The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions
Abstract
1. Introduction
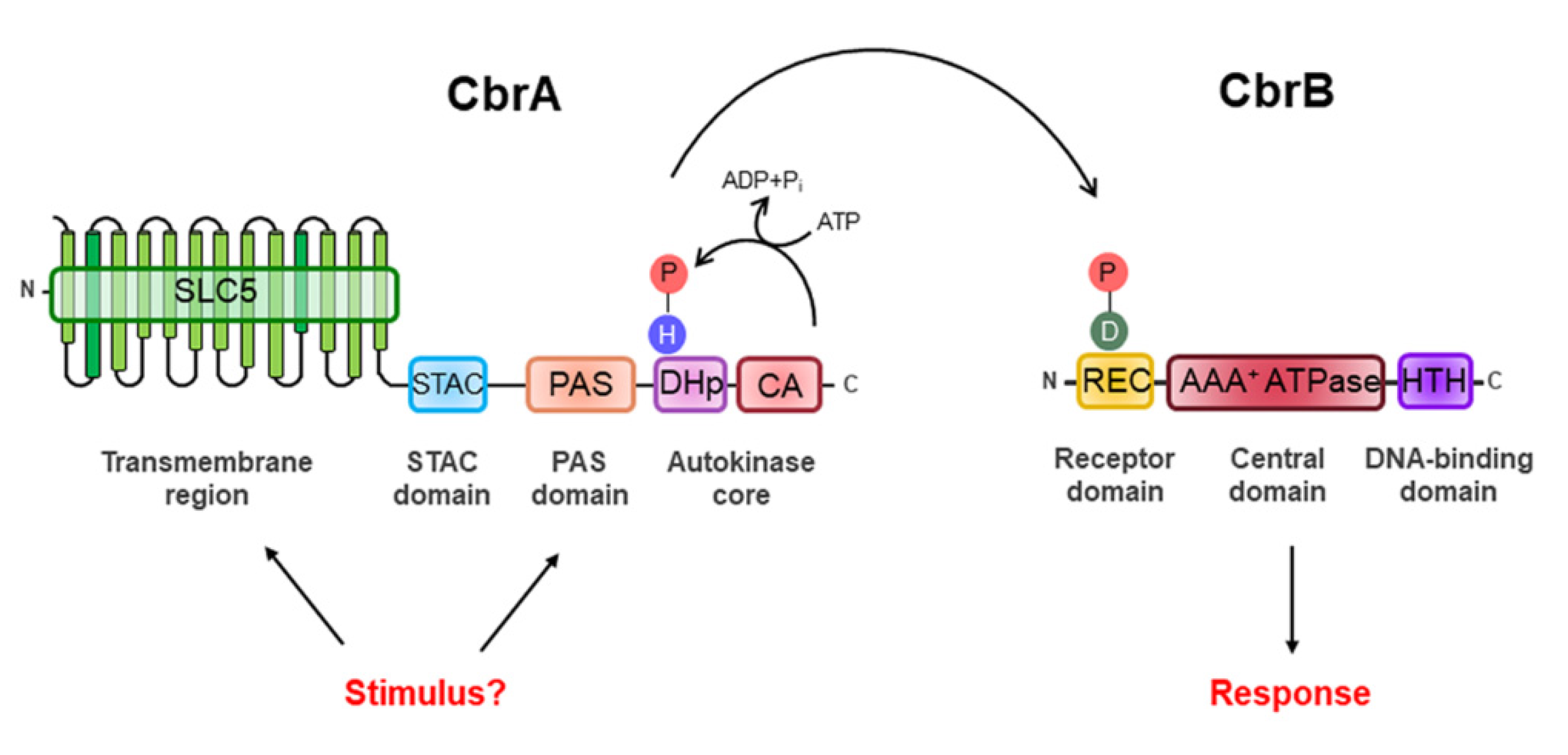
2. The Two-Component CbrAB System
2.1. The Structural Peculiarity of CbrA and the Nature of Activating Signal of the CbrAB System
2.2. CbrB as a Transcriptional Activator of σN-Dependent Promoters
3. Genomic Organization and Expression of the CbrAB TCS
4. Transcriptomic Analyses for the CbrB Regulon Determination
5. The CbrAB-Mediated Control of the Amino Acids Catabolism
5.1. Arginine Catabolism
5.2. Histidine Catabolism
5.3. Proline Catabolism
5.4. Leucine Catabolism
6. Influence of the CbrAB-Mediated Carbon Control on Other Regulatory Systems
6.1. ECF Sigma Factors
6.2. The TCS GacS-GacA
7. Integrated Control of the Virulence in Pseudomonas
8. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galperin, M.Y. A census of membrane-bound and intracellular signal transduction proteins in bacteria: Bacterial IQ, extroverts and introverts. BMC Microbiol. 2005, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 2010, 13, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Weinel, C.; Paulsen, I.T.; Dodson, R.J.; Hilbert, H.; Martins dos Santos, V.A.; Fouts, D.E.; Gill, S.R.; Pop, M.; Holmes, M.; et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 2002, 4, 799–808. [Google Scholar] [CrossRef]
- Cases, I.; de Lorenzo, V.; Ouzounis, C.A. Transcription regulation and environmental adaptation in bacteria. Trends Microbiol. 2003, 11, 248–253. [Google Scholar] [CrossRef]
- Whitworth, D.E.; Cock, P.J.A. Evolution of prokaryotic two-component systems: Insights from comparative genomics. Amino Acids 2009, 37, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Krell, T.; Lacal, J.; Busch, A.; Silva-Jiménez, H.; Guazzaroni, M.-E.; Ramos, J.L. Bacterial Sensor Kinases: Diversity in the Recognition of Environmental Signals. Annu. Rev. Microbiol. 2010, 64, 539–559. [Google Scholar] [CrossRef] [PubMed]
- Mascher, T. Bacterial (intramembrane-sensing) histidine kinases: Signal transfer rather than stimulus perception. Trends Microbiol. 2014, 22, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Matilla, M.A.; Velando, F.; Martín-Mora, D.; Monteagudo-Cascales, E.; Krell, T. A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiol. Rev. 2021, 46, fuab043. [Google Scholar] [CrossRef]
- Bourret, R.B.; Silversmith, R.E. Two-component signal transduction. Curr. Opin. Microbiol. 2010, 13, 113–115. [Google Scholar] [CrossRef]
- Cheung, J.; Hendrickson, W.A.W. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 2010, 13, 116–123. [Google Scholar] [CrossRef]
- Groisman, E.A.E.A. Feedback Control of Two-Component Regulatory Systems. Annu. Rev. Microbiol. 2016, 70, 103–124. [Google Scholar] [CrossRef]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular Mechanisms of Two-Component Signal Transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef]
- Hess, J.F.; Bourret, R.B.; Simon, M.I. Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature 1988, 336, 139–143. [Google Scholar] [CrossRef]
- Ninfa, A.J.; Bennett, R.L. Identification of the site of autophosphorylation of the bacterial protein kinase/phosphatase NRII. J. Biol. Chem. 1991, 266, 6888–6893. [Google Scholar] [CrossRef]
- Sanders, D.A.; Gillece-Castro, B.L.; Burlingame, A.L.; Koshland, D.E. Phosphorylation site of NtrC, a protein phosphatase whose covalent intermediate activates transcription. J. Bacteriol. 1992, 174, 5117–5122. [Google Scholar] [CrossRef][Green Version]
- Ninfa, A.J.; Magasanik, B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc. Natl. Acad. Sci. USA 1986, 83, 5909–5913. [Google Scholar] [CrossRef]
- Song, Y.; Peisach, D.; Pioszak, A.A.; Xu, Z.; Ninfa, A.J. Crystal structure of the C-terminal domain of the two-component system transmitter protein nitrogen regula- tor II (NRII; NtrB), regulator of nitrogen assimilation in Escherichia coli. Biochemistry 2004, 43, 6670–6678. [Google Scholar] [CrossRef]
- Gao, R.; Bouillet, S.; Stock, A.M. Structural basis of response regulator function. Annu. Rev. Microbiol. 2019, 73, 175–197. [Google Scholar] [CrossRef]
- West, A.H.; Stock, A.M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001, 26, 369–376. [Google Scholar] [CrossRef]
- Martín-Mora, D.; Fernández, M.; Velando, F.; Ortega, Á.; Gavira, J.A.; Matilla, M.A.; Krell, T. Functional Annotation of Bacterial Signal Transduction Systems: Progress and Challenges. Int. J. Mol. Sci. 2018, 19, 3755. [Google Scholar] [CrossRef]
- Krell, T. Tackling the bottleneck in bacterial signal transduction research: High-throughput identification of signal molecules. Mol. Microbiol. 2015, 96, 685–688. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Higdon, R.; Kolker, E. Interplay of heritage and habitat in the distribution of bacterial signal transduction systems. Mol. Biosyst. 2010, 6, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Wanchai, V.; Alkam, D.; Nookaew, I.; Ussery, D.W. Conservation of two-component signal transduction systems in E. coli, Salmonella, and across 100,000 bacteria of various bacterial phyla. In Molecular Mechanisms of Microbial Evolution; Rampelotto, P.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 153–174. ISBN 9783319690780. [Google Scholar]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Phyletic distribution and lineage-specific domain architectures of archaeal two-component signal transduction systems. J. Bacteriol. 2018, 200, e00681-17. [Google Scholar] [CrossRef] [PubMed]
- Nishijyo, T.; Haas, D.; Itoh, Y. The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol. Microbiol. 2001, 40, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Garcia-Maurino, S.M.; Perez-Martinez, I.; Santero, E.; Canosa, I.; Lapouge, K. Hierarchical management of carbon sources is regulated similarly by the CbrA/B systems in Pseudomonas aeruginosa and Pseudomonas putida. Microbiology 2014, 160, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Amador, C.I.; López- Sánchez, A.; Govantes, F.; Santero, E.; Canosa, I. A Pseudomonas putida cbrB transposon insertion mutant displays a biofilm hyperproducing phenotype that is resistant to dispersal. Environ. Microbiol. Rep. 2016, 8, 622–629. [Google Scholar] [CrossRef]
- Zhang, X.X.; Rainey, P.B. Dual involvement of CbrAB and NtrBC in the regulation of histidine utilization in Pseudomonas fluorescens SBW25. Genetics 2008, 178, 185–195. [Google Scholar] [CrossRef]
