Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies
Abstract
:1. Introduction
2. Materials and Methods
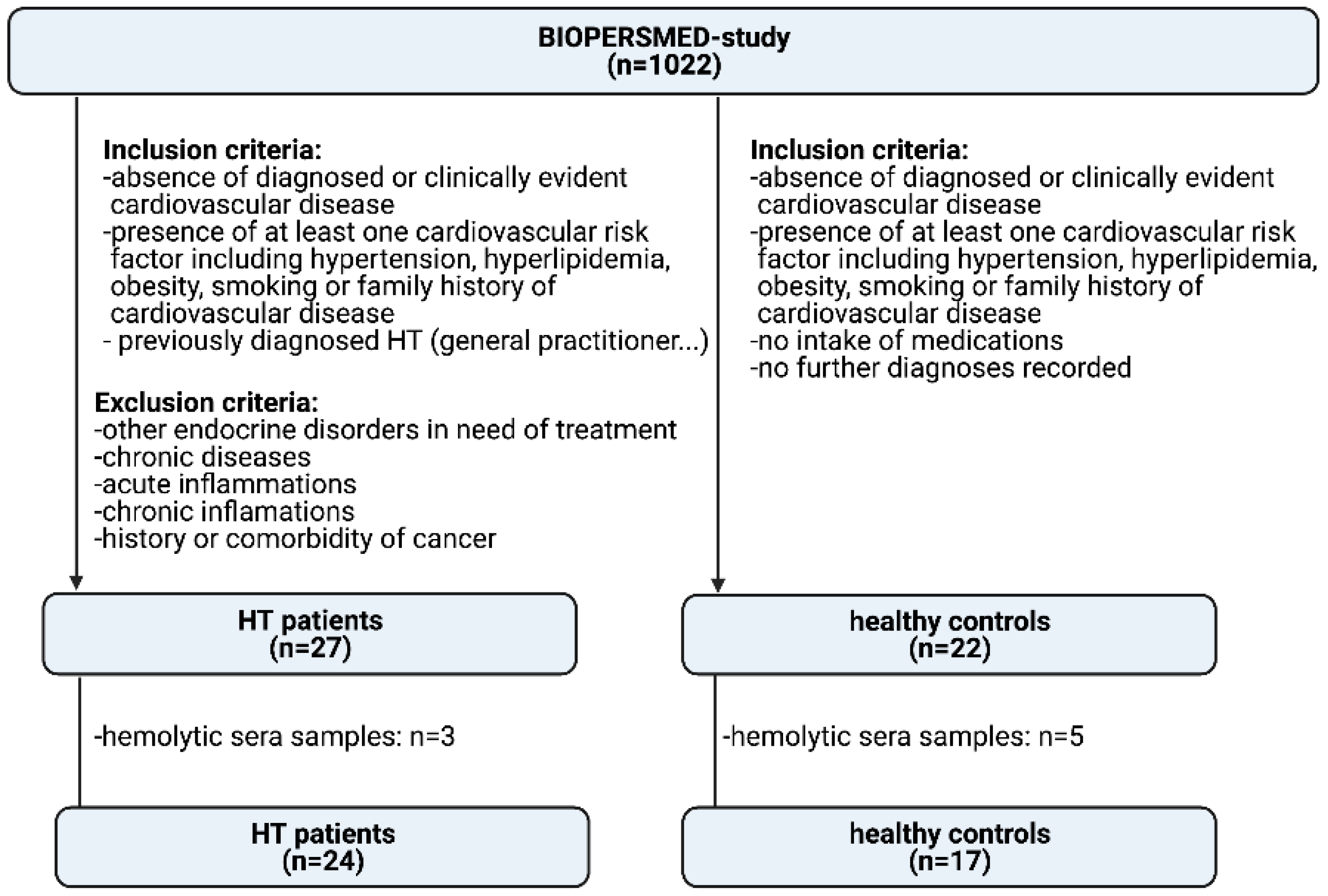
2.1. Study Populations
2.2. Patient Visit
2.3. Selection of miRNAs
2.4. miRNA Isolation and qPCR
2.5. Functional Annotation of miRNAs
2.6. Statistical Analysis
3. Results
3.1. General Results
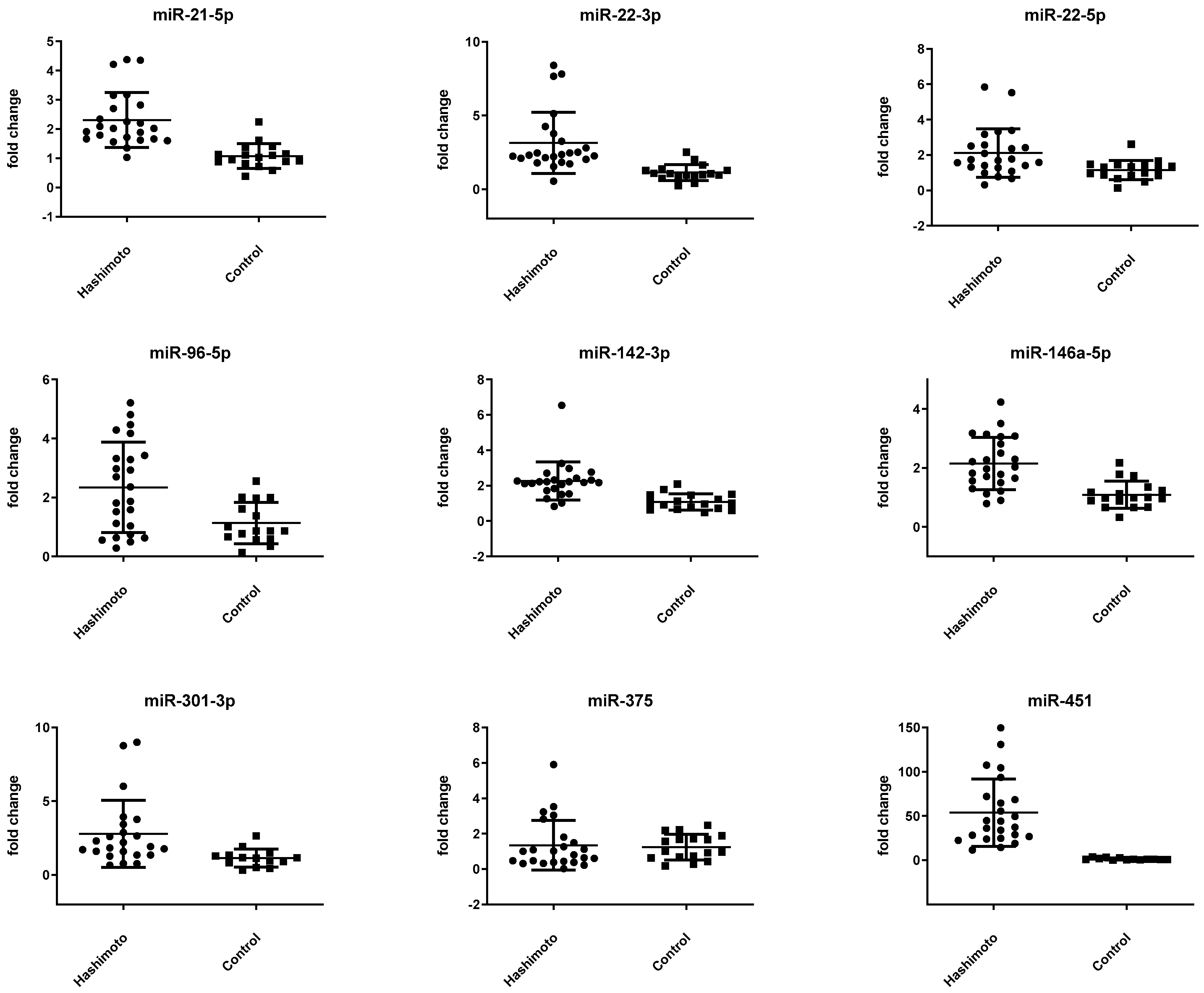
3.2. miRNA Expression Is Altered in Patients with HT
3.3. miR-22-5p and miR-142-3p Are Altered in HT Patients with Higher Levels of Thyroid Antibodies
3.4. Potential Binding Site Targets
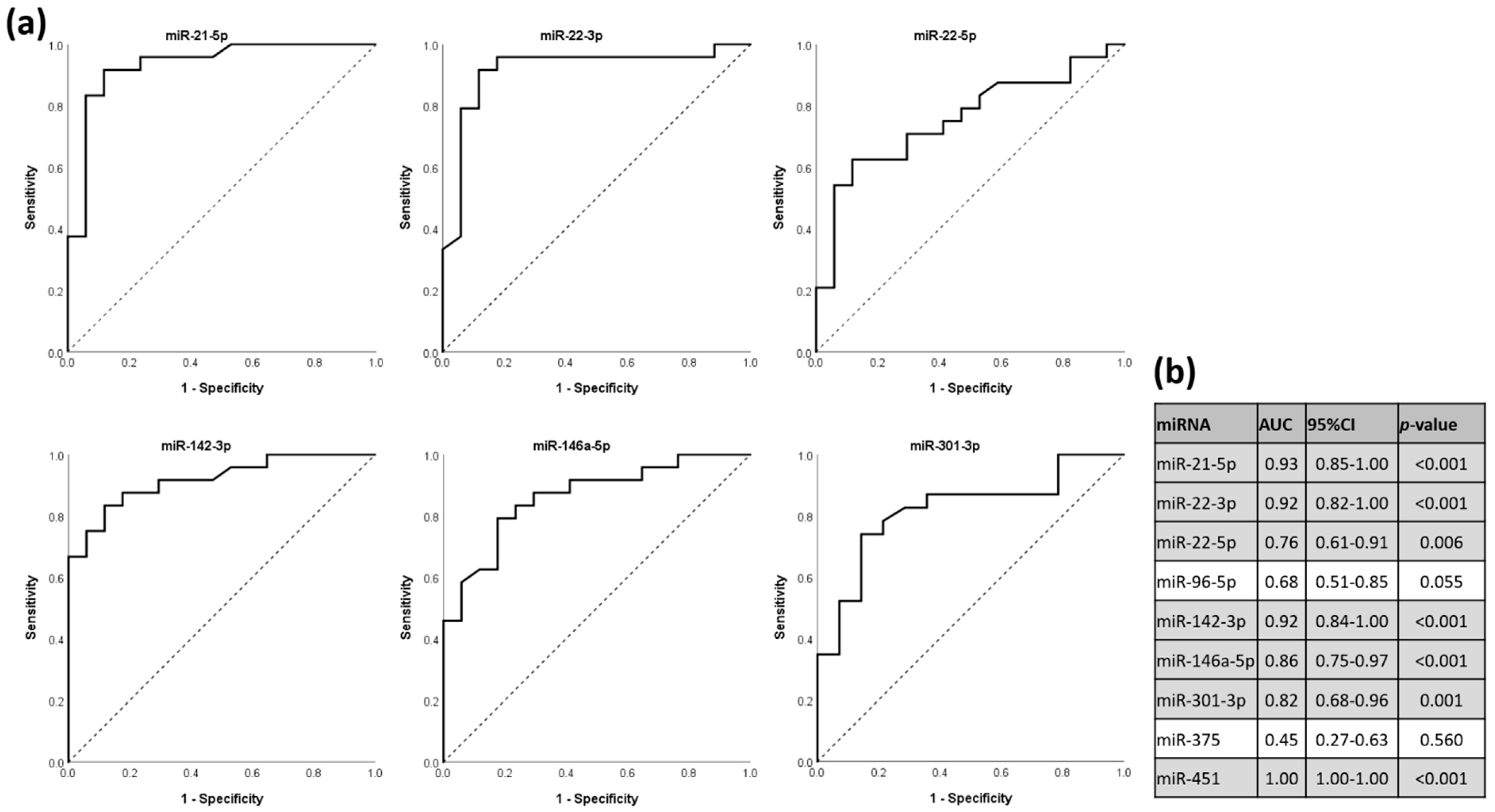
3.5. miRNAs as Discriminators for HT Status in ROC Analysis
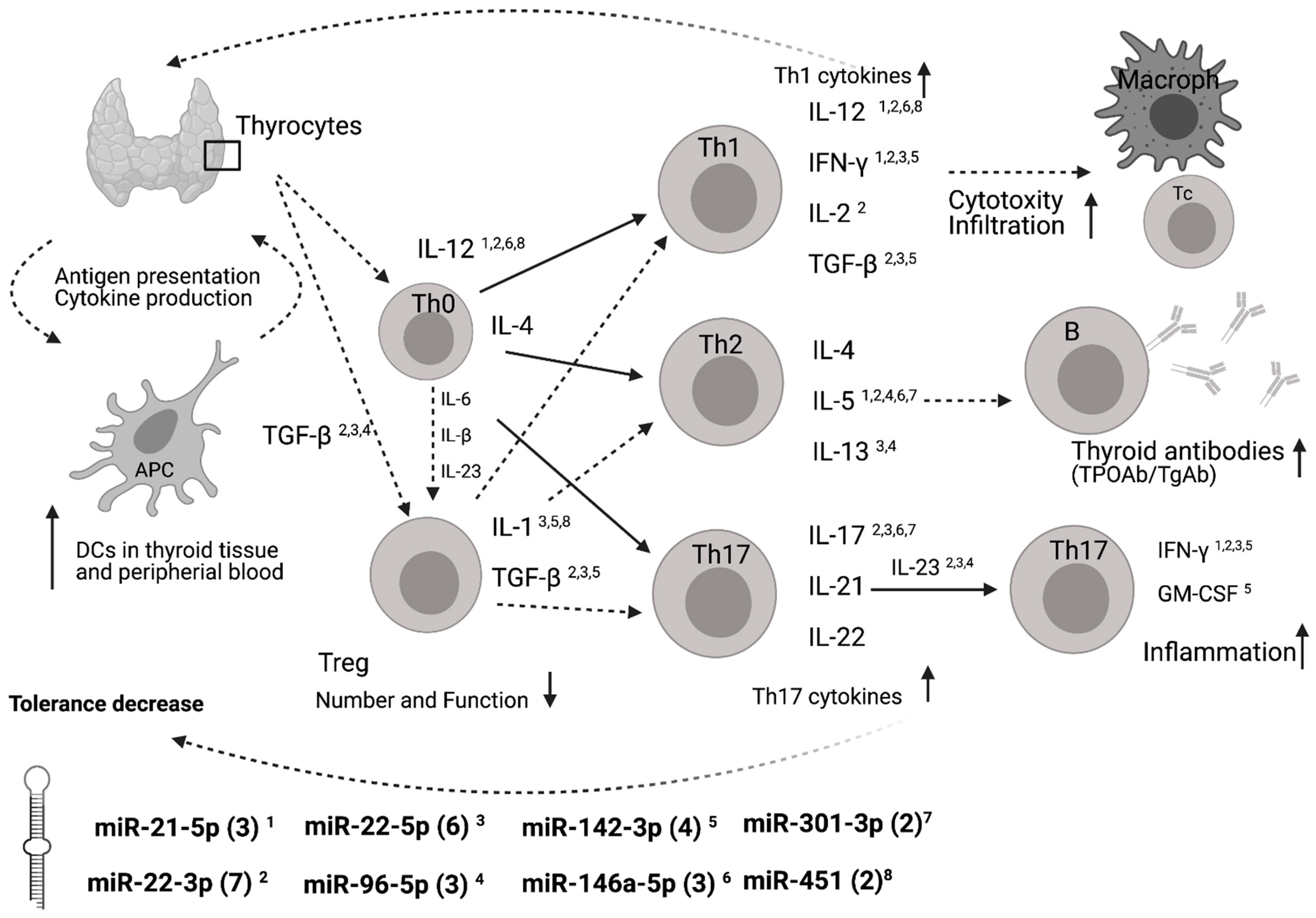
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rapoport, B.; McLachlan, S.M. Thyroid autoimmunity. J. Clin. Investig. 2001, 108, 1253–1259. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Sen, S.; Tandon, N.; Sahoo, M.; Walia, R.P.; Singh, S.; Ganguly, S.K.; Jain, S.K. Familial aggregation of autoimmune thyroiditis in first-degree relatives of patients with juvenile autoimmune thyroid disease. Thyroid 2003, 13, 297–300. [Google Scholar] [CrossRef]
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Baloch, Z.W.; LiVolsi, V.A. Fine-needle aspiration of the thyroid: Today and tomorrow. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 929–939. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.; Shan, X.; Qi, L.W.; Wang, T.; Zhu, J.; Zhu, D.; Huang, Z.; Zhang, L.; Zhang, H.; et al. Differential expression levels of plasma microRNA in Hashimoto’s disease. Gene 2018, 642, 152–158. [Google Scholar] [CrossRef]
