Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine †
Abstract
:1. Cancer Prevention Overview
2. Aspirin and Cancer Prevention
3. Clinical Pharmacology of Aspirin and Non-Invasive Biomarkers for Drug Response Prediction and Monitoring
3.1. Mechanism of Action of Aspirin
3.2. Aspirin Affects Platelet COX-1 at Low-Doses
3.3. Low-Dose Aspirin Causes a Direct Inhibitory Effect on COX-1 Expressed in Colorectal Mucosa
3.4. Low-Dose Aspirin Can Indirectly Prevent COX-2 Induction in Colorectal Mucosa
3.5. Assessment of Biomarkers to Develop a Precision Therapy of CRC with Aspirin
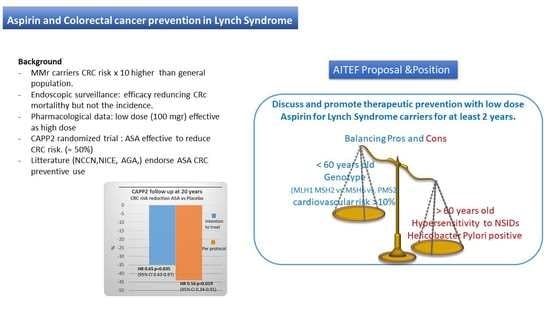
4. Cancer Prevention in Lynch Syndrome Carriers
Guidelines and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; de Schutter, H.; van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal Cancer Incidence, Mortality, and Stage Distribution in European Countries in the Colorectal Cancer Screening Era: An International Population-Based Study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C.; Agoritsas, T.; Lytvyn, L.; Anderson, J.C.; Auer, R.; Murphy, S.B.; Almadi, M.A.; Corley, D.A.; et al. Colorectal Cancer Screening with Faecal Immunochemical Testing, Sigmoidoscopy or Colonoscopy: A Clinical Practice Guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, M.D.; Hoff, G.; Tidemann-Andersen, I.; Bodin, G.E.; Øvervold, S.; Berstad, P. Public Awareness and Perceptions of Colorectal Cancer Prevention: A Cross-Sectional Survey. J. Cancer Educ. 2021, 36, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Ghaddaf, A.A.; Aziz, M.; Alomari, M.S.; Abdulhamid, A.S.; Alharbi, F.A.; Mullah, A.N.; Zaidi, S.F. Influence of Aspirin on Prevention of Colorectal Cancer: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Colorectal Dis. 2021, 36, 1711–1722. [Google Scholar] [CrossRef]
- Veettil, S.K.; Lim, K.G.; Ching, S.M.; Saokaew, S.; Phisalprapa, P.; Chaiyakunapruk, N. Effects of Aspirin and Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs on the Incidence of Recurrent Colorectal Adenomas: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials. BMC Cancer 2017, 17, 763. [Google Scholar] [CrossRef] [Green Version]
- Chudy-Onwugaje, K.; Huang, W.Y.; Su, L.J.; Purdue, M.P.; Johnson, C.C.; Wang, L.; Katki, H.A.; Barry, K.H.; Berndt, S.I. Aspirin, Ibuprofen, and Reduced Risk of Advanced Colorectal Adenoma Incidence and Recurrence and Colorectal Cancer in the PLCO Cancer Screening Trial. Cancer 2021, 127, 3145–3155. [Google Scholar] [CrossRef]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.-P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Fowkes, F.G.R.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of Daily Aspirin on Long-Term Risk of Death Due to Cancer: Analysis of Individual Patient Data from Randomised Trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-Term Effect of Aspirin on Colorectal Cancer Incidence and Mortality: 20-Year Follow-up of Five Randomised Trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of Daily Aspirin on Risk of Cancer Metastasis: A Study of Incident Cancers during Randomised Controlled Trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Price, J.F.; Fowkes, F.G.R.; Zanchetti, A.; Roncaglioni, M.C.; Tognoni, G.; Lee, R.; Belch, J.F.; Wilson, M.; Mehta, Z.; et al. Short-Term Effects of Daily Aspirin on Cancer Incidence, Mortality, and Non-Vascular Death: Analysis of the Time Course of Risks and Benefits in 51 Randomised Controlled Trials. Lancet 2012, 379, 1602–1612. [Google Scholar] [CrossRef]