- Sonnleitner, E.; Valentini, M.; Wenner, N.; Haichar, F.E.Z.; Haas, D.; Lapouge, K. Novel Targets of the CbrAB/Crc Carbon Catabolite Control System Revealed by Transcript Abundance in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e44637. [Google Scholar] [CrossRef]
- Filiatrault, M.J.; Stodghill, P.V.; Wilson, J.; Butcher, B.G.; Chen, H.; Myers, C.R.; Cartinhour, S.W. CrcZ and CrcX regulate carbon source utilization in Pseudomonas syringae pathovar tomato strain DC3000. RNA Biol. 2013, 10, 245–255. [Google Scholar] [CrossRef]
- Barroso, R.; García-Mauriño, S.M.S.M.; Tomás-Gallardo, L.; Andújar, E.; Pérez-Alegre, M.; Santero, E.; Canosa, I. The CbrB Regulon: Promoter dissection reveals novel insights into the CbrAB expression network in Pseudomonas putida. PLoS ONE 2018, 13, e0209191. [Google Scholar] [CrossRef]
- Li, W.; Lu, C.-D.D. Regulation of carbon and nitrogen utilization by CbrAB and NtrBC two-component systems in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5413–5420. [Google Scholar] [CrossRef]
- Amador, C.I.; Canosa, I.; Govantes, F.; Santero, E. Lack of CbrB in Pseudomonas putida affects not only amino acids metabolism but also different stress responses and biofilm development. Environ. Microbiol. 2010, 12, 1748–1761. [Google Scholar] [CrossRef]
- Sánchez, D.G.; Primo, E.D.; Damiani, M.T.M.T.; Lisa, A.T. Pseudomonas aeruginosa gbdR gene is transcribed from a σ54-dependent promoter under the control of NtrC/CbrB, IHF and BetI. Microbiology 2017, 163, 1343–1354. [Google Scholar] [CrossRef]
- Naren, N.; Zhang, X.-X. Role of a local transcription factor in governing cellular carbon/nitrogen homeostasis in Pseudomonas fluorescens. Nucleic Acids Res. 2021, 49, 3204–3216. [Google Scholar] [CrossRef]
- Fléchard, M.; Duchesne, R.; Tahrioui, A.; Bouffartigues, E.; Depayras, S.; Hardouin, J.; Coralie, L.; Maillot, O.; Tortuel, D.; Azuama, C.O.; et al. The absence of SigX results in impaired carbon metabolism and membrane fluidity in Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 17212. [Google Scholar] [CrossRef]
- Garrity, G.; Boone, D.R.; Castenholz, R.W. Bergey’s Manual® of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef]
- Yoneyama, F.; Yamamoto, M.; Hashimoto, W.; Murata, K. Production of polyhydroxybutyrate and alginate from glycerol by Azotobacter vinelandii under nitrogen-free conditions. Bioengineered 2015, 6, 209–217. [Google Scholar] [CrossRef]
- Segura, D.; Guzmán, J.; Espín, G. Azotobacter vinelandii mutants that overproduce poly-β- hydroxybutyrate or alginate. Appl. Microbiol. Biotechnol. 2003, 63, 159–163. [Google Scholar] [CrossRef]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2021, 16, e2000165. [Google Scholar] [CrossRef]
- Weimer, A.; Kohlstedt, M.; Volke, D.C.; Nikel, P.I.; Wittmann, C. Industrial biotechnology of Pseudomonas putida: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7745–7766. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Pseudomonas spp. as cell factories (MCFs) for value-added products: From rational design to industrial applications. Crit. Rev. Biotechnol. 2020, 40, 1232–1249. [Google Scholar] [CrossRef] [PubMed]
- Özen, A.I.; Ussery, D.W. Defining the Pseudomonas genus: Where do we draw the line with Azotobacter? Microb. Ecol. 2012, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Fabiani, F.; Hoyer, E.; Lassak, J. Bacterial transmembrane signalling systems and their engineering for biosensing. Open Biol. 2018, 8, 180023. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Steuber, J. Cation transport by the respiratory NADH:quinone oxidoreductase (complex I): Facts and hypotheses. Biochem. Soc. Trans. 2013, 41, 1280–1287. [Google Scholar] [CrossRef][Green Version]
- Rivera-Ordaz, A.; Bracher, S.; Sarrach, S.; Li, Z.; Shi, L.; Quick, M.; Hilger, D.; Haas, R.; Jung, H. The sodium/proline transporter PutP of Helicobacter pylori. PLoS ONE 2013, 8, e83576. [Google Scholar] [CrossRef]
- Steinmetz, P.A.; Wörner, S.; Unden, G. Differentiation of DctA and DcuS function in the DctA/DcuS sensor complex of Escherichia coli: Function of DctA as an activity switch and of DcuS as the C4 -dicarboxylate sensor. Mol. Microbiol. 2014, 94, 218–229. [Google Scholar] [CrossRef]
- Muda, M.; Rao, N.N.N.; Torriani, A. Role of PhoU in phosphate transport and alkaline phosphatase regulation. J. Bacteriol. 1992, 174, 8057–8064. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef]
- Gardner, S.G.; Johns, K.D.; Tanner, R.; McCleary, W.R. The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J. Bacteriol. 2014, 196, 1741–1752. [Google Scholar] [CrossRef]
- Island, M.D.; Kadner, R.J. Interplay between the membrane-associated UhpB and UhpC regulatory proteins. J. Bacteriol. 1993, 175, 5028–5034. [Google Scholar] [CrossRef]
- Olekhnovich, I.N.; Kadner, R.J. Mutational scanning and affinity cleavage analysis of UhpA-binding sites in the Escherichia coli uhpT promoter. J. Bacteriol. 2002, 184, 2682–2691. [Google Scholar] [CrossRef]
- Gancedo, J.M. The early steps of glucose signalling in yeast. FEMS Microbiol. Rev. 2008, 32, 673–704. [Google Scholar] [CrossRef]
- Wu, B.; Ottow, K.; Poulsen, P.; Gaber, R.F.; Albers, E.; Kielland-Brandt, M.C. Competitive intra- and extracellular nutrient sensing by the transporter homologue Ssy1p. J. Cell Biol. 2006, 173, 327–331. [Google Scholar] [CrossRef]
- Tetsch, L.; Jung, K. The regulatory interplay between membrane-integrated sensors and transport proteins in bacteria. Mol. Microbiol. 2009, 73, 982–991. [Google Scholar] [CrossRef]
- Thevelein, J.M.; Voordeckers, K. Functioning and evolutionary significance of nutrient transceptors. Mol. Biol. Evol. 2009, 26, 2407–2414. [Google Scholar] [CrossRef]
- Tetsch, L.; Jung, K. How are signals transduced across the cytoplasmic membrane? Transport proteins as transmitter of information. Amino Acids 2009, 37, 467–477. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Gauntlett, J.C.; Oldenburg, D.G.; Cook, G.M.; Rainey, P.B. Role of the Transporter-Like Sensor Kinase CbrA in Histidine Uptake and Signal Transduction. J. Bacteriol. 2015, 197, 2867–2878. [Google Scholar] [CrossRef]
- Wirtz, L.; Eder, M.; Schipper, K.; Rohrer, S.; Jung, H. Transport and kinase activities of CbrA of Pseudomonas putida KT2440. Sci. Rep. 2020, 10, 5400. [Google Scholar] [CrossRef]
- Hilger, D.; Böhm, M.; Hackmann, A.; Jung, H. Role of Ser-340 and Thr-341 in transmembrane domain IX of the Na+/proline transporter PutP of Escherichia coli in ligand binding and transport. J. Biol. Chem. 2008, 283, 4921–4929. [Google Scholar] [CrossRef]
