The Interactive Effect of Genetic and Epigenetic Variations in FKBP5 and ApoE Genes on Anxiety and Brain EEG Parameters
Abstract
:1. Introduction
2. Results
2.1. Aging and rs1360780 Alleles
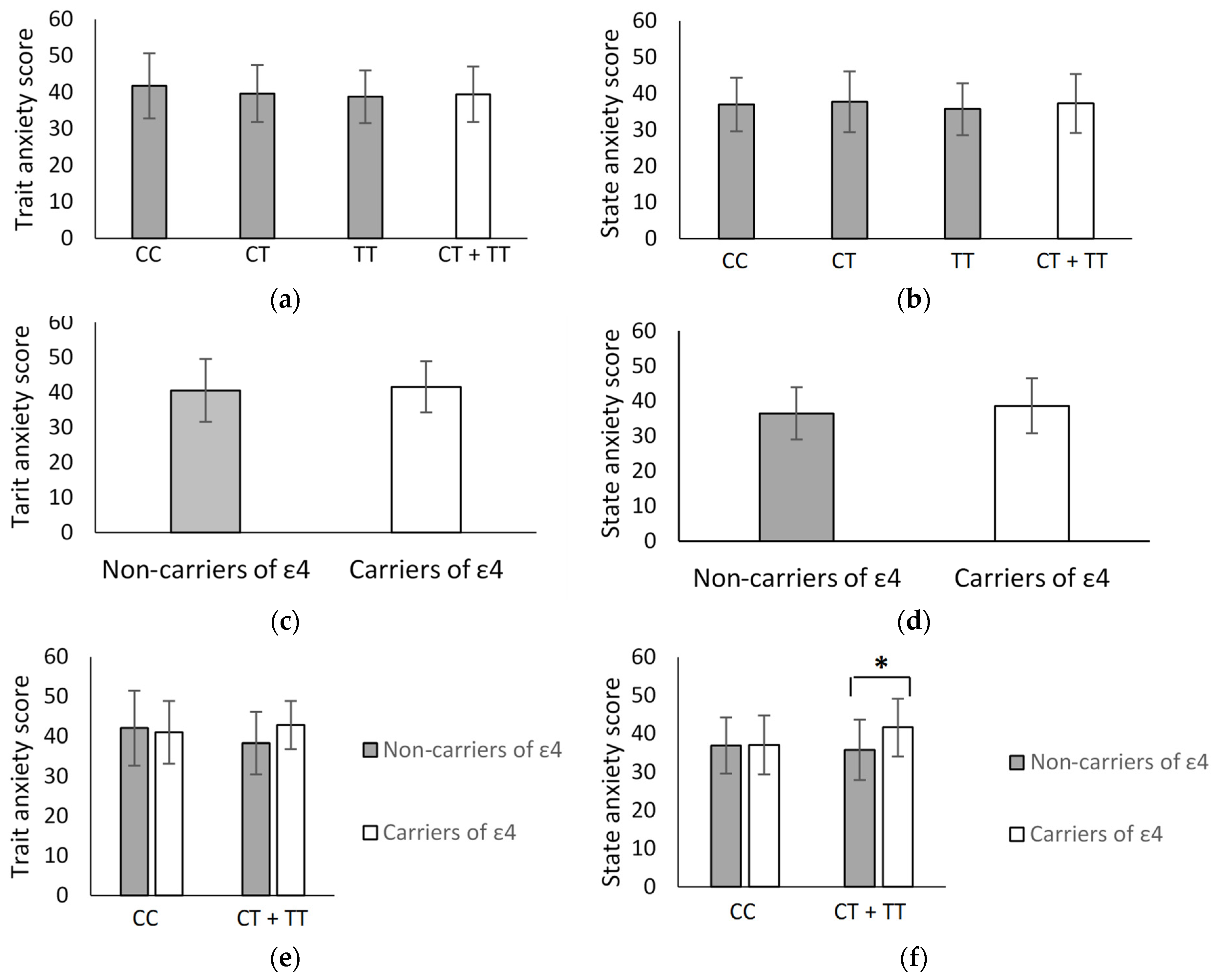
2.2. FKBP5 rs1360780 and ApoE ε2/ε3/ε4 in Association with Anxiety
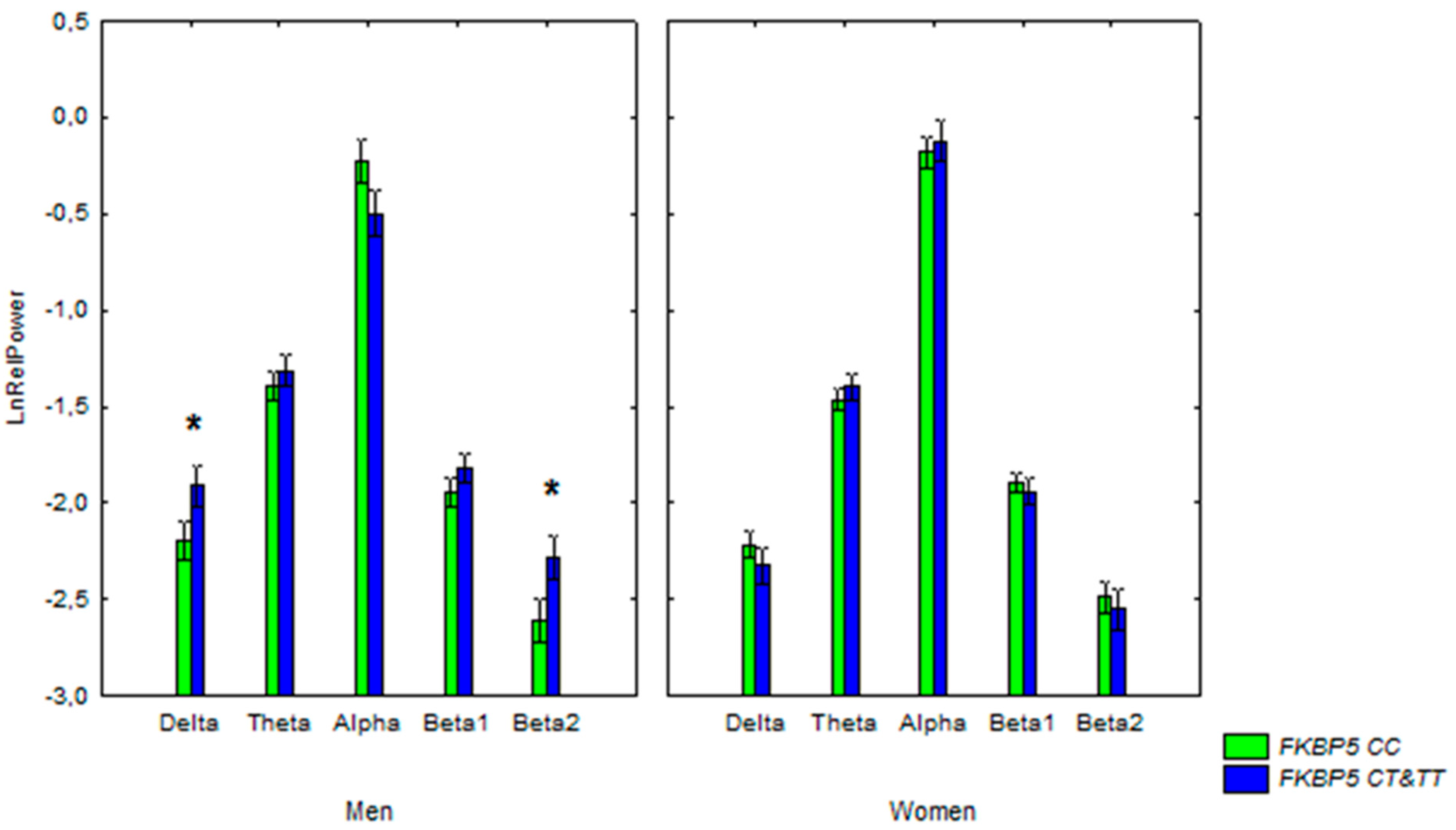
2.3. rs1360780 and ε4 Polymorphisms in Association with the Electrical Activity of the Brain
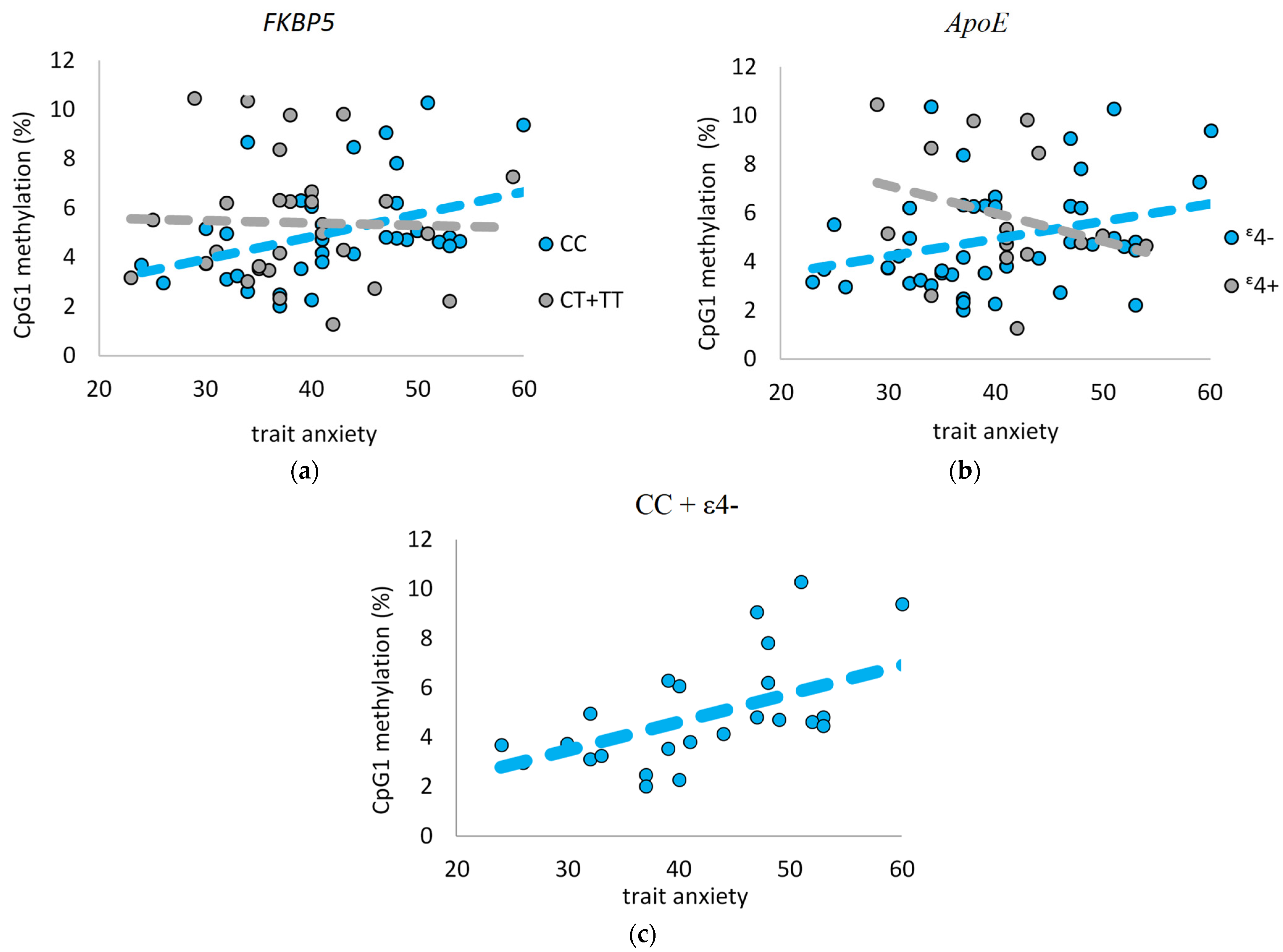
2.4. Interplay between FKBP5 Promotor Methylation and Anxiety
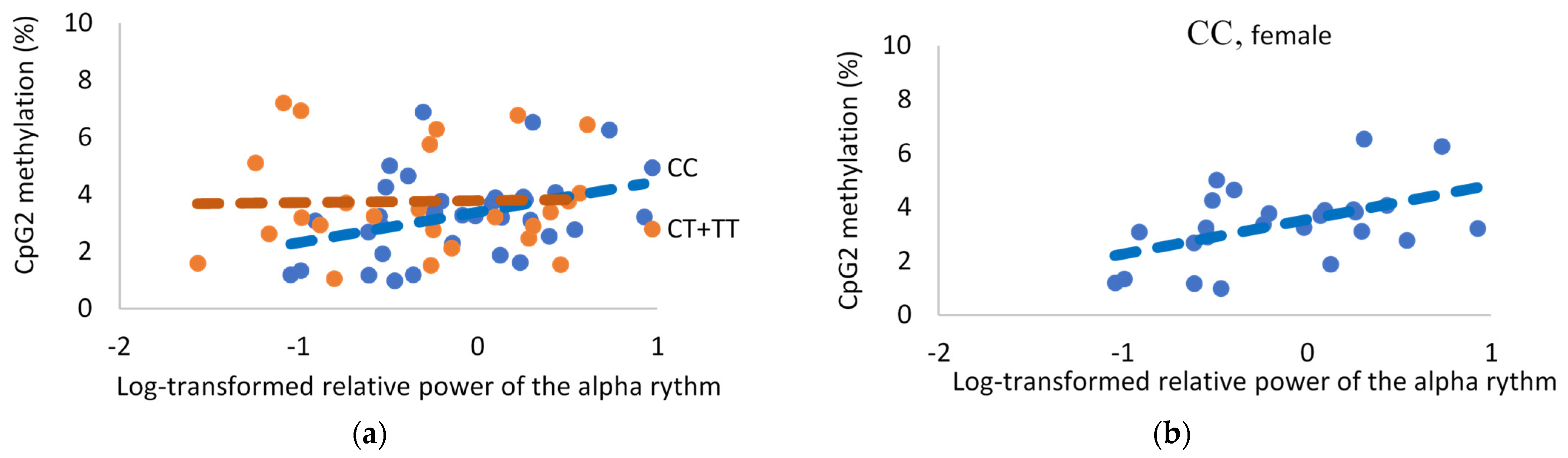
2.5. FKBP5 Methylation and EEG
2.5.1. Delta Rhythm
2.5.2. Theta Rhythm
2.5.3. Alpha Rhythm
2.5.4. Beta1 and Beta2 Rhythm
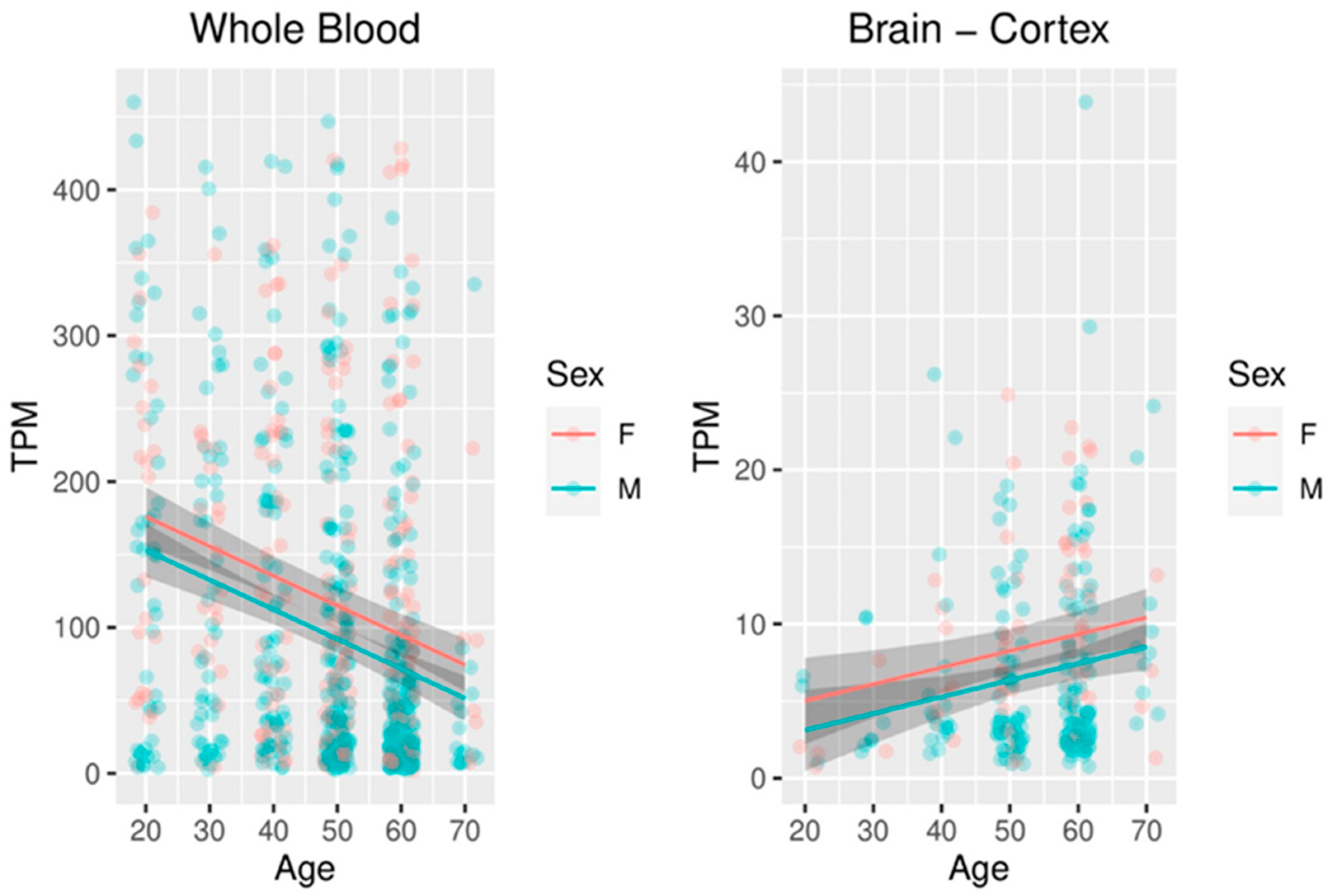
2.6. Meta-Analysis of FKBP5 Expression in the Blood and Brain
3. Discussion
4. Materials and Methods
4.1. Human Samples
4.2. Genotyping
4.3. Methylation
4.4. EEG Procedure
4.5. EEG Analysis
4.6. Statistical Analysis
4.7. FKBP5 Expression Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, S.; Yao, Y.; Ryan, J.; Li, T.; Wang, D.; Zheng, C.; Xu, Y.; Xu, Q. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: A comprehensive meta-analysis. Sci. Rep. 2016, 6, 32687. