Serum Periostin Level and Genetic Polymorphisms Are Associated with Vertebral Fracture in Chinese Postmenopausal Women
Abstract
1. Introduction
2. Methods
2.1. Study Population and Diagnosis
2.2. Determination of Vertebral Fractures
2.3. Basic Information and Clinical Measurements
2.4. SNP Selection and Genotyping
2.5. Measurement of BMD
3. Statistical Analysis
4. Results
4.1. Basic Characteristics of All Participants
4.2. SNPs Distribution of POSTN and Minor Allele Frequencies (MAF)
4.3. Association between Genetic Polymorphisms in POSTN and Serum Periostin Levels, BMD and the Prevalence of Vertebral Fracture
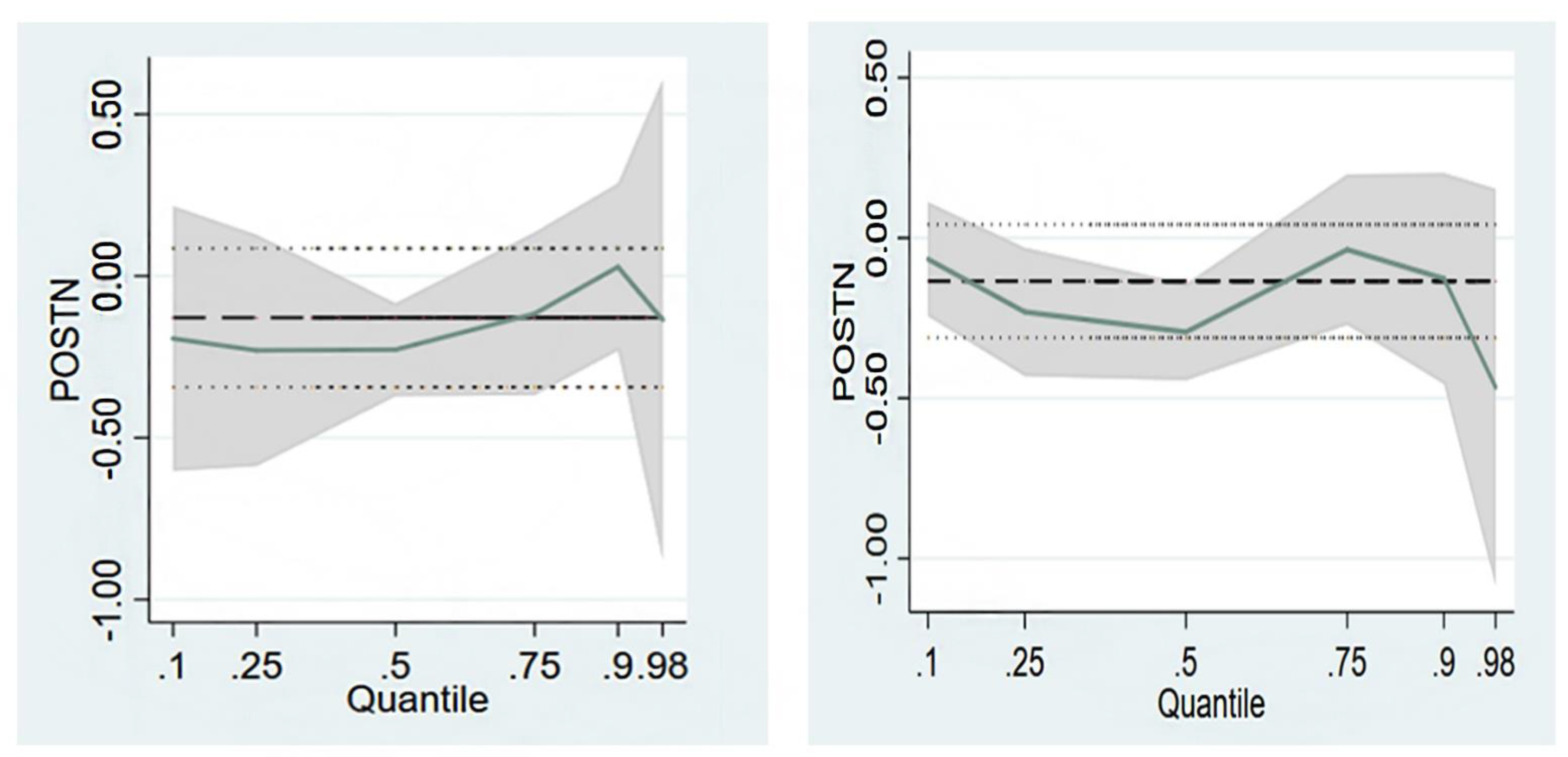
4.4. Association between Serum Periostin Levels and the Prevalence of Vertebral Fractures and BMD
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delmas, P.D.; van de Langerijt, L.; Watts, N.B.; Eastell, R.; Genant, H.; Grauer, A.; Cahall, D.L. Underdiagnosis of vertebral fractures is a worldwide problem: The IMPACT study. J. Bone Miner. Res. 2005, 20, 557–563. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata-Andía, J.B.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; John, B.; Mohan, S.; Paul, T.V. Vertebral fracture assessment by dual-energy X-ray absorptiometry along with bone mineral density in the evaluation of postmenopausal osteoporosis. Arch. Osteoporos. 2020, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Nakajima, A.; Seki, N.; Okawa, A.; Kato, M.; Moriya, H.; Amizuka, N.; Einhorn, T.A.; Yamazaki, M. Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J. Orthop. Res. 2004, 22, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Barbe, M.F.; Amin, N.; Rani, S.; Popoff, S.N.; Safadi, F.F.; Litvin, J. Immunolocalization of Periostin-like factor and Periostin during embryogenesis. J. Histochem. Cytochem. 2008, 56, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Gossiel, F.; Scott, J.R.; Paggiosi, M.A.; Eastell, R. Effect of age and gender on serum periostin: Relationship to cortical measures, bone turnover and hormones. Bone 2017, 99, 8–13. [Google Scholar] [CrossRef]
- Rousseau, J.C.; Sornay-Rendu, E.; Bertholon, C.; Chapurlat, R.; Garnero, P. Serum periostin is associated with fracture risk in postmenopausal women: A 7-year prospective analysis of the OFELY study. J. Clin. Endocrinol. Metab. 2014, 99, 2533–2539. [Google Scholar] [CrossRef]