- Henriquez, T.; Wirtz, L.; Su, D.; Jung, H. Prokaryotic Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family. Int. J. Mol. Sci. 2021, 22, 1880. [Google Scholar] [CrossRef]
- Monteagudo-Cascales, E.; García-Mauriño, S.M.; Santero, E.; Canosa, I. Unraveling the role of the CbrA histidine kinase in the signal transduction of the CbrAB two-component system in Pseudomonas putida. Sci. Rep. 2019, 9, 9110. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, E.; Lupas, A.N. Characterization of the CrbS/R two-component system in Pseudomonas fluorescens reveals a new set of genes under its control and a DNA motif required for CrbR-mediated transcriptional activation. Front. Microbiol. 2017, 8, 2287. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Purdy, A.E.; Robins, W.P.; Wang, Z.; Mandal, M.; Chang, S.; Mekalanos, J.J.; Watnick, P.I. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 2014, 16, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Rasmussen, A.; Tyler, P.; Servos, M.M.; Sylla, M.; Prado, C.; Daniele, E.; Sharp, J.S.; Purdy, A.E. Regulation of acetyl-CoA synthetase transcription by the CrbS/R two-component system is conserved in genetically diverse environmental pathogens. PLoS ONE 2017, 12, e0177825. [Google Scholar] [CrossRef]
- Bueno, R.; Pahel, G.; Magasanik, B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J. Bacteriol. 1985, 164, 816–822. [Google Scholar] [CrossRef]
- Santero, E.; Hervas, A.B.; Canosa, I.; Govantes, F. Glutamate Dehydrogenases: Enzymology, Physiological Role and Biotechnological Relevance. In Dehydrogenases; Canuto, R.A., Ed.; INTECH: Rijeka, Croatia, 2012; Volume 1, pp. 289–318. ISBN 9789533070193. [Google Scholar]
- Bush, M.; Dixon, R. The role of bacterial enhancer binding proteins as specialized activators of sigma54-dependent transcription. Microbiol. Mol. Biol. Rev. 2012, 76, 497–529. [Google Scholar] [CrossRef]
- Ghosh, T.; Bose, D.; Zhang, X. Mechanisms for activating bacterial RNA polymerase. FEMS Microbiol. Rev. 2010, 34, 611–627. [Google Scholar] [CrossRef]
- Rappas, M.; Bose, D.; Zhang, X. Bacterial enhancer-binding proteins: Unlocking σ54-dependent gene transcription. Curr. Opin. Struct. Biol. 2007, 17, 110–116. [Google Scholar] [CrossRef]
- García-Mauriño, S.M.; Pérez-Martínez, I.; Amador, C.I.; Canosa, I.; Santero, E. Transcriptional activation of the CrcZ and CrcY regulatory RNAs by the CbrB response regulator in Pseudomonas putida. Mol. Microbiol. 2013, 89, 189–205. [Google Scholar] [CrossRef]
- Mao, F.; Dam, P.; Chou, J.; Olman, V.; Xu, Y. DOOR: A database for prokaryotic operons. Nucleic Acids Res. 2009, 37, D459–D463. [Google Scholar] [CrossRef]
- Moreno, R.; Fonseca, P.; Rojo, F. Two small RNAs, CrcY and CrcZ, act in concert to sequester the Crc global regulator in Pseudomonas putida, modulating catabolite repression. Mol. Microbiol. 2012, 83, 24–40. [Google Scholar] [CrossRef]
- Sonnleitner, E.; Abdou, L.; Haas, D. Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2009, 106, 21866–21871. [Google Scholar] [CrossRef]
- Martínez-Valenzuela, M.; Guzmán, J.; Moreno, S.; Ahumada-Manuel, C.L.; Espín, G.; Núñez, C. Expression of the sRNAs CrcZ and CrcY modulate the strength of carbon catabolite repression under diazotrophic or non-diazotrophic growing conditions in Azotobacter vinelandii. PLoS ONE 2018, 13, e0208975. [Google Scholar] [CrossRef]
- Liu, Y.; Gokhale, C.S.; Rainey, P.B.; Zhang, X.-X. Unravelling the complexity and redundancy of carbon catabolic repression in Pseudomonas fluorescens SBW25. Mol. Microbiol. 2017, 105, 589–605. [Google Scholar] [CrossRef]
- Hernández Arranz, S.; Sánchez Hevia, D.; Rojo, F.; Moreno, R. Effect of Crc and Hfq proteins on the transcription, processing, and stability of the Pseudomonas putida CrcZ sRNA. RNA 2016, 22, 1902–1917. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Hevia, D.L.; Yuste, L.; Moreno, R.; Rojo, F. Influence of the Hfq and Crc global regulators on the control of iron homeostasis in Pseudomonas putida. Environ. Microbiol. 2018, 20, 3484–3503. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Jan, M.; Kong, D.; Pan, J.; Zhang, X. The global regulator Hfq exhibits far more extensive and intensive regulation than Crc in Pseudomonas protegens H78. Mol. Plant Pathol. 2021, 22, 921–938. [Google Scholar] [CrossRef]
- Park, S.M.; Lu, C.D.; Abdelal, A.T. Cloning and characterization of argR, a gene that participates in regulation of arginine biosynthesis and catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 1997, 179, 5300–5308. [Google Scholar] [CrossRef]
- Itoh, Y. Cloning and characterization of the aru genes encoding enzymes of the catabolic arginine succinyltransferase pathway in Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 7280–7290. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, C.D. Functional genomics enables identification of genes of the arginine transaminase pathway in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 3945–3953. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Rainey, P.B. Genetic analysis of the histidine utilization (hut) genes in Pseudomonas fluorescens SBW25. Genetics 2007, 176, 2165–2176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naren, N.; Zhang, X.X. Global regulatory roles of the histidine-responsive transcriptional repressor HutC in Pseudomonas fluorescens SBW25. J. Bacteriol. 2020, 202, e00792-19. [Google Scholar] [CrossRef] [PubMed]
- Abdou, L.; Chou, H.-T.T.; Haas, D.; Lu, C.-D.D. Promoter Recognition and Activation by the Global Response Regulator CbrB in Pseudomonas aeruginosa. J. Bacteriol. 2011, 193, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Gerritse, G.; Dankmeyer, L.; Quax, W.J. Characterization of the promoter and Upstream Activating Sequence from the Pseudomonas alcaligenes lipase gene. J. Biotechnol. 2001, 86, 9–17. [Google Scholar] [CrossRef]
- Krzeslak, J.; Gerritse, G.; van Merkerk, R.; Cool, R.H.; Quax, W.J. Lipase Expression in Pseudomonas alcaligenes Is under the Control of a Two-Component Regulatory System. Appl. Environ. Microbiol. 2008, 74, 1402. [Google Scholar] [CrossRef]
- Itoh, Y.; Nishijyo, T.; Nakada, Y. Histidine catabolism and catabolite regulation. In Pseudomonas; Ramos, J.-L., Filloux, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 5, pp. 371–395. ISBN 9781402060960. [Google Scholar]