- Gracias, D.T.; Katsikis, P.D. MicroRNAs: Key components of immune regulation. Adv. Exp. Med. Biol. 2011, 780, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Ma, X.; Xu, L.; Wang, D.; Jiang, X.; Zhu, W.; Cui, B.; Ning, G.; Lin, D.; Wang, S. Differential microRNA expression in peripheral blood mononuclear cells from Graves’ disease patients. J. Clin. Endocrinol. Metab. 2012, 97, E968–E972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef]
- Heegaard, N.H.H.; Carlsen, A.L.; Skovgaard, K.; Heegaard, P.M.H. Circulating extracellular microRNA in systemic autoimmunity. EXS 2015, 106, 171–195. [Google Scholar] [PubMed]
- Nakamachi, Y.; Kawano, S.; Takenokuchi, M.; Nishimura, K.; Sakai, Y.; Chin, T.; Saura, R.; Kurosaka, M.; Kumagai, S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patientswith rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1294–1304. [Google Scholar] [CrossRef]
- Wang, H.; Peng, W.; Ouyang, X.; Li, W.; Dai, Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl. Res. 2012, 160, 198–206. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef]
- Xia, P.; Fang, X.; Zhang, Z.H.; Huang, Q.; Yan, K.X.; Kang, K.F.; Han, L.; Zheng, Z.Z. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol. Lett. 2012, 148, 151–162. [Google Scholar] [CrossRef]