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B. Association of FKBP5 Polymorphisms and Childhood Abuse with Risk of Posttraumatic Stress Disorder Symptoms in Adults. JAMA J. Am. Med. Assoc. 2008, 299, 1291–1305. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Pütz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319–1325. [Google Scholar] [CrossRef]
- Szczepankiewicz, A.; Leszczyńska-Rodziewicz, A.; Pawlak, J.; Narozna, B.; Rajewska-Rager, A.; Wiłkość-Dębczyńska, M.; Zaremba, D.; Maciukiewicz, M.; Twarowska-Hauser, J. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J. Affect. Disord. 2014, 164, 33–37. [Google Scholar] [CrossRef]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.; Pariante, C.M.; Pace, T.W.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Tozzi, L.; Farrell, C.; Booij, L.; Doolin, K.; Nemoda, Z.; Szyf, M.; Pomares, F.B.; Chiarella, J.; O’Keane, V.; Frodl, T. Epigenetic Changes of FKBP5 as a Link Connecting Genetic and Environmental Risk Factors with Structural and Functional Brain Changes in Major Depression. Neuropsychopharmacology 2018, 43, 1138–1145. [Google Scholar] [CrossRef]
- Saito, T.; Shinozaki, G.; Koga, M.; Tanichi, M.; Takeshita, S.; Nakagawa, R.; Nagamine, M.; Cho, H.R.; Morimoto, Y.; Kobayashi, Y.; et al. Effect of interaction between a specific subtype of child abuse and the FKBP5 rs1360780 SNP on DNA methylation among patients with bipolar disorder. J. Affect. Disord. 2020, 272, 417–422. [Google Scholar] [CrossRef]