- Flossmann, E.; Rothwell, P.M. Effect of Aspirin on Long-Term Risk of Colorectal Cancer: Consistent Evidence from Randomised and Observational Studies. Lancet 2007, 369, 1603–1613. [Google Scholar] [CrossRef]
- Bosetti, C.; Santucci, C.; Gallus, S.; Martinetti, M.; la Vecchia, C. Aspirin and the Risk of Colorectal and Other Digestive Tract Cancers: An Updated Meta-Analysis through 2019. Ann. Oncol. 2020, 31, 558–568. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Jacobs, E.J.; Newton, C.C.; Guinter, M.A.; Cance, W.G.; Campbell, P.T. Associations of Aspirin and Non-Aspirin Non-Steroidal Anti-Inflammatory Drugs with Colorectal Cancer Mortality After Diagnosis. J. Natl. Cancer Inst. 2021, 113, 833–840. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, A.T.; Meyerhardt, J.A.; Giovannucci, E.L. Timing of Aspirin Use in Colorectal Cancer Chemoprevention: A Prospective Cohort Study. J. Natl. Cancer Inst. 2021, 113, 841–851. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Liu, E.; Jovani, M.; Li, S.X.; Takvorian, K.; Suthahar, N.; Cheng, S.; Splansky, G.L.; Januzzi, J.L.; et al. Cardiovascular Risk Factors Are Associated with Future Cancer. JACC Cardio Oncol. 2021, 3, 48–58. [Google Scholar] [CrossRef]
- Kannel, W.B.; Feinleib, M.; Mcnamara, P.M.; Garrison, R.J.; Castelli, W.P. An investigation of coronary heart disease in families. Am. J. Epidemiol. 1979, 110, 281–290. [Google Scholar] [CrossRef]
- Hillege, H.L.; Fidler, V.; Diercks, G.F.H.; van Gilst, W.H.; de Zeeuw, D.; van Veldhuisen, D.J.; Gans, R.O.B.; Janssen, W.M.T.; Grobbee, D.E.; de Jong, P.E. Urinary Albumin Excretion Predicts Cardiovascular and Noncardiovascular Mortality in General Population. Circulation 2002, 106, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Coglianese, E.E.; Vasan, R.S.; Wang, T.J. Long-term Cardiovascular Risks Associated with an Elevated Heart Rate: The Framingham Heart Study. J. Am. Heart Assoc. 2014, 3, e000668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Gillman, M.; Harper, D.M.; Kemper, A.R.; Krist, A.H.; et al. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guirguis-Blake, J.M.; Evans, C.V.; Perdue, L.A.; Bean, S.I.; Senger, C.A. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force; Evidence Synthesis No. 211; AHRQ Publication: Rockville, MD, USA, 2021. [Google Scholar]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Reid, C.M.; Kirpach, B.; Wolfe, R.; Storey, E.; Shah, R.C.; Lockery, J.E.; Tonkin, A.M.; et al. Effect of Aspirin on Disability-Free Survival in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Cafferty, F.; Hubner, R.; Swinson, D.; Sothi, S.; Gupta, K.; Falk, S.; Patel, K.; Warner, N.; Kunene, V.; et al. Aspirin as an Adjuvant Treatment for Cancer: Feasibility Results from the Add-Aspirin Randomised Trial. Lancet Gastroenterol. Hepatol. 2019, 4, 854–862. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, P.; Patrono, C. Cyclooxygenase Inhibitors: From Pharmacology to Clinical Read-Outs. Biochim. Biophys. Acta 2015, 1851, 422–432. [Google Scholar] [CrossRef]
- Dovizio, M.; Bruno, A.; Tacconelli, S.; Patrignani, P. Mode of action of aspirin as a chemopreventive agent. In Prospects for Chemoprevention of Colorectal Neoplasia; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–65. [Google Scholar]
- Patrignani, P.; Tacconelli, S.; Piazuelo, E.; di Francesco, L.; Dovizio, M.; Sostres, C.; Marcantoni, E.; Guillem-Llobat, P.; del Boccio, P.; Zucchelli, M.; et al. Reappraisal of the Clinical Pharmacology of Low-Dose Aspirin by Comparing Novel Direct and Traditional Indirect Biomarkers of Drug Action. J. Thromb. Haemost. 2014, 12, 1320–1330. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.K.; FitzGerald, G.A. Dose-Related Kinetics of Aspirin. N. Engl. J. Med. 1984, 311, 1206–1211. [Google Scholar] [CrossRef]