- Kim, B.J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.K.; Kim, D.Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.-Y.; et al. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef]
- Guo, R.J.; Huang, E.; Ezaki, T.; Patel, N.; Sinclair, K.; Wu, J.; Klein, P.; Suh, E.R.; Lynch, J.P. Cdx1 inhibits human colon cancer cell proliferation by reducing beta-catenin/T-cell factor transcriptional activity. J. Biol. Chem. 2004, 279, 36865–36875. [Google Scholar] [CrossRef]
- Pepe, J.; Bonnet, N.; Herrmann, F.R.; Biver, E.; Rizzoli, R.; Chevalley, T.; Ferrari, S.L. Interaction between LRP5 and periostin gene polymorphisms on serum periostin levels and cortical bone microstructure. Osteoporos. Int. 2018, 29, 339–346. [Google Scholar] [CrossRef]
- Xiao, S.M.; Gao, Y.; Cheung, C.L.; Bow, C.H.; Lau, K.S.; Sham, P.C.; Tan, K.C.B.; Kung, A.W.C. Association of CDX1 binding site of periostin gene with bone mineral density and vertebral fracture risk. Osteoporos. Int. 2012, 23, 1877–1887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Zhang, Z.; Zhang, H.; He, J.W.; Gu, J.M.; Hu, W.W.; Hu, Y.Q.; Li, M.; Liu, Y.-J.; Fu, W.-Z.; et al. Susceptibility genes for osteoporotic fracture in postmenopausal Chinese women. J. Bone Miner. Res. 2012, 27, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Sanfélix-Genovés, J.; Arana, E.; Sanfélix-Gimeno, G.; Peiró, S.; Graells-Ferrer, M.; Vega-Martínez, M. Agreement between semi-automatic radiographic morphometry and Genant semi-quantitative method in the assessment of vertebral fractures. Osteoporos. Int. 2012, 23, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, J.W.; Gao, G.; Yue, H.; Yu, J.B.; Hu, W.W.; Gu, J.-M.; Hu, Y.-Q.; Li, M.; Fu, W.-Z.; et al. Polymorphisms in the HOXD4 gene are not associated with peak bone mineral density in Chinese nuclear families. Acta Pharmacol. Sin. 2010, 31, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, Z.L.; Zhang, H.; Hu, W.W.; Huang, Q.R.; Lu, J.H.; Hu, Y.-Q.; Li, M.; Liu, Y.-J.; He, J.-W.; et al. Hip axis length changes in 10,554 males and females and the association with femoral neck fracture. J. Clin. Densitom. 2008, 11, 360–366. [Google Scholar] [CrossRef]
- Merle, B.; Garnero, P. The multiple facets of periostin in bone metabolism. Osteoporos. Int. 2012, 23, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 1999, 14, 1239–1249. [Google Scholar] [CrossRef]