- Martínez-Hernández, R.; Sampedro-Núñez, M.; Serrano-Somavilla, A.; Ramos-Leví, A.M.; de la Fuente, H.; Triviño, J.C.; Sanz-García, A.; Sánchez-Madrid, F.; Marazuela, M. A MicroRNA Signature for Evaluation of Risk and Severity of Autoimmune Thyroid Diseases. J. Clin. Endocrinol. Metab. 2018, 103, 1139–1150. [Google Scholar] [CrossRef]
- Yamada, H.; Itoh, M.; Hiratsuka, I.; Hashimoto, S. Circulating microRNAs in autoimmune thyroid diseases. Clin. Endocrinol. 2014, 81, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in our Understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernecker, C.; Lenz, L.; Ostapczuk, M.S.; Schinner, S.; Willenberg, H.; Ehlers, M.; Vordenbaumen, S.; Feldkamp, J.; Schott, M. MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid 2012, 22, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liu, Y.; Tian, J.; Ma, J.; Tang, X.; Yang, J.; Rui, K.; Zhang, Y.; Mao, C.; Lu, L.; et al. Decreased expression of microRNA-125a-3p upregulates interleukin-23 receptor in patients with Hashimoto’s thyroiditis. Immunol. Res. 2015, 62, 129–136. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Pathogenesis of thyroid autoimmune disease: The role of cellular mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- Li, F.; He, H. Assessing the Accuracy of Diagnostic Tests. Shanghai Arch. Psychiatry 2018, 30, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Zhang, W.; Zhang, W.; Fan, L.; Wang, L.; Liu, Y.; Liu, S.; Guo, Y.; Wang, Y.; et al. MicroRNA-142-5p contributes to Hashimoto’s thyroiditis by targeting CLDN1. J. Transl. Med. 2016, 14, 166. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA spectrum between serum and plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef]