- Yeung, A.T.Y.; Bains, M.; Hancock, R.E.W. The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa. J. Bacteriol. 2011, 193, 918–931. [Google Scholar] [CrossRef]
- Santero, E.; Hoover, T.R.; North, A.K.; Berger, D.K.; Porter, S.C.; Kustu, S. Role of integration host factor in stimulating transcription from the sigma 54-dependent nifH promoter. J. Mol. Biol. 1992, 227, 602–620. [Google Scholar] [CrossRef]
- Perez-Martin, J.; De Lorenzo, V. Integration host factor suppresses promiscuous activation of the sigma 54-dependent promoter Pu of Pseudomonas putida. Proc. Natl. Acad. Sci. USA 1995, 92, 7277–7281. [Google Scholar] [CrossRef]
- Allison, S.L.; Phillips, A.T. Nucleotide sequence of the gene encoding the repressor for the histidine utilization genes of Pseudomonas putida. J. Bacteriol. 1990, 172, 5470–5476. [Google Scholar] [CrossRef]
- Rella, M.; Mercenier, A.; Haas, D. Transposon insertion mutagenesis of Pseudomonas aeruginosa with a Tn5 derivative: Application to physical mapping of the arc gene cluster. Gene 1985, 33, 293–303. [Google Scholar] [CrossRef]
- Li, G.; Lu, C.-D. Molecular characterization and regulation of operons for asparagine and aspartate uptake and utilization in Pseudomonas aeruginosa. Microbiology 2018, 164, 205–216. [Google Scholar] [CrossRef]
- Kennedy, C.; Gamal, R.; Humphrey, R.; Ramos, J.; Brigle, K.; Dean, D. Characterisation by Tn5 mutagenesis and isolation from pLAFR1 gene banks. Mol. Genet. Genom. 1986, 205, 318–325. [Google Scholar] [CrossRef]
- Quiroz-Rocha, E.; Moreno, R.; Hernández-Ortíz, A.; Fragoso-Jiménez, J.C.; Muriel-Millán, L.F.; Guzmán, J.; Espín, G.; Rojo, F.; Núñez, C. Glucose uptake in Azotobacter vinelandii occurs through a GluP transporter that is under the control of the CbrA/CbrB and Hfq-Crc systems. Sci. Rep. 2017, 7, 858. [Google Scholar] [CrossRef]
- Nikel, P.I.; Chavarría, M.; Fuhrer, T.; Sauer, U.; de Lorenzo, V. Pseudomonas putida KT2440 Strain Metabolizes Glucose through a Cycle Formed by Enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and Pentose Phosphate Pathways. J. Biol. Chem. 2015, 290, 25920. [Google Scholar] [CrossRef]
- Quiroz-Rocha, E.; Bonilla-Badía, F.; García-Aguilar, V.; López-Pliego, L.; Serrano-Román, J.; Cocotl-Yañez, M.; Guzmán, J.; Ahumada-Manuel, C.L.; Muriel-Millán, L.F.; Castañeda, M.; et al. Two-component system CbrA/CbrB controls alginate production in Azotobacter vinelandii. Microbiology 2017, 163, 1105–1115. [Google Scholar] [CrossRef]
- Molina, L.; La Rosa, R.; Nogales, J.; Rojo, F. Influence of the Crc global regulator on substrate uptake rates and the distribution of metabolic fluxes in Pseudomonas putida KT2440 growing in a complete medium. Environ. Microbiol. 2019, 21, 4446–4459. [Google Scholar] [CrossRef]
- Moreno, R.; Hernández-Arranz, S.; La Rosa, R.; Yuste, L.; Madhushani, A.; Shingler, V.; Rojo, F.; Hernandez-Arranz, S.; La Rosa, R.; Yuste, L.; et al. The Crc and Hfq proteins of Pseudomonas putida cooperate in catabolite repression and formation of ribonucleic acid complexes with specific target motifs. Environ. Microbiol. 2015, 17, 105–118. [Google Scholar] [CrossRef]
- Moreno, R.; Martinez-Gomariz, M.; Yuste, L.; Gil, C.; Rojo, F. The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: Evidence from proteomic and genomic analyses. Proteomics 2009, 9, 2910–2928. [Google Scholar] [CrossRef]
- Itoh, Y.; Nakada, Y. Arginine and Polyamine Metabolism. In Pseudomonas: Volume 3 Biosynthesis of Macromolecules and Molecular Metabolism; Ramos, J.-L., Ed.; Springer US: Boston, MA, USA, 2004; pp. 243–272. ISBN 978-1-4419-9088-4. [Google Scholar]
- Nishijyo, T.; Park, S.M.; Lu, C.D.; Itoh, Y.; Abdelal, A.T. Molecular characterization and regulation of an operon encoding a system for transport of arginine and ornithine and the ArgR regulatory protein in Pseudomonas aeruginosa. J. Bacteriol. 1998, 180, 5559–5566. [Google Scholar] [CrossRef]
- Lu, C.D.; Abdelal, A.T. The gdhB gene of Pseudomonas aeruginosa encodes an arginine-inducible NAD+-dependent glutamate dehydrogenase which is subject to allosteric regulation. J. Bacteriol. 2001, 183, 490–499. [Google Scholar] [CrossRef]
- Gamper, M.; Zimmermann, A.; Haas, D. Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J. Bacteriol. 1991, 173, 4742–4750. [Google Scholar] [CrossRef]
- Stalon, V.; Mercenier, A. L-arginine utilization by Pseudomonas species. J. Gen. Microbiol. 1984, 130, 69–76. [Google Scholar] [CrossRef]
- Tricot, C.; Stalon, V.; Legrain, C. Isolation and characterization of Pseudomonas putida mutants affected in arginine, ornithine and citrulline catabolism: Function of the arginine oxidase and arginine succinyltransferase pathways. J. Gen. Microbiol. 1991, 137, 2911–2918. [Google Scholar] [CrossRef][Green Version]
- Bender, R.A. Regulation of the Histidine Utilization (Hut) System in Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 565–584. [Google Scholar] [CrossRef]
- Lessie, T.G.; Neidhart, F.C. Formation and Operation of the Histidine-degrading Pathway in Pseudomonas aeruginosa. J. Bacteriol. 1967, 93, 1794–1799. [Google Scholar] [CrossRef]
- Wirtz, L.; Eder, M.; Brand, A.K.; Jung, H. HutT functions as the major L-histidine transporter in Pseudomonas putida KT2440. FEBS Lett. 2021, 595, 2113–2126. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chang, H.; Tran, S.L.; Gauntlett, J.C.; Cook, G.M.; Rainey, P.B. Variation in transport explains polymorphism of histidine and urocanate utilization in a natural Pseudomonas population. Environ. Microbiol. 2012, 14, 1941–1951. [Google Scholar] [CrossRef]
- Hahn, D.R.; Myers, R.S.; Kent, C.R.; Maloy, S.R. Regulation of proline utilization in Salmonella typhimurium: Molecular characterization of the put operon, and DNA sequence of the put control region. Mol. Genet. Genom. MGG 1988, 213, 125–133. [Google Scholar] [CrossRef]
- Cairney, J.; Higgins, C.F.; Booth, I.R. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J. Bacteriol. 1984, 160, 22–27. [Google Scholar] [CrossRef]
- Vílchez, S.; Manzanera, M.; Ramos, J.L. Control of expression of divergent Pseudomonas putida put promoters for proline catabolism. Appl. Environ. Microbiol. 2000, 66, 5221–5225. [Google Scholar] [CrossRef]