- Patrono, C.; García Rodríguez, L.A.; Landolfi, R.; Baigent, C. Low-Dose Aspirin for the Prevention of Atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383. [Google Scholar] [CrossRef] [Green Version]
- The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: Randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998, 351, 233–241. [Google Scholar]
- Clarke, R.J.; Mayo, G.; Price, P.; FitzGerald, G.A. Suppression of Thromboxane A2 but Not of Systemic Prostacyclin by Controlled-Release Aspirin. N. Engl. J. Med. 1991, 325, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Lee, I.M.; Zhang, S.M.; Moorthy, M.V.; Buring, J.E. Alternate-Day, Low-Dose Aspirin and Cancer Risk: Long-Term Observational Follow-up of a Randomized Trial. Ann. Intern. Med. 2013, 159, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, A.T. Collaborative Meta-Analysis of Randomised Trials of Antiplatelet Therapy for Prevention of Death, Myocardial Infarction, and Stroke in High Risk Patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovizio, M.; Sacco, A.; Patrignani, P. Curbing Tumorigenesis and Malignant Progression through the Pharmacological Control of the Wound Healing Process. Vasc. Pharmacol. 2017, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Buchanan, F.G.; Wang, H.; Dey, S.K.; DuBois, R.N. Prostaglandin E2 Enhances Intestinal Adenoma Growth via Activation of the Ras-Mitogen-Activated Protein Kinase Cascade. Cancer Res. 2005, 65, 1822–1829. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; DuBois, R.N. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, P.; Sacco, A.; Sostres, C.; Bruno, A.; Dovizio, M.; Piazuelo, E.; di Francesco, L.; Contursi, A.; Zucchelli, M.; Schiavone, S.; et al. Low-Dose Aspirin Acetylates Cyclooxygenase-1 in Human Colorectal Mucosa: Implications for the Chemoprevention of Colorectal Cancer. Clin. Pharmacol. Ther. 2017, 102, 52–61. [Google Scholar] [CrossRef]
- Tacconelli, S.; Contursi, A.; Falcone, L.; Mucci, M.; D’Agostino, I.; Fullone, R.; Sacco, A.; Zucchelli, M.; Bruno, A.; Ballerini, P.; et al. Characterization of Cyclooxygenase-2 Acetylation and Prostanoid Inhibition by Aspirin in Cellular Systems. Biochem. Pharmacol. 2020, 178, 114094. [Google Scholar] [CrossRef]
- Dovizio, M.; Ballerini, P.; Fullone, R.; Tacconelli, S.; Contursi, A.; Patrignani, P. Multifaceted Functions of Platelets in Cancer: From Tumorigenesis to Liquid Biopsy Tool and Drug Delivery System. Int. J. Mol. Sci. 2020, 21, 9585. [Google Scholar] [CrossRef]
- Johnson, J.C.; Schmidt, C.R.; Shrubsole, M.J.; Billheimer, D.D.; Joshi, P.R.; Morrow, J.D.; Heslin, M.J.; Washington, M.K.; Ness, R.M.; Zheng, W.; et al. Urine PGE-M: A Metabolite of Prostaglandin E2 as a Potential Biomarker of Advanced Colorectal Neoplasia. Clin. Gastroenterol. Hepatol. 2006, 4, 1358–1365. [Google Scholar] [CrossRef]