- Sunderland, N.; Skroblin, P.; Barwari, T.; Huntley, R.P.; Lu, R.; Joshi, A.; Lovering, R.C.; Mayr, M. MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens. Circ. Res. 2017, 120, 418–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussbacher, M.; Schrottmaier, W.C.; Salzmann, M.; Brostjan, C.; Schmid, J.A.; Starlinger, P.; Assinger, A. Optimized plasma preparation is essential to monitor platelet-stored molecules in humans. PLoS ONE 2017, 12, e0188921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.P.; Di Liao, C.; Fu, B.Y.; Lam, L.C.; Tang, N.L. Interindividual and interethnic variation in genomewide gene expression: Insights into the biological variation of gene expression and clinical implications. Clin. Chem. 2009, 55, 774–785. [Google Scholar] [CrossRef] [Green Version]
- Wu j Cai, H.; Xiang, Y.-B.; Matthews, C.E.; Ye, F.; Zheng, W.; Cai, Q.; Shu, X.-O. Intra-individual variation of miRNA expression levels in human plasma samples. Biomarkers 2018, 23, 339–346. [Google Scholar] [CrossRef]
- Khan, J.; Lieberman, J.A.; Lockwood, C.M. Variability in, variability out: Best practice recommendations to standardize pre-analytical variables in the detection of circulating and tissue microRNAs. Clin. Chem. Lab Med. 2017, 55, 608–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraldi, M.; Gomarasca, M.; Sansoni, V.; Perego, S.; Banfi, G.; Lombardi, G. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 2019, 9, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzenbach, H.; Machado da Silva, A.; Calin, G.; Pantel, K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef] [PubMed]

| micro RNAs | Sequence | Reference |
|---|---|---|
| hsa-miR-21-5p | 5′UAGCUUAUCAGACUGAUGUUGA | [15] |
| hsa-miR-22-3p | 5′AAGCUGCCAGUUGAAGAACUGU | [16] |
| hsa-miR-22-5p | 5′AGUUCUUCAGUGGCAAGCUUUA | [16] |
| hsa-miR-96-5p | 5′UUUGGCACUAGCACAUUUUUGCU | [15] |
| hsa-miR-142-3p | 5′UGUAGUGUUUCCUACUUUAUGGA | [15] |
| hsa-miR-146a-5p | 5′UGAGAACUGAAUUCCAUGGGUU | [15] |
| hsa-miR-301-3p | 5′CAGUGCAAUAGUAUUGUCAAAGC | [15] |
| hsa-miR-375 | 5′UUUGUUCGUUCGGCUCGCGUGA | [16] |
| hsa-miR-451 | 5′AAACCGUUACCAUUACUGAGUU | [16] |
| Patients with HT | Healthy Subjects | |
|---|---|---|
| n | 24 | 17 |
| Sex female (n) | 22 (91.7%) | 11 (64.7%) |
| Age (yr) | 57.9 ± 7.4 | 61.1 ± 5.8 |
| BMI (kg/m2) | 25.2 ± 4.2 | 23.7 ± 2.34 |
| fT3 (pmol/L) | 4.4 ± 0.5 | 4.8 ± 0.4 |
| fT4 (pmol/L) | 15.9 ± 2.3 | 16.3 ± 2.0 |
| TSH (µU/mL) | 1.60 ± 0.87 | 1.83 ± 0.73 |
| TgAb (IU/mL) | 213.8 ± 365.6 | |
| TPOAb (IU/mL) | 212.2 ± 199.1 | |
| Levothyroxine treatment | 7 (29%) |
| hsa-miRNA | Patients with HT (n = 24) | Healthy Subjects (n = 17) | p-Value |
|---|---|---|---|
| miR-21-5p | −0.43 ± 0.54 | 0.68 ± 0.58 | <0.001 * |
| miR-22-3p | 1.58 ± 0.85 | 2.98 ± 0.80 | <0.001 * |
| miR-22-5p | 6.42 ± 0.95 | 7.22 ± 0.91 | 0.010 |
| miR-96-5p | 8.40 ± 1.21 | 9.22 ± 1.22 | 0.040 |