- Meile, L.; Leisinger, T. Purification and Properties of the Bifunctional Proline Dehydrogenase/1-Pyrroline-5-Carboxylate Dehydrogenase from Pseudomonas aeruginosa. Eur. J. Biochem. 1982, 129, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, S.; Molina, L.; Ramos, C.; Ramos, J.L. Proline catabolism by Pseudomonas putida: Cloning, characterization, and expression of the put genes in the presence of root exudates. J. Bacteriol. 2000, 182, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.W.; Senti-willis, A.; Maloy, S.R. DNA sequence of the putA gene from Salmonella typhimurium: A bifunctional membrane-associated dehydrogenase that binds DNA. Nucleic Acids Res. 1993, 21, 1676. [Google Scholar] [CrossRef] [PubMed]
- de Spicer, P.O.; O’Brien, K.; Maloy, S. Regulation of proline utilization in Salmonella typhimurium: A membrane-associated dehydrogenase binds DNA in vitro. J. Bacteriol. 1991, 173, 211–219. [Google Scholar] [CrossRef]
- Bharwad, K.; Rajkumar, S. Rewiring the functional complexity between Crc, Hfq and sRNAs to regulate carbon catabolite repression in Pseudomonas. World J. Microbiol. Biotechnol. 2019, 35, 140. [Google Scholar] [CrossRef]
- Díaz-Pérez, A.L.; Núñez, C.; Meza Carmen, V.; Campos-García, J. The expression of the genes involved in leucine catabolism of Pseudomonas aeruginosa is controlled by the transcriptional regulator LiuR and by the CbrAB/Crc system. Res. Microbiol. 2018, 169, 324–334. [Google Scholar] [CrossRef]
- Gruber, T.M.; Gross, C.A. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 2003, 57, 441–466. [Google Scholar] [CrossRef]
- Potvin, E.; Sanschagrin, F.; Levesque, R.C. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2008, 32, 38–55. [Google Scholar] [CrossRef]
- Otero-Asman, J.R.; Wettstadt, S.; Bernal, P.; Llamas, M.A. Diversity of extracytoplasmic function sigma (σECF) factor-dependent signaling in Pseudomonas. Mol. Microbiol. 2019, 112, 356–373. [Google Scholar] [CrossRef]
- Ho, T.D.T.D.; Ellermeier, C.D.C.D. Extra cytoplasmic function σ factor activation. Curr. Opin. Microbiol. 2012, 15, 182–188. [Google Scholar] [CrossRef][Green Version]
- Butcher, B.G.; Mascher, T.; Helmann, J.D. Environmental sensing and the role of extracytoplasmic function (ECF) sigma factors. In Bacterial Physiology—A Molecular Approach; El-Sharoud, W.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 233–261. [Google Scholar]
- Helmann, J.D. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 2002, 46, 47–110. [Google Scholar]
- Mascher, T. Signaling diversity and evolution of extracytoplasmic function (ECF) σ factors. Curr. Opin. Microbiol. 2013, 16, 148–155. [Google Scholar] [CrossRef]
- Edgar, R.J.; Xu, X.; Shirley, M.; Konings, A.F.; Martin, L.W.; Ackerley, D.F.; Lamont, I.L. Interactions between an anti-sigma protein and two sigma factors that regulate the pyoverdine signaling pathway in Pseudomonas aeruginosa. BMC Microbiol. 2014, 14, 287. [Google Scholar] [CrossRef]
- Cao, M.; Helmann, J.D. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J. Bacteriol. 2004, 186, 1136–1146. [Google Scholar] [CrossRef]
- Blanka, A.; Schulz, S.; Eckweiler, D.; Franke, R.; Bielecka, A.; Nicolai, T.; Casilag, F.; Düvel, J.; Abraham, W.R.; Kaever, V.; et al. Identification of the alternative sigma factor SigX regulon and its implications for Pseudomonas aeruginosa pathogenicity. J. Bacteriol. 2014, 196, 345–356. [Google Scholar] [CrossRef]
- Duchesne, R.; Bouffartigues, E.; Oxaran, V.; Maillot, O.; Bénard, M.; Feuilloley, M.G.J.; Orange, N.; Chevalier, S. A proteomic approach of SigX function in Pseudomonas aeruginosa outer membrane composition. J. Proteom. 2013, 94, 451–459. [Google Scholar] [CrossRef]
- Bouffartigues, E.; Gicquel, G.; Bazire, A.; Bains, M.; Maillot, O.; Vieillard, J.; Feuilloley, M.G.J.; Orange, N.; Hancock, R.E.W.; Dufour, A.; et al. Transcription of the oprF gene of Pseudomonas aeruginosa is dependent mainly on the SigX sigma factor and is sucrose induced. J. Bacteriol. 2012, 194, 4301–4311. [Google Scholar] [CrossRef]
- Brinkman, F.S.L.L.; Schoofs, G.; Hancock, R.E.W.W.; De Mot, R. Influence of a Putative ECF Sigma Factor on Expression of the Major Outer Membrane Protein, OprF, in Pseudomonas aeruginosa and Pseudomonas fluorescens. J. Bacteriol. 1999, 181, 4746–4754. [Google Scholar] [CrossRef]
- Winsor, G.L.; Griffiths, E.J.; Lo, R.; Dhillon, B.K.; Shay, J.A.; Brinkman, F.S. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016, 44, D646–D653. [Google Scholar] [CrossRef]
- Bass, R.B.; Strop, P.; Barclay, M.; Rees, D.C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 2002, 298, 1582–1587. [Google Scholar] [CrossRef]
- Staroń, A.; Sofia, H.J.; Dietrich, S.; Ulrich, L.E.; Liesegang, H.; Mascher, T. The third pillar of bacterial signal transduction: Classification of the extracytoplasmic function (ECF) σ factor protein family. Mol. Microbiol. 2009, 74, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Bouffartigues, E.; Tortuel, D.; Maillot, O.; Dubot, V.; Lesouhaitier, O.; Orange, N.; Feuilloley, M.; Cornelis, P.; Chevalier, S. New insights into the molecular mechanisms regulating SigX activity in Pseudomonas aeruginosa. In Proceedings of the 16th International Conference on Pseudomonas, Liverpool, UK, 5–9 September 2017. [Google Scholar]
- Gicquel, G.; Bouffartigues, E.; Bains, M.; Oxaran, V.; Rosay, T.; Lesouhaitier, O.; Connil, N.; Bazire, A.; Maillot, O.; Bénard, M.; et al. The extra-cytoplasmic function sigma factor sigX modulates biofilm and virulence-related properties in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e80407. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Eckweiler, D.; Bielecka, A.; Nicolai, T.; Franke, R.; Dötsch, A.; Hornischer, K.; Bruchmann, S.; Düvel, J.; Häussler, S. Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog. 2015, 11, e1004744. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.C.; Eckweiler, D.; Schulz, S.; Bielecka, A.; Nicolai, T.; Franke, R.; Häussler, S.; Meyer-Hermann, M. Functional modules of sigma factor regulons guarantee adaptability and evolvability. Sci. Rep. 2016, 6, 22212. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bazire, A.; Tahrioui, A.; Duchesne, R.; Tortuel, D.; Maillot, O.; Clamens, T.; Orange, N.; Feuilloley, M.G.J.J.; et al. Extracytoplasmic function sigma factors in Pseudomonas aeruginosa. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 706–721. [Google Scholar] [CrossRef]
- Latour, X. The Evanescent GacS Signal. Microorganisms 2020, 8, 1746. [Google Scholar] [CrossRef]
- Sonnleitner, E.; Bläsi, U. Regulation of Hfq by the RNA CrcZ in Pseudomonas aeruginosa carbon catabolite repression. PLoS Genet. 2014, 10, e1004440. [Google Scholar] [CrossRef]
