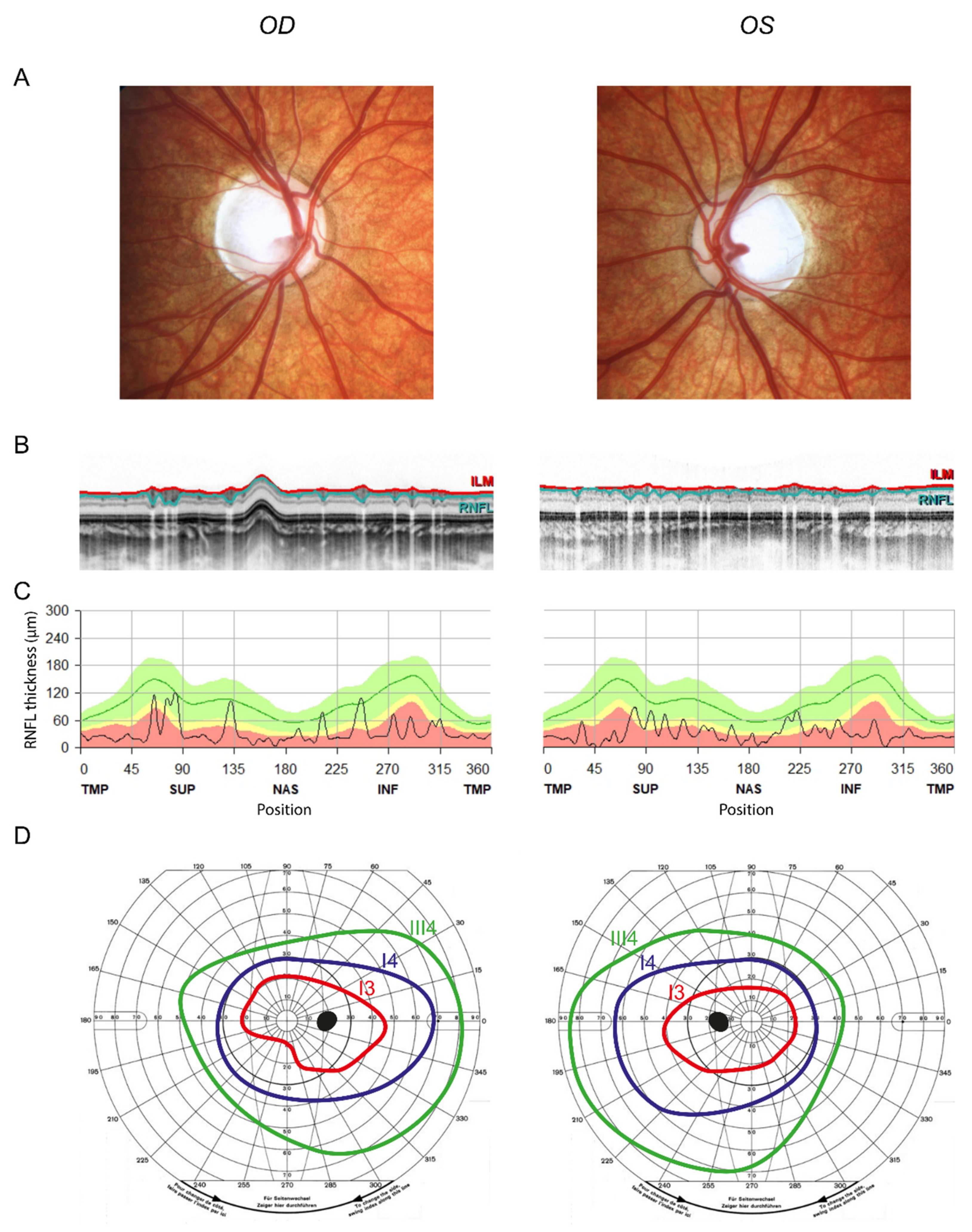
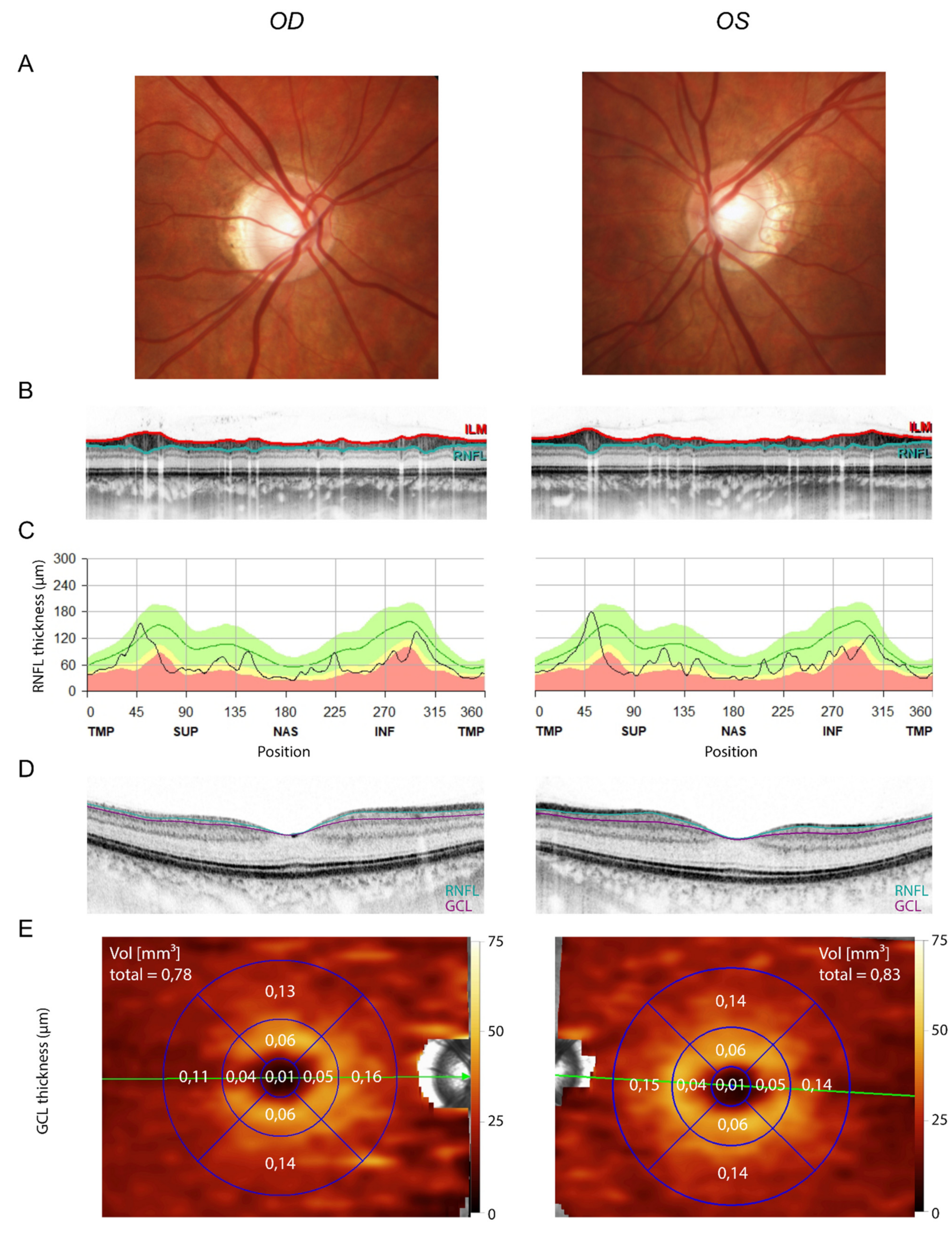
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferré, M.; Bonneau, D.; Milea, D.; Chevrollier, A.; Verny, C.; Dollfus, H.; Ayuso, C.; Defoort, S.; Vignal, C.; Zanlonghi, X.; et al. Molecular Screening of 980 Cases of Suspected Hereditary Optic Neuropathy with a Report on 77 Novel OPA1 Mutations. Hum. Mutat. 2009, 30, E692–E705. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.J. Hereditary Optic Neuropathies: From the Mitochondria to the Optic Nerve. Am. J. Ophthalmol. 2005, 140, 517.e1–517.e9. [Google Scholar] [CrossRef] [PubMed]
- Cipolat, S.; Martins de Brito, O.; Dal Zilio, B.; Scorrano, L. OPA1 Requires Mitofusin 1 to Promote Mitochondrial Fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Trenell, M.I.; Hollingsworth, K.G.; Griffiths, P.G.; Chinnery, P.F. OPA1 Mutations Impair Mitochondrial Function in Both Pure and Complicated Dominant Optic Atrophy. Brain 2011, 134, e164. [Google Scholar] [CrossRef] [PubMed]
- Lenaers, G.; Reynier, P.; Elachouri, G.; Soukkarieh, C.; Olichon, A.; Belenguer, P.; Baricault, L.; Ducommun, B.; Hamel, C.; Delettre, C. OPA1 Functions in Mitochondria and Dysfunctions in Optic Nerve. Int. J. Biochem. Cell Biol. 2009, 41, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, H.; Fiket, M.; Alexander, C.; Chan, D.C. OPA1 Processing Controls Mitochondrial Fusion and Is Regulated by MRNA Splicing, Membrane Potential, and Yme1L. J. Cell Biol. 2007, 178, 749–755. [Google Scholar] [CrossRef]
- Delettre, C.; Lenaers, G.; Pelloquin, L.; Belenguer, P.; Hamel, C.P. OPA1 (Kjer Type) Dominant Optic Atrophy: A Novel Mitochondrial Disease. Mol. Genet. Metab. 2002, 75, 97–107. [Google Scholar] [CrossRef]
- Skidd, P.M.; Lessell, S.; Cestari, D.M. Autosomal Dominant Hereditary Optic Neuropathy (ADOA): A Review of the Genetics and Clinical Manifestations of ADOA and ADOA+. Semin. Ophthalmol. 2013, 28, 422–426. [Google Scholar] [CrossRef]
- Hoyt, C.S. Autosomal Dominant Optic Atrophy. Ophthalmology 1980, 87, 245–251. [Google Scholar] [CrossRef]
