The Effects of FGF4 Retrogenes on Canine Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Data Collection
2.2. Measurements
2.3. Genotyping
2.4. Statistical Analysis
3. Results
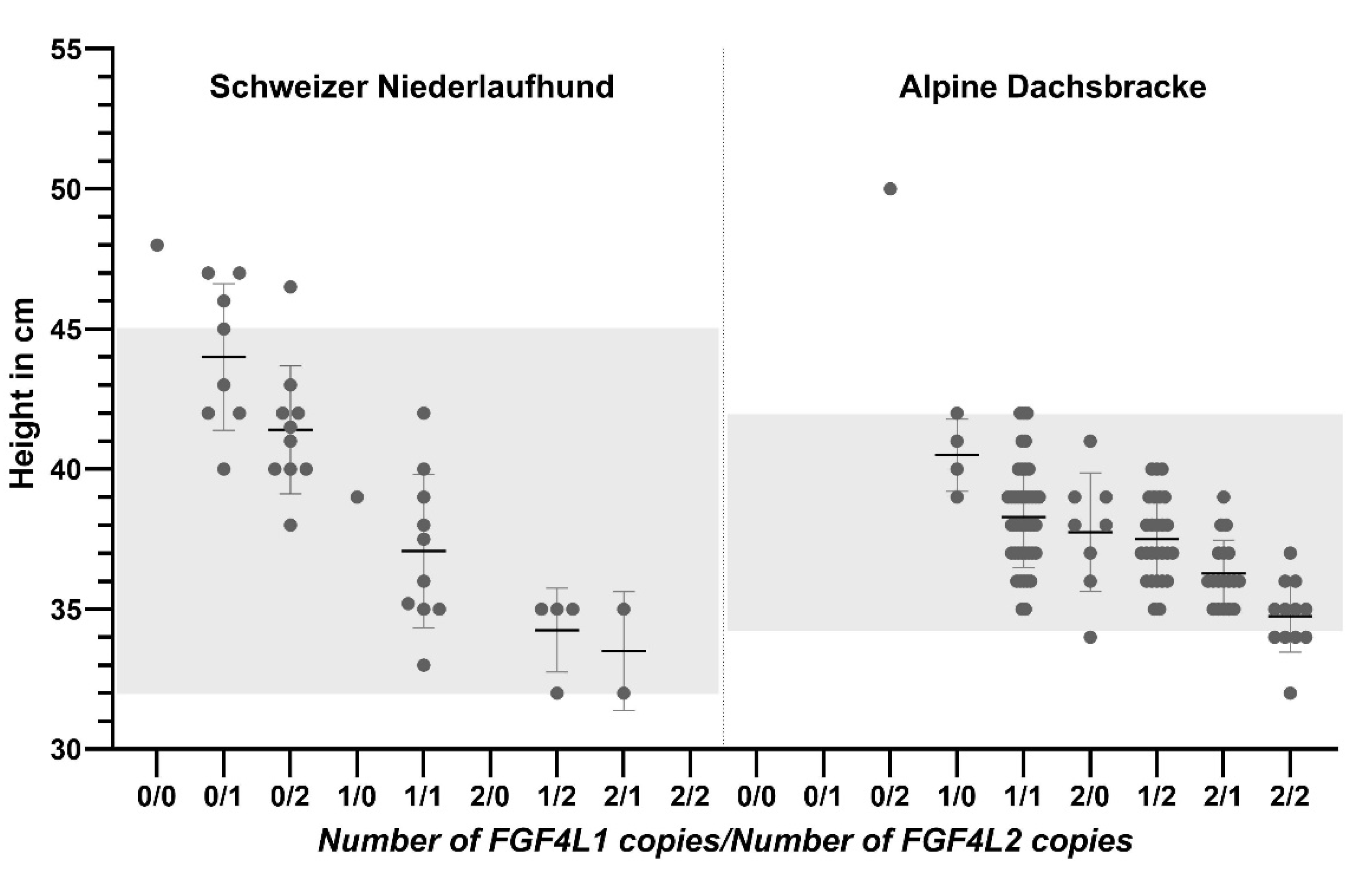
3.1. Evaluation of Height at the Shoulder
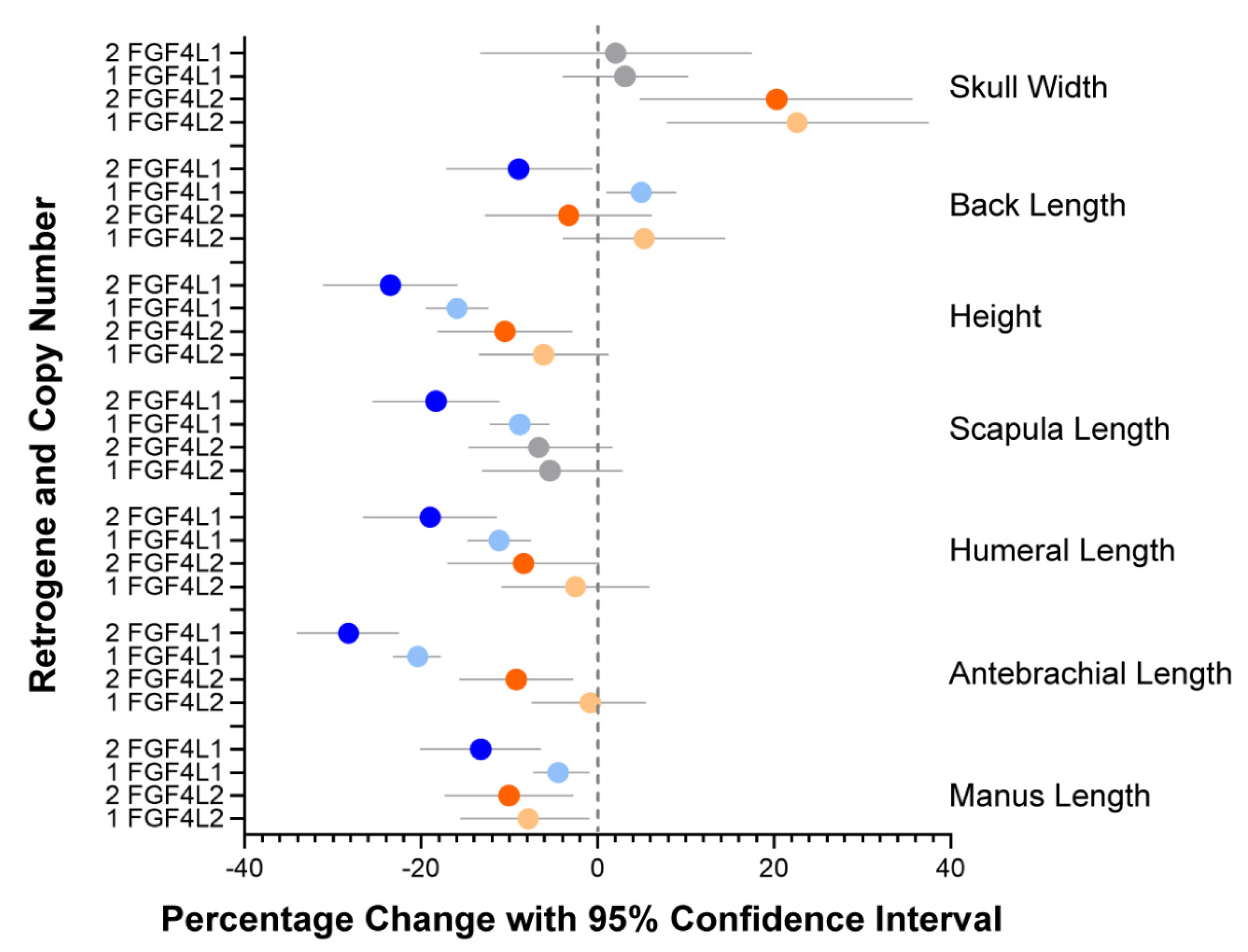
3.2. Evaluation of Specific Morphologic Parameters in the Schweizer Niederlaufhund
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.A.; Dickinson, P.J.; Mansour, T.; Sturges, B.K.; Aguilar, M.; Young, A.E.; Korff, C.; Lind, J.; Ettinger, C.L.; Varon, S.; et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc. Natl. Acad. Sci. USA 2017, 114, 11476–11481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G. An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiang, R.; Thompson, L.M.; Zhu, Y.-Z.; Church, D.M.; Fielder, T.J.; Bocian, M.; Winokur, S.T.; Wasmuth, J.J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 1994, 78, 335–342. [Google Scholar] [CrossRef]
- Jurka, J. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc. Natl. Acad. Sci. USA 1997, 94, 1872–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batcher, K.; Dickinson, P.; Maciejczyk, K.; Brzeski, K.; Rasouliha, S.H.; Letko, A.; Drogemuller, C.; Leeb, T.; Bannasch, D. Multiple FGF4 Retrocopies Recently Derived within Canids. Genes 2020, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.