- Brencic, A.; McFarland, K.A.; McManus, H.R.; Castang, S.; Mogno, I.; Dove, S.L.; Lory, S. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 2009, 73, 434–445. [Google Scholar] [CrossRef]
- Heurlier, K.; Williams, F.; Heeb, S.; Dormond, C.; Pessi, G.; Singer, D.; Cámara, M.; Williams, P.; Haas, D. Positive Control of Swarming, Rhamnolipid Synthesis, and Lipase Production by the Posttranscriptional RsmA/RsmZ System in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2004, 186, 2936–2945. [Google Scholar] [CrossRef]
- Morris, E.R.; Hall, G.; Li, C.; Heeb, S.; Kulkarni, R.V.; Lovelock, L.; Silistre, H.; Messina, M.; Cámara, M.; Emsley, J.; et al. Structural rearrangement in an RsmA/CsrA Ortholog of Pseudomonas aeruginosa creates a dimeric RNA-binding protein, RsmN. Structure 2013, 21, 1659–1671. [Google Scholar] [CrossRef]
- Kay, E.; Humair, B.; Dénervaud, V.; Riedel, K.; Spahr, S.; Eberl, L.; Valverde, C.; Haas, D. Two GacA-Dependent Small RNAs Modulate the Quorum-Sensing Response in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 6026–6033. [Google Scholar] [CrossRef]
- Moll, S.; Schneider, D.J.; Stodghill, P.; Myers, C.R.; Cartinhour, S.W.; Filiatrault, M.J. Construction of an rsmX co-variance model and identification of five rsmX non-coding RNAs in Pseudomonas syringae pv. tomato DC3000. RNA Biol. 2010, 7, 508–516. [Google Scholar] [CrossRef][Green Version]
- Laskowski, M.A.; Kazmierczak, B.I. Mutational analysis of RetS, an unusual sensor kinase-response regulator hybrid required for Pseudomonas aeruginosa virulence. Infect. Immun. 2006, 74, 4462–4473. [Google Scholar] [CrossRef]
- Ventre, I.; Goodman, A.L.; Vallet-Gely, I.; Vasseur, P.; Soscia, C.; Molin, S.; Bleves, S.; Lazdunski, A.; Lory, S.; Filloux, A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 2006, 103, 171–176. [Google Scholar] [CrossRef]
- Goodman, A.L.; Kulasekara, B.; Rietsch, A.; Boyd, D.; Smith, R.S.; Lory, S. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 2004, 7, 745–754. [Google Scholar] [CrossRef]
- Broder, U.N.; Jaeger, T.; Jenal, U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat. Microbiol. 2016, 2, 16184. [Google Scholar] [CrossRef]
- Mancl, J.M.; Ray, W.K.; Helm, R.F.; Schubot, F.D. Helix Cracking Regulates the Critical Interaction between RetS and GacS in Pseudomonas aeruginosa. Structure 2019, 27, 785–793. [Google Scholar] [CrossRef]
- Kong, W.; Chen, L.; Zhao, J.; Shen, T.; Surette, M.G.; Shen, L.; Duan, K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol. Microbiol. 2013, 88, 784–797. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Pydi, S.P.; Li, Y.; Lin, C.; Kong, W.; Chelikani, P.; Duan, K. Characterization of the Direct Interaction between Hybrid Sensor Kinases PA1611 and RetS That Controls Biofilm Formation and the Type III Secretion System in Pseudomonas aeruginosa. ACS Infect. Dis. 2017, 3, 162–175. [Google Scholar] [CrossRef]
- Gooderham, W.J.; Hancock, R.E.W. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2009, 33, 279–294. [Google Scholar] [CrossRef]
- Lapouge, K.; Schubert, M.; Allain, F.H.-T.T.; Haas, D. Gac/Rsm signal transduction pathway of γ-proteobacteria: From RNA recognition to regulation of social behaviour. Mol. Microbiol. 2008, 67, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Francis, V.I.; Stevenson, E.C.; Porter, S.L. Two-Component Systems Required for Virulence in Pseudomonas aeruginosa; FEMS Microbiology Letters; Oxford University Press: Oxford, UK, 2017; Volume 364. [Google Scholar]
- Moscoso, J.A.; Mikkelsen, H.; Heeb, S.; Williams, P.; Filloux, A. The Pseudomonas aeruginosa sensor RetS switches Type III and Type VI secretion via c-di-GMP signalling. Environ. Microbiol. 2011, 13, 3128–3138. [Google Scholar] [CrossRef]
- Frangipani, E.; Visaggio, D.; Heeb, S.; Kaever, V.; Cámara, M.; Visca, P.; Imperi, F. The Gac/Rsm and cyclic-di-GMP signalling networks coordinately regulate iron uptake in Pseudomonas aeruginosa. Environ. Microbiol. 2014, 16, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Lalaouna, D.; Fochesato, S.; Sanchez, L.; Schmitt-Kopplin, P.; Haas, D.; Heulin, T.; Achouak, W. Phenotypic switching in Pseudomonas brassicacearum involves GacS- and GacA-dependent Rsm small RNAs. Appl. Environ. Microbiol. 2012, 78, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Arya, R.; Kim, K.K. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int. J. Mol. Sci. 2021, 22, 12152. [Google Scholar] [CrossRef] [PubMed]
- Segura, D.; Núñez, C.; Espín, G. Azotobacter Cysts. In eLS; Major Reference Works; Wiley: Hoboken, NJ, USA, 2014; ISBN 0-470-01617-5. [Google Scholar]
- Galindo, E.; Peña, C.; Núñez, C.; Segura, D.; Espín, G. Molecular and bioengineering strategies to improve alginate and polydydroxyalkanoate production by Azotobacter vinelandii. Microb. Cell Fact. 2007, 6, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernandez-Eligio, A.; Moreno, S.; Castellanos, M.; Castañeda, M.; Nuñez, C.; Muriel-Millan, L.F.; Espín, G. RsmA post-transcriptionally controls PhbR expression and polyhydroxybutyrate biosynthesis in Azotobacter vinelandii. Microbiology 2012, 158, 1953–1963. [Google Scholar] [CrossRef]
- López-Pliego, L.; Lara-Flores, N.; Molina-Romero, D.; May-Compañ, G.; Carreño-López, R.; Núñez, C.E.; Castañeda, M. The GacS/A-Rsm Pathway Positively Regulates Motility and Flagella Synthesis in Azotobacter vinelandii. Curr. Microbiol. 2022, 79, 17. [Google Scholar] [CrossRef]
- Krishna, S.N.; Luan, C.-H.; Mishra, R.K.; Xu, L.; Scheidt, K.A.; Anderson, W.F.; Bergan, R.C. A fluorescence-based thermal shift assay identifies inhibitors of mitogen activated protein kinase kinase 4. PLoS ONE 2013, 8, e81504. [Google Scholar] [CrossRef]
- Gallarato, L.A.; Sánchez, D.G.; Olvera, L.; Primo, E.D.; Garrido, M.N.; Beassoni, P.R.; Morett, E.; Lisa, A.T. Exopolyphosphatase of Pseudomonas aeruginosa is essential for the production of virulence factors, and its expression is controlled by NtrC and PhoB acting at two interspaced promoters. Microbiology 2014, 160, 406–417. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Janot, L.; Pena, O.M.; Neidig, A.; Kukavica-Ibrulj, I.; Hilchie, A.; Levesque, R.C.; Overhage, J.; Hancock, R.E.W. Requirement of the Pseudomonas aeruginosa CbrA sensor kinase for full virulence in a murine acute lung infection model. Infect. Immun. 2014, 82, 1256–1267. [Google Scholar] [CrossRef]
- Reva, O.N.; Weinel, C.; Weinel, M.; Böhm, K.; Stjepandic, D.; Hoheisel, J.D.; Tümmler, B. Functional genomics of stress response in Pseudomonas putida KT2440. J. Bacteriol. 2006, 188, 4079–4092. [Google Scholar] [CrossRef]