- Roberts, S.; Keers, R.; Breen, G.; Coleman, J.R.I.; Jöhren, P.; Kepa, A.; Lester, K.J.; Margraf, J.; Scheider, S.; Teismann, T.; et al. DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 150–158. [Google Scholar] [CrossRef]
- Roberts, S.; Keers, R.; Lester, K.; Coleman, J.; Breen, G.; Arendt, K.; Blatter-Meunier, J.; Cooper, P.; Creswell, C.; Fjermestad, K.; et al. Hpa Axis Related Genes and Response to Psychological Therapies: Genetics and Epigenetics. Depress. Anxiety 2015, 32, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, A.; Aiello, A.E.; Guffanti, G.; Galea, S.; Wildman, D.E.; Uddin, M. FKBP5 DNA methylation does not mediate the association between childhood maltreatment and depression symptom severity in the Detroit Neighborhood Health Study. J. Psychiatr. Res. 2018, 96, 39–48. [Google Scholar] [CrossRef]
- Alexander, N.; Kirschbaum, C.; Stalder, T.; Muehlhan, M.; Vogel, S. No association between FKBP5 gene methylation and acute and long-term cortisol output. Transl. Psychiatry 2020, 10, 175. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Xu, Y.; Wang, J.; Liu, M.; Dou, L.; Deng, R.; Wang, C.; Williams, K.E.; Stewart, R.B.; Xie, Z.; et al. Loss of FKBP5 Affects Neuron Synaptic Plasticity: An Electrophysiology Insight. Neuroscience 2019, 402, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Ota, M.; Hori, H.; Hattori, K.; Teraishi, T.; Sasayama, D.; Higuchi, T.; Kunugi, H. Association between the common functional FKBP5 variant (rs1360780) and brain structure in a non-clinical population. J. Psychiatr. Res. 2014, 58, 96–101. [Google Scholar] [CrossRef]
- Fani, N.; Gutman, D.; Tone, E.; Almli, L.; Mercer, K.B.; Davis, J.; Glover, E.; Jovanovic, T.; Bradley, B.; Dinov, I.; et al. FKBP5 and Attention Bias for Threat. JAMA Psychiatry 2013, 70, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Pagliaccio, D.; Luby, J.L.; Bogdan, R.; Agrawal, A.; Gaffrey, M.S.; Belden, A.; Botteron, K.N.; Harms, M.; Barch, D.M. Stress-System Genes and Life Stress Predict Cortisol Levels and Amygdala and Hippocampal Volumes in Children. Neuropsychopharmacology 2014, 39, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Zobel, A.; Schuhmacher, A.; Jessen, F.; Höfels, S.; Von Widdern, O.; Metten, M.; Pfeiffer, U.; Hanses, C.; Becker, T.; Rietschel, M.; et al. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int. J. Neuropsychopharmacol. 2010, 13, 649–660. [Google Scholar] [CrossRef]