- Liao, X.; Lochhead, P.; Nishihara, R.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Imamura, Y.; Qian, Z.R.; Baba, Y.; Shima, K.; et al. Aspirin Use, Tumor PIK3CA Mutation, and Colorectal-Cancer Survival. N. Engl. J. Med. 2012, 367, 1596–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, H.; Hutter, C.M.; Lin, Y.; Jacobs, E.J.; Ulrich, C.M.; White, E.; Baron, J.A.; Berndt, S.I.; Brenner, H.; Butterbach, K.; et al. Association of Aspirin and NSAID Use with Risk of Colorectal Cancer According to Genetic Variants. JAMA 2015, 313, 1133. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Broeke, S.W.; Plazzer, J.-P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer Risks by Gene, Age, and Gender in 6350 Carriers of Pathogenic Mismatch Repair Variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasen, H.F.A. Progress Report: New Insights into the Prevention of CRC by Colonoscopic Surveillance in Lynch Syndrome. Fam. Cancer 2021, 21, 49–56. [Google Scholar] [CrossRef]
- Ahadova, A.; von Knebel Doeberitz, M.; Bläker, H.; Kloor, M. CTNNB1-Mutant Colorectal Carcinomas with Immediate Invasive Growth: A Model of Interval Cancers in Lynch Syndrome. Fam. Cancer 2016, 15, 579–586. [Google Scholar] [CrossRef]
- Ahadova, A.; Gallon, R.; Gebert, J.; Ballhausen, A.; Endris, V.; Kirchner, M.; Stenzinger, A.; Burn, J.; von Knebel Doeberitz, M.; Bläker, H.; et al. Three Molecular Pathways Model Colorectal Carcinogenesis in Lynch Syndrome. Int. J. Cancer 2018, 143, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Burn, J.; Bishop, D.T.; Mecklin, J.-P.; Macrae, F.; Möslein, G.; Olschwang, S.; Bisgaard, M.-L.; Ramesar, R.; Eccles, D.; Maher, E.R.; et al. Effect of Aspirin or Resistant Starch on Colorectal Neoplasia in the Lynch Syndrome. N. Engl. J. Med. 2008, 359, 2567–2578. [Google Scholar] [CrossRef] [Green Version]
- Burn, J.; Gerdes, A.-M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-Term Effect of Aspirin on Cancer Risk in Carriers of Hereditary Colorectal Cancer: An Analysis from the CAPP2 Randomised Controlled Trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer Prevention with Aspirin in Hereditary Colorectal Cancer (Lynch Syndrome), 10-Year Follow-up and Registry-Based 20-Year Data in the CAPP2 Study: A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Burn, J.; Mathers, J.C.; Bishop, D.T. Chemoprevention in Lynch Syndrome. Fam. Cancer 2013, 12, 707–718. [Google Scholar] [CrossRef]
- Clinical Guidelines. Chemopreventive Candidate Agents; Clinical Guidelines: Melbourne, VIC, Australia, 2019. [Google Scholar]
- Milton, S.; McIntosh, J.; Yogaparan, T.; Alphonse, P.; Saya, S.; Karnchanachari, N.; Nguyen, P.; Lau, P.; MacRae, F.; Emery, J. Clinicians’ Opinions on Recommending Aspirin to Prevent Colorectal Cancer to Australians Aged 50-70 Years: A Qualitative Study. BMJ Open 2021, 11, e042261. [Google Scholar] [CrossRef] [PubMed]
- Milton, S.; McIntosh, J.; Macrae, F.; Chondros, P.; Trevena, L.; Jenkins, M.; Walter, F.M.; Taylor, N.; Boyd, L.; Saya, S.; et al. An RCT of a Decision Aid to Support Informed Choices about Taking Aspirin to Prevent Colorectal Cancer and Other Chronic Diseases: A Study Protocol for the SITA (Should I Take Aspirin?) Trial. Trials 2021, 22, 452. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the Management of Hereditary Colorectal Cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care. Excellence NICE-Effectiveness of Aspirin in the Prevention of Colorectal Cancer in People with Lynch Syndrome; National Institute for Health and Care: London, UK, 2020. [Google Scholar]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European Guidelines from the EHTG and ESCP for Lynch Syndrome: An Updated Third Edition of the Mallorca Guidelines Based on Gene and Gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer Risk and Survival in Path_MMR Carriers by Gene and Gender up to 75 Years of Age: A Report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Movahedi, M.; Bishop, D.T.