- Norris, R.A.; Moreno-Rodriguez, R.A.; Sugi, Y.; Hoffman, S.; Amos, J.; Hart, M.M.; Potts, J.D.; Goodwin, R.L.; Markwald, R.R. Periostin regulates atrioventricular valve maturation. Dev. Biol. 2008, 316, 200–213. [Google Scholar] [CrossRef]
- Litvin, J.; Selim, A.H.; Montgomery, M.O.; Lehmann, K.; Rico, M.C.; Devlin, H.; Bednarik, D.P.; Safadi, F.F. Expression and function of periostin-isoforms in bone. J. Cell Biochem. 2004, 92, 1044–1061. [Google Scholar] [CrossRef]
- Bonnet, N.; Biver, E.; Chevalley, T.; Rizzoli, R.; Garnero, P.; Ferrari, S.L. Serum Levels of a Cathepsin-K Generated Periostin Fragment Predict Incident Low-Trauma Fractures in Postmenopausal Women Independently of BMD and FRAX. J. Bone Miner. Res. 2017, 32, 2232–2238. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Li, H.; Chen, L.; Bian, Y.Q.; Zhao, B.; Han, H.X.; Han, S.Z.; Han, L.R.; Wang, D.W.; et al. Circulating periostin levels increase in association with bone density loss and healing progression during the early phase of hip fracture in Chinese older women. Osteoporos. Int. 2017, 28, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Luijk, R.; Zhernakova, D.V.; Moed, M.; Deelen, P.; Vermaat, M.; van Iterson, M.; van Dijk, F.; van Galen, M.; Bot, J.; et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat. Genet. 2017, 49, 131–138. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Subjects (n = 385) | Vertebral Fracture Subgroup (n = 70) | Control Subgroup (n = 315) | p Value |
|---|---|---|---|---|
| Age (year) | 65.74 ± 9.70 | 68.10 ± 9.38 | 65.22 ± 9.71 | 0.024 * |
| Height (cm) | 154.30 ± 6.03 | 152.70 ± 5.92 | 154.65 ± 6.01 | 0.018 * |
| Body weight (kg) | 56.33 ± 8.09 | 56.01 ± 8.56 | 56.40 ± 8.00 | 0.727 |
| BMI (kg/m2) | 23.65 ± 3.11 | 23.99 ± 3.28 | 23.58 ± 3.07 | 0.336 |
| Serum calcium (mmol/L) | 2.38 ± 0.48 | 2.44 ± 0.79 | 2.36 ± 0.38 | 0.257 |
| Serum phosphorus (mmol/L) | 1.17 ± 0.15 | 1.13 ± 0.15 | 1.18 ± 0.15 | 0.023 * |
| Adjusted # | 1.16 ± 0.20 | 1.18 ± 0.35 | 1.13 ± 0.18 | 0.037 * |
| Serum parathyroid hormone (mmol/L) | 46.62 ± 19.24 | 46.46 ± 25.26 | 46.65 ± 17.68 | 0.953 |
| Serum alkaline phosphatase (mmol/L) | 76.75 ± 22.45 | 81.67 ± 26.96 | 75.63 ± 21.18 | 0.042 * |
| Adjusted # | 78.72 ± 28.85 | 81.85 ± 52.19 | 75.59 ± 25.10 | 0.036 * |
| Serum creatine (mmol/L) | 58.13 ± 11.41 | 57.63 ± 11.96 | 58.27 ± 11.29 | 0.730 |
| Serum urea (mmol/L) | 5.27 ± 1.56 | 5.16 ± 1.24 | 5.30 ± 1.64 | 0.562 |