- Cohn, A.C.; Toomes, C.; Potter, C.; Towns, K.V.; Hewitt, A.W.; Inglehearn, C.F.; Craig, J.E.; Mackey, D.A. Autosomal Dominant Optic Atrophy: Penetrance and Expressivity in Patients with OPA1 Mutations. Am. J. Ophthalmol. 2007, 143, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.C.; Toomes, C.; Hewitt, A.W.; Kearns, L.S.; Inglehearn, C.F.; Craig, J.E.; Mackey, D.A. The Natural History of OPA1-Related Autosomal Dominant Optic Atrophy. Br. J. Ophthalmol. 2008, 92, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Votruba, M.; Moore, A.T.; Bhattacharya, S.S. Clinical Features, Molecular Genetics, and Pathophysiology of Dominant Optic Atrophy. J. Med. Genet. 1998, 35, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Gorman, G.S.; Lourenco, C.M.; Wright, A.F.; Auer-Grumbach, M.; Toscano, A.; Musumeci, O.; Valentino, M.L.; Caporali, L.; et al. Multi-System Neurological Disease Is Common in Patients with OPA1 Mutations. Brain 2010, 133, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, D.; Colin, E.; Oca, F.; Ferré, M.; Chevrollier, A.; Guéguen, N.; Desquiret-Dumas, V.; N’Guyen, S.; Barth, M.; Zanlonghi, X.; et al. Early-Onset Behr Syndrome Due to Compound Heterozygous Mutations in OPA1. Brain 2014, 137, e301. [Google Scholar] [CrossRef]
- Barboni, P.; Valentino, M.L.; La Morgia, C.; Carbonelli, M.; Savini, G.; De Negri, A.; Simonelli, F.; Sadun, F.; Caporali, L.; Maresca, A.; et al. Idebenone Treatment in Patients with OPA1-Mutant Dominant Optic Atrophy. Brain 2013, 136, e231. [Google Scholar] [CrossRef]
- Delettre-Cribaillet, C.; Hamel, C.P.; Lenaers, G. Optic Atrophy Type 1. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Mirzaa, G., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Acuna-Hidalgo, R.; Veltman, J.A.; Hoischen, A. New Insights into the Generation and Role of de Novo Mutations in Health and Disease. Genome Biol. 2016, 17, 241. [Google Scholar] [CrossRef]
- Baris, O.; Delettre, C.; Amati-Bonneau, P.; Surget, M.-O.; Charlin, J.-F.; Catier, A.; Derieux, L.; Guyomard, J.-L.; Dollfus, H.; Jonveaux, P.; et al. Fourteen Novel OPA1 Mutations in Autosomal Dominant Optic Atrophy Including Two de Novo Mutations in Sporadic Optic Atrophy. Hum. Mutat. 2003, 21, 656. [Google Scholar] [CrossRef]
- Cohen, L.; Tzur, S.; Goldenberg-Cohen, N.; Bormans, C.; Behar, D.M.; Reinstein, E. Exome Sequencing Identified a Novel de Novo OPA1 Mutation in a Consanguineous Family Presenting with Optic Atrophy. Genet. Res. 2016, 98, e10. [Google Scholar] [CrossRef]