- Chakravarthy, S.; Butcher, B.G.; Liu, Y.; D’Amico, K.; Coster, M.; Filiatrault, M.J. Virulence of Pseudomonas syringae pv. Tomato DC3000 is influenced by the catabolite repression control protein Crc. Mol. Plant-Microbe Interact. 2017, 30, 283–294. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Gibbs, K.A.; Hager, P.W.; Phibbs, P.V., Jr.; Kolter, R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 425–431. [Google Scholar] [CrossRef]
- Huang, J.; Sonnleitner, E.; Ren, B.; Xu, Y.; Haas, D. Catabolite repression control of pyocyanin biosynthesis at an intersection of primary and secondary metabolism in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 5016–5020. [Google Scholar] [CrossRef]
- Hester, K.L.; Lehman, J.; Najar, F.; Song, L.; Roe, B.A.; MacGregor, C.H.; Hager, P.W.; Phibbs, J.; Sokatch, J.R. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 1144–1149. [Google Scholar] [CrossRef]
- Yang, N.; Ding, S.; Chen, F.; Zhang, X.; Xia, Y.; Di, H.; Cao, Q.; Deng, X.; Wu, M.; Wong, C.C.L.; et al. The Crc protein participates in down-regulation of the Lon gene to promote rhamnolipid production and rhl quorum sensing in Pseudomonas aeruginosa. Mol. Microbiol. 2015, 96, 526–547. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Chen, W.; Qin, H.; Hengzhuang, W.; Chen, Y.; Yang, L.; Zhang, G. Regulation of pqs quorum sensing via catabolite repression control in Pseudomonas aeruginosa. Microbiology 2013, 159, 1931–1936. [Google Scholar] [CrossRef]
- Reales-Calderón, J.A.; Corona, F.; Monteoliva, L.; Gil, C.; Martínez, J.L. Quantitative proteomics unravels that the post-transcriptional regulator Crc modulates the generation of vesicles and secreted virulence determinants of Pseudomonas aeruginosa. J. Proteom. 2015, 127, 352–364. [Google Scholar] [CrossRef]
- Linares, J.F.; Moreno, R.; Fajardo, A.; Martinez-Solano, L.; Escalante, R.; Rojo, F.; Martinez, J.L. The global regulator Crc modulates metabolism, susceptibility to antibiotics and virulence in Pseudomonas aeruginosa. Environ. Microbiol. 2010, 12, 3196–3212. [Google Scholar] [CrossRef]
- Davies, J.A.; Harrison, J.J.; Marques, L.L.R.; Foglia, G.R.; Stremick, C.A.; Storey, D.G.; Turner, R.J.; Olson, M.E.; Ceri, H. The GacS sensor kinase controls phenotypic reversion of small colony variants isolated from biofilms of Pseudomonas aeruginosa PA14. FEMS Microbiol. Ecol. 2007, 59, 32–46. [Google Scholar] [CrossRef]
- Romero, M.; Silistre, H.; Lovelock, L.; Wright, V.J.; Chan, K.G.; Hong, K.W.; Williams, P.; Cámara, M.; Heeb, S. Genome-wide mapping of the RNA targets of the Pseudomonas aeruginosa riboregulatory protein RsmN. Nucleic Acids Res. 2018, 46, 6823–6840. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, E.; Schuster, M.; Sorger-Domenigg, T.; Greenberg, E.P.; Bläsi, U. Hfq-dependent alterations of the transcriptome profile and effects on quorum sensing in Pseudomonas aeruginosa. Mol. Microbiol. 2006, 59, 1542–1558. [Google Scholar] [CrossRef] [PubMed]
- Zha, D.; Xu, L.; Zhang, H.; Yan, Y. The two-component GacS-GacA system activates lipA translation by RsmE but not RsmA in Pseudomonas protegens Pf-5. Appl. Environ. Microbiol. 2014, 80, 6627–6637. [Google Scholar] [CrossRef] [PubMed]
- Floyd, M.; Winn, M.; Cullen, C.; Sil, P.; Chassaing, B.; Yoo, D.G.; Gewirtz, A.T.; Goldberg, J.B.; McCarter, L.L.; Rada, B. Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1005987. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Horna, G.; Ruiz, J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021, 246, 126719. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Marvig, R.L.; Sommer, L.M.; Molin, S.; Johansen, H.K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 2015, 47, 57–64. [Google Scholar] [CrossRef]
- Yoon, S.S.; Hennigan, R.F.; Hilliard, G.M.; Ochsner, U.A.; Parvatiyar, K.; Kamani, M.C.; Allen, H.L.; DeKievit, T.R.; Gardner, P.R.; Schwab, U.; et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: Relationships to cystic fibrosis pathogenesis. Dev. Cell 2002, 3, 593–603. [Google Scholar] [CrossRef]
- Kamath, K.S.; Krisp, C.; Chick, J.; Pascovici, D.; Gygi, S.P.; Molloy, M.P. Pseudomonas aeruginosa proteome under hypoxic stress conditions mimicking the cystic fibrosis lung. J. Proteome Res. 2017, 16, 3917–3928. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Sheng, Y.H. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Dubern, J.; Cigana, C.; De Simone, M.; Lazenby, J.; Juhas, M.; Schwager, S.; Bianconi, I.; Döring, G.; Eberl, L.; Williams, P.; et al. Integrated whole-genome screening for Pseudomonas aeruginosa virulence genes using multiple disease models reveals that pathogenicity is host specific. Environ. Microbiol. 2015, 17, 4379–4393. [Google Scholar] [CrossRef]
- Fernández, M.; Porcel, M.; de la Torre, J.; Molina-Henares, M.A.; Daddaoua, A.; Llamas, M.A.; Roca, A.; Carriel, V.; Garzón, I.; Ramos, J.L.; et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015, 6, 871. [Google Scholar] [CrossRef]
- Nandi, M.; Selin, C.; Brassinga, A.K.C.; Belmonte, M.F.; Fernando, W.G.D.; Loewen, P.C.; De Kievit, T.R. Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE 2015, 10, e0123184. [Google Scholar] [CrossRef]
- Migiyama, Y.; Yanagihara, K.; Kaku, N.; Harada, Y.; Yamada, K.; Nagaoka, K.; Morinaga, Y.; Akamatsu, N.; Matsuda, J.; Izumikawa, K.; et al. Pseudomonas aeruginosa Bacteremia among Immunocompetent and Immunocompromised Patients: RelationtoInitial Antibiotic Therapy and Survival. Jpn. J. Infect. Dis. 2016, 69, 91–96. [Google Scholar] [CrossRef]
- Turner, K.H.; Everett, J.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Requirements for Pseudomonas aeruginosa Acute Burn and Chronic Surgical Wound Infection. PLOS Genet. 2014, 10, e1004518. [Google Scholar] [CrossRef]
- Reyes, E.A.; Bale, M.J.; Cannon, W.H.; Matsen, J.M. Identification of Pseudomonas aeruginosa by pyocyanin production on Tech agar. J. Clin. Microbiol. 1981, 13, 456–458. [Google Scholar] [CrossRef]
- Johnson, M.K.; Boese-Marrazzo, D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 1980, 29, 1028–1033. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T.; Givskov, M.; Alhede, M. Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Adv. Appl. Microb. 2014, 86, 1–40. [Google Scholar]
- Jensen, P.Ø.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 292–305. [Google Scholar] [CrossRef]