| Serum 25-hydroxylvitaminD (mmol/L) | 11.64 ± 6.95 | 12.13 ± 7.30 | 11.53 ± 6.88 | 0.515 |
| Serum periostin (ng/mL) | 55.09 ± 21.03 | 59.57 ± 26.05 | 54.10 ± 19.65 | 0.049 * |
| Adjusted # | 56.64 ± 27.14 | 59.07 ± 49.21 | 54.21 ± 23.09 | 0.081 |
| Serum PINP (ng/mL) | 57.95 (40.06–70.66) | 57.42 (46.76–78.76) | 54.56 (38.96–69.65) | 0.162 1 |
| Serum β-CTX (ng/mL) | 464.80 ± 225.97 | 430.09 ± 213.94 | 472.52 ± 228.16 | 0.156 |
| BMD at lumbar spine 1–4 (g/cm2) | 0.86(0.79–0.94) | 0.78(0.63–0.88) | 0.87(0.80–0.94) | <0.001 ** 1 |
| Sqrt L1-L4 BMD Adjusted # | 0.92 ± 0.11 | 0.90 ± 0.20 | 0.94 ± 0.07 | 0.001 * |
| BMD at trochanter (g/cm2) | 0.59 ± 0.10 | 0.53 ± 0.12 | 0.60 ± 0.09 | <0.001 ** |
| Adjusted # | 0.57 ± 0.11 | 0.54 ± 0.23 | 0.60 ± 0.11 | <0.001 ** |
| BMD at femoral neck (g/cm2) | 0.71 ± 0.11 | 0.65 ± 0.13 | 0.72 ± 0.10 | <0.001 ** |
| Adjusted # | 0.69 ± 0.13 | 0.67 ± 0.25 | 0.72 ± 0.11 | <0.001 ** |
| BMD at total hip (g/cm2) | 0.75 ± 0.12 | 0.69 ± 0.15 | 0.77 ± 0.10 | <0.001 ** |
| Adjusted # | 0.73 ± 0.13 | 0.70 ± 0.25 | 0.77 ± 0.11 | <0.001 ** |
| SNP | Alleles | Minor Allele | MAF in This Study | MAF in CHB ^ |
|---|---|---|---|---|
| rs9547952 | C/T | T | 0.088 | 0.068 |
| rs9603226 | A/G | A | 0.371 | 0.422 |
| rs7322993 | C/T | T | 0.139 | 0.126 |
| rs9547965 | A/G | A | 0.045 | 0.068 |
| rs1924285 | A/T | A | 0.139 | 0.126 |
| rs7338244 | C/G | G | 0.273 | 0.262 |
| rs3923854 | C/G | G | 0.134 | 0.136 |
| rs2025405 | C/T | T | 0.273 | 0.262 |
| rs3829365 | G/C | C | 0.355 | 0.316 |
| rs9547970 | A/G | G | 0.226 | 0.194 |
| SNP | Allelic OR (95% CI) | Genotypic OR (95% CI) | n |
|---|---|---|---|
| rs1924285 | 0.642 (0.354–1.163) | T/T 1 | 288 |
| A/T 0.795 (0.418–1.510) | 87 | ||
| A/A NA | 10 | ||
| rs2025405 | 0.827 ( 0.542–1.264) | C/C 1 | 212 |
| C/T 0.894 (0.512–1.560) | 136 | ||
| T/T 0.652 (0.239–1.775) | 37 | ||
| rs3829365 | 0.654 (0.437–0.978) | G/G 1 | 158 |
| G/C 0.647 (0.375–1.114) | 181 | ||
| C/C 0.413 (0.152–1.124) | 46 | ||
| rs3923854 | 1.098 (0.649–1.860) | C/C 1 | 295 |
| C/G 1.460 (0.795–2.681) | 77 | ||
| G/G 0.399 (0.051–3.135) | 13 | ||
| rs7322993 | 0.642 (0.354–1.163) | C/C 1 | 288 |
| C/T 0.795 (0.418–1.510) | 87 | ||
| T/T NA | 10 | ||
| rs7338244 | 0.827 (0.542–1.264) | C/C 1 | 212 |
| C/G 0.894 (0.512–1.560) | 136 | ||
| G/G 0.652 (0.239–1.775) | 37 | ||
| rs9547952 | 0.961 (0.501–1.845) | C/C 1 | 324 |
| C/T 1.021 (0.486–2.145) | 54 | ||