- Zannas, A.S.; Jia, M.; Hafner, K.; Baumert, J.; Wiechmann, T.; Pape, J.C.; Arloth, J.; Ködel, M.; Martinelli, S.; Roitman, M.; et al. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB–driven inflammation and cardiovascular risk. Proc. Natl. Acad. Sci. USA 2019, 116, 11370–11379. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, J.J.; O’Leary, J.C.; Blair, L.J.; Klengel, T.; Nordhues, B.A.; Fontaine, S.N.; Binder, E.B.; Dickey, C.A. Age-Associated Epigenetic Upregulation of the FKBP5 Gene Selectively Impairs Stress Resiliency. PLoS ONE 2014, 9, e107241. [Google Scholar] [CrossRef]
- Gusev, F.E.; Reshetov, D.A.; Mitchell, A.C.; Andreeva, T.V.; Dincer, A.; Grigorenko, A.P.; Fedonin, G.; Halene, T.; Aliseychik, M.; Goltsov, A.Y.; et al. Epigenetic-genetic chromatin footprinting identifies novel and subject-specific genes active in prefrontal cortex neurons. FASEB J. 2019, 33, 8161–8173. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Imbimbo, B.P.; Santamato, A.; Vendemiale, G.; Seripa, D.; Pilotto, A.; et al. Late-Life Depression, Mild Cognitive Impairment, and Dementia: Possible Continuum? Am. J. Geriatr. Psychiatry 2010, 18, 98–116. [Google Scholar] [CrossRef]