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Obesity, Aspirin, and Risk of Colorectal Cancer in Carriers of Hereditary Colorectal Cancer: A Prospective Investigation in the CAPP2 Study. J. Clin. Oncol. 2015, 33, 3591–3597. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Cook, N.R.; Gaziano, J.M.; Price, J.F.; Belch, J.F.F.; Roncaglioni, M.C.; Morimoto, T.; Mehta, Z. Effects of Aspirin on Risks of Vascular Events and Cancer According to Bodyweight and Dose: Analysis of Individual Patient Data from Randomised Trials. Lancet 2018, 392, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Harmon, B.E.; Little, M.A.; Ollberding, N.J.; Kolonel, L.N.; Henderson, B.E.; le Marchand, L.; Wilkens, L.R. Excess Body Weight and Colorectal Cancer Survival: The Multiethnic Cohort. Cancer Causes Control. 2015, 26, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Aukrust, P.; Halvorsen, B.; Ueland, T.; Michelsen, A.E.; Skjelland, M.; Gullestad, L.; Yndestad, A.; Otterdal, K. Activated Platelets and Atherosclerosis. Expert Rev. Cardiovasc. Ther. 2010, 8, 1297–1307. [Google Scholar] [CrossRef]
- Grinstein, J.; Cannon, C.P. Aspirin Resistance: Current Status and Role of Tailored Therapy. Clin. Cardiol. 2012, 35, 673–680. [Google Scholar] [CrossRef]
| Organization | Reccomandations |
|---|---|
| United States Preventive Services Task Force | Adults aged 50–69 years with 10 years CVD ≥10% * Low-dose aspirin (81–100 mg daily) Treatment for 5 to 10 years |
| Cancer Council Australia | People aged 50–70 years with average risk of colorectal cancer ** Dose from 100 to 300 mg daily Lynch Syndrome carriers aged ≥25 years Dose from 100 to 600 mg daily Treatment for at least 2.5 years |
| National Institute for Health and Care Excellence (NICE guideline) | People with Lynch syndrome Treatment for more than 2 years |
| BSG/ACPGBI/UKCGG | Lynch Syndrome carriers 100 mg per daily *** |
| EHTG/ESCP | Lynch Syndrome carriers 75–100 mg daily |
| Variable | Pros | Cons |
|---|---|---|
| Age | <60 years old | ≥60 years old |
| Genotype | MLH1 MSH2 MSH6 (PMS2 *) | |
| Sex | M/F | Child bearing or desire of pregnancy |
| Allergy | No | Known NSAIDs ipersensibility |
| Helicobacter Pylori | Negative | HP positive |
| Cancer history ** | Previous syndromic cancer including CRC | protocolectomy |
| Cardiovascular risk *** | ≥10% | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, D.; Patrignani, P.; Stigliano, V.; Turchetti, D.; Sciallero, S.; Roviello, F.; D’Arpino, A.; Grattagliano, I.; Testa, S.; Oliani, C.; et al. Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine. Genes 2022, 13, 460. https://doi.org/10.3390/genes13030460
Serrano D, Patrignani P, Stigliano V, Turchetti D, Sciallero S, Roviello F, D’Arpino A, Grattagliano I, Testa S, Oliani C, et al. Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine. Genes. 2022; 13(3):460. https://doi.org/10.3390/genes13030460
Chicago/Turabian StyleSerrano, Davide, Paola Patrignani, Vittoria Stigliano, Daniela Turchetti, Stefania Sciallero, Franco Roviello, Alessandro D’Arpino, Ignazio Grattagliano, Salvo Testa, Cristina Oliani, and et al. 2022. "Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine" Genes 13, no. 3: 460. https://doi.org/10.3390/genes13030460
APA StyleSerrano, D., Patrignani, P., Stigliano, V., Turchetti, D., Sciallero, S., Roviello, F., D’Arpino, A., Grattagliano, I., Testa, S., Oliani, C., Bertario, L., & Bonanni, B. (2022). Aspirin Colorectal Cancer Prevention in Lynch Syndrome: Recommendations in the Era of Precision Medicine. Genes, 13(3), 460. https://doi.org/10.3390/genes13030460