| T/T 0.749 (0.088–6.335) | 7 | ||
| rs9547965 | 1.354 (0.602–3.046) | G/G 1 | 351 |
| G/A 1.492(0.643–3.462) | 33 | ||
| A/A NA | 1 | ||
| rs9547970 | 0.743 (0.467–1.181) | A/A 1 | 237 |
| G/A 0.681 (0.377–1.232) | 122 | ||
| G/G 0.716 (0.236–2.175) | 26 | ||
| rs9603226 | 1.722 (1.190–2.493) p = 0.037 *,& | G/G 1 | 151 |
| G/A 1.617 (0.732–3.575) | 182 | ||
| A/A 3.371 (1.318–8.617) *,# p = 0.011 | 52 |
| SNP (Genotype) | n | Serum Periostin Level (ng/mL) | BMD at Lumbar Spine 1–4 (g/cm2) | BMD at Femoral Neck (g/cm2) | BMD at Trochanter (g/cm2) | BMD at Total Hip (g/cm2) |
|---|---|---|---|---|---|---|
| rs1924285 | ||||||
| T/T | 288 | 55.06 ± 20.64 | 0.85 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.11 | 0.75 ± 0.12 |
| A/T | 87 | 55.19 ± 22.65 | 0.84 ± 0.22 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.76 ± 0.10 |
| A/A | 10 | 55.09 ± 19.43 | 0.86 ± 0.15 | 0.72 ± 0.15 | 0.62 ± 0.10 | 0.78 ± 0.15 |
| rs2025405 | ||||||
| C/C | 212 | 54.81 ± 21.09 | 0.84 ± 0.20 | 0.71 ± 0.11 | 0.58 ± 0.11 | 0.75 ± 0.12 |
| C/T | 136 | 54.02 ± 21.44 | 0.85 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.76 ± 0.10 |
| T/T | 37 | 60.66 ± 18.67 | 0.87 ± 0.21 | 0.71 ± 0.12 | 0.59 ± 0.11 | 0.76 ± 0.13 |
| rs3829365 | ||||||
| G/G | 158 | 54.47 ± 20.82 | 0.84 ± 0.21 | 0.71 ± 0.11 | 0.59 ± 0.10 | 0.75 ± 0.12 |
| C/G | 181 | 53.58 ± 21.89 | 0.84 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.11 | 0.76 ± 0.12 |
| C/C | 46 | 52.89 ± 17.58 | 0.85 ± 0.20 | 0.71 ± 0.09 | 0.59 ± 0.10 | 0.75 ± 0.11 |
| rs3923854 | ||||||
| C/C | 294 | 54.57 ± 21.44 | 0.84 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.10 | 0.75 ± 0.12 |
| C/G | 77 | 54.34 ± 18.23 | 0.85 ± 0.23 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.75 ± 0.11 |
| G/G | 13 | 67.26 ± 19.90 *,&,# | 0.92 ± 0.09 | 0.74 ± 0.11 | 0.60 ± 0.11 | 0.77 ± 0.12 |
| rs7322993 | ||||||
| C/C | 288 | 55.06 ± 20.64 | 0.85 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.11 | 0.75 ± 0.12 |
| C/T | 87 | 55.19 ± 22.65 | 0.84 ± 0.22 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.76 ± 0.10 |
| T/T | 10 | 55.09 ± 19.43 | 0.86 ± 0.15 | 0.72 ± 0.15 | 0.61 ± 0.10 | 0.78 ± 0.15 |
| rs7338244 | ||||||
| C/C | 212 | 54.81 ± 21.09 | 0.84 ± 0.20 | 0.71 ± 0.11 | 0.58 ± 0.11 | 0.75 ± 0.12 |
| C/G | 136 | 54.02 ± 21.44 | 0.85 ± 0.20 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.76 ± 0.10 |