-J. A pathologic-anatomical study on disc degeneration in dog: With special reference to the so-called enchondrosis intervertebralis. Acta Orthop. Scand. 1952, 23, 1–130. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.J.; Castelhano, M.G.; Oliveira, K.C.; Corey, E.; Balkman, C.; Baxter, T.L.; Casal, M.L.; Center, S.A.; Fang, M.; Garrison, S.J.; et al. Complex disease and phenotype mapping in the domestic dog. Nat. Commun. 2016, 7, 10460. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.J.; White, M.E.; Boyle, M.; Shannon, L.M.; Casal, M.L.; Castelhano, M.G.; Center, S.A.; Meyers-Wallen, V.N.; Simpson, K.W.; Sutter, N.B.; et al. Imputation of canine genotype array data using 365 whole-genome sequences improves power of genome-wide association studies. PLoS Genet. 2019, 15, e1008003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plassais, J.; Kim, J.; Davis, B.W.; Karyadi, D.M.; Hogan, A.N.; Harris, A.C.; Decker, B.; Parker, H.G.; Ostrander, E.A. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat. Commun. 2019, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Batcher, K.; Dickinson, P.; Giuffrida, M.; Sturges, B.; Vernau, K.; Knipe, M.; Rasouliha, S.H.; Drogemuller, C.; Leeb, T.; Maciejczyk, K.; et al. Phenotypic Effects of FGF4 Retrogenes on Intervertebral Disc Disease in Dogs. Genes 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- American Kennel Club. Available online: https://www.akc.org/ (accessed on 2 January 2022).
- Frischknecht, M.; Niehof-Oellers, H.; Jagannathan, V.; Owczarek-Lipska, M.; Drogemuller, C.; Dietschi, E.; Dolf, G.; Tellhelm, B.; Lang, J.; Tiira, K.; et al. A COL11A2 mutation in Labrador retrievers with mild disproportionate dwarfism. PLoS ONE 2013, 8, e60149. [Google Scholar] [CrossRef] [Green Version]
- Jeanes, E.C.; Oliver, J.A.C.; Ricketts, S.L.; Gould, D.J.; Mellersh, C.S. Glaucoma-causing ADAMTS17 mutations are also reproducibly associated with height in two domestic dog breeds: Selection for short stature may have contributed to increased prevalence of glaucoma. Canine Genet. Epidemiol. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, K.K.L.; Lingaas, F. Short and sweet: Foreleg abnormalities in Havanese and the role of the FGF4 retrogene. Canine Med. Genet. 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Veterinary_Genetics_Laboratory. Chondrodystrophy (CDDY and IVDD Risk) and Chondrodysplasia (CDPA). Available online: https://vgl.ucdavis.edu/test/cddy-cdpa (accessed on 21 December 2021).