| miR-142-3p | −0.84 ± 0.58 | 1.03 ± 0.60 | <0.001 * |
| miR-146a-5p | 2.71 ± 0.63 | 3.69 ± 0.65 | <0.001 * |
| miR-301-3p | 6.09 ± 1.01 | 7.21 ± 0.82 | 0.001 * |
| miR-375 | 8.52 ± 1.67 | 8.21 ± 1.10 | 0.503 |
| miR-451 | −2.60 ± 1.00 | 2.82 ± 1.35 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trummer, O.; Foessl, I.; Schweighofer, N.; Arifi, E.; Haudum, C.W.; Reintar, S.; Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Zirlik, A.; et al. Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies. Genes 2022, 13, 171. https://doi.org/10.3390/genes13020171
Trummer O, Foessl I, Schweighofer N, Arifi E, Haudum CW, Reintar S, Pilz S, Theiler-Schwetz V, Trummer C, Zirlik A, et al. Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies. Genes. 2022; 13(2):171. https://doi.org/10.3390/genes13020171
Chicago/Turabian StyleTrummer, Olivia, Ines Foessl, Natascha Schweighofer, Edi Arifi, Christoph W. Haudum, Sharmaine Reintar, Stefan Pilz, Verena Theiler-Schwetz, Christian Trummer, Andreas Zirlik, and et al. 2022. "Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies" Genes 13, no. 2: 171. https://doi.org/10.3390/genes13020171
APA StyleTrummer, O., Foessl, I., Schweighofer, N., Arifi, E., Haudum, C. W., Reintar, S., Pilz, S., Theiler-Schwetz, V., Trummer, C., Zirlik, A., Schmidt, A., Colantonio, C., Kolesnik, E., Verheyen, N., Pieber, T. R., & Obermayer-Pietsch, B. (2022). Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies. Genes, 13(2), 171. https://doi.org/10.3390/genes13020171