| G/G | 37 | 60.66 ± 18.67 | 0.87 ± 0.21 | 0.71 ± 0.12 | 0.59 ± 0.11 | 0.76 ± 0.13 |
| rs9547952 | ||||||
| C/C | 324 | 54.83 ± 21.30 | 0.84 ± 0.21 | 0.71 ± 0.11 | 0.59 ± 0.10 | 0.75 ± 0.12 |
| C/T | 54 | 55.75 ± 19.81 | 0.87 ± 0.16 | 0.70 ± 0.11 | 0.58 ± 0.09 | 0.75 ± 0.11 |
| T/T | 7 | 62.15 ± 18.18 | 0.90 ± 0.10 | 0.78 ± 0.07 | 0.65 ± 0.10 | 0.82 ± 0.08 |
| rs9547965 | ||||||
| G/G | 351 | 54.78 ± 21.16 | 0.84 ± 0.19 | 0.71 ± 0.11 | 0.59 ± 0.10 | 0.76 ± 0.12 |
| G/A | 33 | 58.08 ± 19.81 | 0.84 ± 0.30 | 0.71 ± 0.11 | 0.58 ± 0.10 | 0.74 ± 0.12 |
| A/A | 1 | 67.87 | 0.96 | 0.75 | 0.64 | 0.83 |
| rs9547970 | ||||||
| A/A | 237 | 54.43 ± 20.72 | 0.84 ± 0.21 | 0.71 ± 0.11 | 0.58 ± 0.11 | 0.75 ± 0.12 |
| G/A | 122 | 56.19 ± 22.55 | 0.86 ± 0.21 | 0.71 ± 0.11 | 0.59 ± 0.09 | 0.76 ± 0.11 |
| G/G | 26 | 55.95 ± 16.36 | 0.86 ± 0.12 | 0.71 ± 0.12 | 0.60 ± 0.11 | 0.76 ± 0.13 |
| rs9603226 | ||||||
| G/G | 151 | 54.75 ± 20.40 | 0.85 ± 0.20 | 0.71 ± 0.10 | 0.59 ± 0.09 | 0.76 ± 0.11 |
| G/A | 182 | 54.60 ± 21.03 | 0.85 ± 0.20 | 0.71 ± 0.12 | 0.59 ± 0.11 | 0.76 ± 0.12 |
| A/A | 52 | 57.83 ± 22.93 | 0.81 ± 0.22 | 0.70 ± 0.11 | 0.58 ± 0.10 | 0.74 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-M.; Cheng, J.-H.; Zhang, H.; He, J.-W.; Yue, H.; Hu, W.-W.; Gu, J.-M.; Hu, Y.-Q.; Fu, W.-Z.; Wang, C.; et al. Serum Periostin Level and Genetic Polymorphisms Are Associated with Vertebral Fracture in Chinese Postmenopausal Women. Genes 2022, 13, 439. https://doi.org/10.3390/genes13030439
Guo Y-M, Cheng J-H, Zhang H, He J-W, Yue H, Hu W-W, Gu J-M, Hu Y-Q, Fu W-Z, Wang C, et al. Serum Periostin Level and Genetic Polymorphisms Are Associated with Vertebral Fracture in Chinese Postmenopausal Women. Genes. 2022; 13(3):439. https://doi.org/10.3390/genes13030439
Chicago/Turabian StyleGuo, Yi-Ming, Jian-Hao Cheng, Hao Zhang, Jin-Wei He, Hua Yue, Wei-Wei Hu, Jie-Mei Gu, Yun-Qiu Hu, Wen-Zhen Fu, Chun Wang, and et al. 2022. "Serum Periostin Level and Genetic Polymorphisms Are Associated with Vertebral Fracture in Chinese Postmenopausal Women" Genes 13, no. 3: 439. https://doi.org/10.3390/genes13030439
APA StyleGuo, Y.-M., Cheng, J.-H., Zhang, H., He, J.-W., Yue, H., Hu, W.-W., Gu, J.-M., Hu, Y.-Q., Fu, W.-Z., Wang, C., & Zhang, Z.-L. (2022). Serum Periostin Level and Genetic Polymorphisms Are Associated with Vertebral Fracture in Chinese Postmenopausal Women. Genes, 13(3), 439. https://doi.org/10.3390/genes13030439