- Taylor, P.K.; Yeung, A.T.Y.; Hancock, R.E.W. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa Can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007, 10, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Starkey, M.; Edwards, A.N.; Wozniak, D.J.; Romeo, T.; Parsek, M.R. Pseudomonas aeruginosa biofilm matrix polysaccharide Psl is regulated transcriptionally by RpoS and post-transcriptionally by RsmA. Mol. Microbiol. 2010, 78, 158–172. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Harrison, J.J.; Almblad, H.; Irie, Y.; Wolter, D.J.; Eggleston, H.C.; Randall, T.E.; Kitzman, J.O.; Stackhouse, B.; Emerson, J.C.; McNamara, S.; et al. Elevated exopolysaccharide levels in Pseudomonas aeruginosa flagellar mutants have implications for biofilm growth and chronic infections. PLoS Genet. 2020, 16, e1008848. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Taktikos, J.; Stark, H.; Zaburdaev, V. How the Motility Pattern of Bacteria Affects Their Dispersal and Chemotaxis. PLoS ONE 2013, 8, e81936. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Köhler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechère, J.-C. Swarming of Pseudomonas aeruginosa Is Dependent on Cell-to-Cell Signaling and Requires Flagella and Pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef]
- Bai, F.; Branch, R.W.; Nicolau, D.V.; Pilizota, T.; Steel, B.C.; Maini, P.K.; Berry, R.M. Conformational Spread as a Mechanism for Cooperativity in the Bacterial Flagellar Switch. Science 2010, 327, 685–689. [Google Scholar] [CrossRef]
- Rashid, M.H.; Kornberg, A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 4885–4890. [Google Scholar] [CrossRef]
- Tremblay, J.; Richardson, A.-P.; Lépine, F.; Déziel, E. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environ. Microbiol. 2007, 9, 2622–2630. [Google Scholar] [CrossRef]
- Dasgupta, N.; Wolfgang, M.C.; Goodman, A.L.; Arora, S.K.; Jyot, J.; Lory, S.; Ramphal, R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003, 50, 809–824. [Google Scholar] [CrossRef]
- Leal-Morales, A.; Pulido-Sánchez, M.; López-Sánchez, A.; Govantes, F. Transcriptional organization and regulation of the Pseudomonas putida flagellar system. Environ. Microbiol. 2021. [Google Scholar] [CrossRef]
- Sivakumar, R.; Ranjani, J.; Vishnu, U.S.; Jayashree, S.; Lozano, G.L.; Miles, J.; Broderick, N.A.; Guan, C.; Gunasekaran, P.; Handelsman, J.; et al. Evaluation of InSeq to Identify Genes Essential for Pseudomonas aeruginosa PGPR2 Corn Root Colonization. G3 Genes Genomes Genet. 2019, 9, 651–661. [Google Scholar] [CrossRef]
- Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Inactivation of CbrAB two-component system hampers root colonization in rhizospheric strain of Pseudomonas aeruginosa PGPR2. Biochim. Biophys. Acta-Gene Regul. Mech. 2021, 1864, 194763. [Google Scholar] [CrossRef]
- Nair, C.; Shoemark, A.; Chan, M.; Ollosson, S.; Dixon, M.; Hogg, C.; Alton, E.W.F.W.; Davies, J.C.; Williams, H.D. Cyanide levels found in infected cystic fibrosis sputum inhibit airway ciliary function. Eur. Respir. J. 2014, 44, 1253–1261. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Zamioudis, C.; Berendsen, R.L.; Bakker, P.A.H.M.; Pieterse, C.M.J. Type III secretion system of beneficial rhizobacteria Pseudomonas simiae WCS417 and Pseudomonas defensor WCS374. Front. Microbiol. 2019, 10, 1631. [Google Scholar] [CrossRef]
- Rezzonico, F.; Binder, C.; Défago, G.; Moënne-Loccoz, Y. The type III secretion system of biocontrol Pseudomonas fluorescens KD targets the phytopathogenic chromista Pythium ultimum and promotes cucumber protection. Mol. Plant-Microbe Interact. 2005, 18, 991–1001. [Google Scholar] [CrossRef]
- Songwattana, P.; Noisangiam, R.; Teamtisong, K.; Prakamhang, J.; Teulet, A.; Tittabutr, P.; Piromyou, P.; Boonkerd, N.; Giraud, E.; Teaumroong, N. Type 3 secretion system (T3SS) of Bradyrhizobium sp. DOA9 and its roles in legume symbiosis and rice endophytic association. Front. Microbiol. 2017, 8, 1810. [Google Scholar] [CrossRef]
- Lombardi, C.; Tolchard, J.; Bouillot, S.; Signor, L.; Gebus, C.; Liebl, D.; Fenel, D.; Teulon, J.M.; Brock, J.; Habenstein, B.; et al. Structural and functional characterization of the type three secretion system (T3SS) needle of Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Büttner, D.; He, S.Y. Type III Protein Secretion in Plant Pathogenic Bacteria. Plant Physiol. 2009, 150, 1656–1664. [Google Scholar] [CrossRef]
- Alfano, J.R.; Charkowski, A.O.; Deng, W.L.; Badel, J.L.; Petnicki-Ocwieja, T.; Van Dijk, K.; Collmer, A. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in pl. Proc. Natl. Acad. Sci. USA 2000, 97, 4856–4861. [Google Scholar] [CrossRef]
- Arnold, D.L.; Pitman, A.; Jackson, R.W. Pathogenicity and other genomic islands in plant pathogenic bacteria. Mol. Plant Pathol. 2003, 4, 407–420. [Google Scholar] [CrossRef]
- Shao, X.; Tan, M.; Xie, Y.; Yao, C.; Wang, T.; Huang, H.; Zhang, Y.; Ding, Y.; Liu, J.; Han, L.; et al. Integrated regulatory network in Pseudomonas syringae reveals dynamics of virulence. Cell Rep. 2021, 34, 108920. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shao, X.; Deng, X. Regulation of type III secretion system in Pseudomonas syringae. Environ. Microbiol. 2019, 21, 4465–4477. [Google Scholar] [CrossRef]
- Rietsch, A.; Wolfgang, M.C.; Mekalanos, J.J. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect. Immun. 2004, 72, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Zhang, X.F.; Zhang, L.H. The global regulator Crc plays a multifaceted role in modulation of type III secretion system in Pseudomonas aeruginosa. Microbiologyopen 2013, 2, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, e3. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteagudo-Cascales, E.; Santero, E.; Canosa, I. The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions. Genes 2022, 13, 375. https://doi.org/10.3390/genes13020375
Monteagudo-Cascales E, Santero E, Canosa I. The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions. Genes. 2022; 13(2):375. https://doi.org/10.3390/genes13020375
Chicago/Turabian StyleMonteagudo-Cascales, Elizabet, Eduardo Santero, and Inés Canosa. 2022. "The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions" Genes 13, no. 2: 375. https://doi.org/10.3390/genes13020375
APA StyleMonteagudo-Cascales, E., Santero, E., & Canosa, I. (2022). The Regulatory Hierarchy Following Signal Integration by the CbrAB Two-Component System: Diversity of Responses and Functions. Genes, 13(2), 375. https://doi.org/10.3390/genes13020375