- Marchant, T.W.; Johnson, E.J.; McTeir, L.; Johnson, C.I.; Gow, A.; Liuti, T.; Kuehn, D.; Svenson, K.; Bermingham, M.L.; Drogemuller, M.; et al. Canine Brachycephaly Is Associated with a Retrotransposon-Mediated Missplicing of SMOC2. Curr. Biol. 2017, 27, 1573–1584.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N | Mean | 95% CI | Percent Change | p | |
|---|---|---|---|---|---|
| FGF4L1 | 2.20 × 10−16 | ||||
| 0 copies | 1 | 50.4 | 47.7 to 53.1 | - | |
| 1 copy | 72 | 38.7 | 33.4 to 43.9 | −23.48% | 6.1 × 10−15 |
| 2 copies | 38 | 36.5 | 31.2 to 41.7 | −27.90% | 2.0 × 10−16 |
| FGF4L2 | 1.7 × 10−07 | ||||
| 0 copies | 12 | 50.4 | 47.7 to 53.1 | - | |
| 1 copy | 60 | 49.2 | 45.6 to 52.7 | −3.21% | 3.9 × 10−03 |
| 2 copies | 39 | 48.0 | 44.5 to 51.6 | −6.13% | 3.1 × 10−07 |
| Sex | 1.64 × 10−12 | ||||
| Female | 53 | 50.4 | 47.7 to 53.1 | - | |
| Male | 58 | 52.4 | 49.2 to 55.6 | 5.42% |
| N | Mean | 95% CI | Percent Change | p | |
|---|---|---|---|---|---|
| FGF4L1 | 2.7 × 10−10 | ||||
| 0 copies | 19 | 46.2 | 42.7 to 49.6 | - | |
| 1 copy | 15 | 38.8 | 33.7 to 43.8 | −15.9% | 3.4 × 10−10 |
| 2 copies | 2 | 35.3 | 28.4 to 42.2 | −23.5% | 6.1 × 10−7 |
| FGF4L2 | 0.01 | ||||
| 0 copies | 2 | 46.2 | 42.7 to 49.6 | - | |
| 1 copy | 20 | 43.3 | 36.3 to 50.1 | −6.1% | 0.10 |
| 2 copies | 14 | 41.3 | 28.4 to 48.3 | −10.5% | 0.009 |
| Sex | 0.03 | ||||
| Female | 25 | 46.2 | 42.7 to 49.6 | - | |
| Male | 11 | 48.3 | 42.9 to 53.5 | 4.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannasch, D.; Batcher, K.; Leuthard, F.; Bannasch, M.; Hug, P.; Marcellin-Little, D.J.; Dickinson, P.J.; Drögemüller, M.; Drögemüller, C.; Leeb, T. The Effects of FGF4 Retrogenes on Canine Morphology. Genes 2022, 13, 325. https://doi.org/10.3390/genes13020325
Bannasch D, Batcher K, Leuthard F, Bannasch M, Hug P, Marcellin-Little DJ, Dickinson PJ, Drögemüller M, Drögemüller C, Leeb T. The Effects of FGF4 Retrogenes on Canine Morphology. Genes. 2022; 13(2):325. https://doi.org/10.3390/genes13020325
Chicago/Turabian StyleBannasch, Danika, Kevin Batcher, Fabienne Leuthard, Michael Bannasch, Petra Hug, Denis J. Marcellin-Little, Peter J. Dickinson, Michaela Drögemüller, Cord Drögemüller, and Tosso Leeb. 2022. "The Effects of FGF4 Retrogenes on Canine Morphology" Genes 13, no. 2: 325. https://doi.org/10.3390/genes13020325
APA StyleBannasch, D., Batcher, K., Leuthard, F., Bannasch, M., Hug, P., Marcellin-Little, D. J., Dickinson, P. J., Drögemüller, M., Drögemüller, C., & Leeb, T. (2022). The Effects of FGF4 Retrogenes on Canine Morphology. Genes, 13(2), 325. https://doi.org/10.3390/genes13020325