- Le Roux, B.; Lenaers, G.; Zanlonghi, X.; Amati-Bonneau, P.; Chabrun, F.; Foulonneau, T.; Caignard, A.; Leruez, S.; Gohier, P.; Procaccio, V.; et al. OPA1: 516 Unique Variants and 831 Patients Registered in an Updated Centralized Variome Database. Orphanet J. Rare Dis. 2019, 14, 214. [Google Scholar] [CrossRef]
- Chen, J.; Xu, K.; Zhang, X.; Jiang, F.; Liu, L.; Dong, B.; Ren, Y.; Li, Y. Mutation Screening of Mitochondrial DNA as Well as OPA1 and OPA3 in a Chinese Cohort with Suspected Hereditary Optic Atrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6987–6995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pesch, U.E.; Leo-Kottler, B.; Mayer, S.; Jurklies, B.; Kellner, U.; Apfelstedt-Sylla, E.; Zrenner, E.; Alexander, C.; Wissinger, B. OPA1 Mutations in Patients with Autosomal Dominant Optic Atrophy and Evidence for Semi-Dominant Inheritance. Hum. Mol. Genet. 2001, 10, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Schimpf-Linzenbold, S.; Mazzola, P.; Kieninger, S.; Xiao, T.; Kellner, U.; Neuhann, T.; Kelbsch, C.; Tonagel, F.; Wilhelm, H.; et al. Mutation Spectrum of the OPA1 Gene in a Large Cohort of Patients with Suspected Dominant Optic Atrophy: Identification and Classification of 48 Novel Variants. PLoS ONE 2021, 16, e0253987. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Karri, R.; Danesh-Meyer, H.; Drummond, K.; Symons, A. A Normative Database of A-Scan Data Using the Heidelberg Spectralis Spectral Domain Optical Coherence Tomography Machine. PLoS ONE 2021, 16, e0253720. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Tomasini, L.; Mariani, J.; Zhou, B.; Roychowdhury, T.; Franjic, D.; Pletikos, M.; Pattni, R.; Chen, B.-J.; Venturini, E.; et al. Different Mutational Rates and Mechanisms in Human Cells at Pregastrulation and Neurogenesis. Science 2018, 359, 550–555. [Google Scholar] [CrossRef]
- Moog, U.; Felbor, U.; Has, C.; Zirn, B. Disorders Caused by Genetic Mosaicism. Dtsch. Arztebl. Int. 2020, 116, 119–125. [Google Scholar] [CrossRef]
- Tai, E.L.M.; Ling, J.L.; Gan, E.H.; Adil, H.; Wan-Hazabbah, W.-H. Comparison of Peripapillary Retinal Nerve Fiber Layer Thickness between Myopia Severity Groups and Controls. Int. J. Ophthalmol. 2018, 11, 274–278. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.W.; Im, S.K.; Lee, S.H.; Ahn, M.D. Effect of Myopia on the Thickness of the Retinal Nerve Fiber Layer Measured by Cirrus HD Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4075–4083. [Google Scholar] [CrossRef]
- Aydogan, T.; Akçay, B.İ.S.; Kardeş, E.; Ergin, A. Evaluation of Spectral Domain Optical Coherence Tomography Parameters in Ocular Hypertension, Preperimetric, and Early Glaucoma. Indian J. Ophthalmol. 2017, 65, 1143–1150. [Google Scholar] [CrossRef]
- Savini, G.; Barboni, P.; Parisi, V.; Carbonelli, M. The Influence of Axial Length on Retinal Nerve Fibre Layer Thickness and Optic-Disc Size Measurements by Spectral-Domain OCT. Br. J. Ophthalmol. 2012, 96, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.M.; Sparkes, R.S. Segmental Neurofibromatosis. Arch. Dermatol. 1977, 113, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Tinschert, S.; Naumann, I.; Stegmann, E.; Buske, A.; Kaufmann, D.; Thiel, G.; Jenne, D.E. Segmental Neurofibromatosis Is Caused by Somatic Mutation of the Neurofibromatosis Type 1 (NF1) Gene. Eur. J. Hum. Genet. 2000, 8, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; Tan, E.-C.; Wei, H.; Zhao, Y.; Koh, M.J.A. Epidermolytic Ichthyosis in a Child and Systematized Epidermolytic Nevi in the Mosaic Parent Associated with a KRT1 Variant. Eur. J. Med. Genet. 2021, 64, 104324. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alter, S.; Farassat, N.; Küchlin, S.; Lagrèze, W.A.; Fischer, J. First Description of Inheritance of a Postzygotic OPA1 Mosaic Variant. Genes 2022, 13, 478. https://doi.org/10.3390/genes13030478
Alter S, Farassat N, Küchlin S, Lagrèze WA, Fischer J. First Description of Inheritance of a Postzygotic OPA1 Mosaic Variant. Genes. 2022; 13(3):478. https://doi.org/10.3390/genes13030478
Chicago/Turabian StyleAlter, Svenja, Navid Farassat, Sebastian Küchlin, Wolf A. Lagrèze, and Judith Fischer. 2022. "First Description of Inheritance of a Postzygotic OPA1 Mosaic Variant" Genes 13, no. 3: 478. https://doi.org/10.3390/genes13030478
APA StyleAlter, S., Farassat, N., Küchlin, S., Lagrèze, W. A., & Fischer, J. (2022). First Description of Inheritance of a Postzygotic OPA1 Mosaic Variant. Genes, 13(3), 478. https://doi.org/10.3390/genes13030478