- Mah, L.; Binns, M.; Steffens, D.C. Anxiety Symptoms in Amnestic Mild Cognitive Impairment Are Associated with Medial Temporal Atrophy and Predict Conversion to Alzheimer Disease. Am. J. Geriatr. Psychiatry 2015, 23, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Banning, L.C.; Ramakers, I.H.; Deckers, K.; Verhey, F.R.; Aalten, P. Apolipoprotein E and affective symptoms in mild cognitive impairment and Alzheimer’s disease dementia: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 302–315. [Google Scholar] [CrossRef]
- Delano-Wood, L.; Houston, W.S.; Emond, J.A.; Marchant, N.; Salmon, D.P.; Jeste, D.V.; Thal, L.J.; Bondi, M.W. APOE genotype predicts depression in women with Alzheimer’s disease: A retrospective study. Int. J. Geriatr. Psychiatry 2007, 23, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Averill, L.A.; Abdallah, C.G.; Levey, D.F.; Han, S.; Harpaz-Rotem, I.; Kranzler, H.R.; Southwick, S.M.; Krystal, J.H.; Gelernter, J.; Pietrzak, R.H. Apolipoprotein E gene polymorphism, posttraumatic stress disorder, and cognitive function in older U.S. veterans: Results from the National Health and Resilience in Veterans Study. Depress. Anxiety 2019, 36, 834–845. [Google Scholar] [CrossRef]
- Arlt, S.; Demiralay, C.; Tharun, B.; Geisel, O.; Storm, N.; Eichenlaub, M.; Lehmbeck, J.T.; Wiedemann, K.; Leuenberger, B.; Jahn, H. Genetic risk factors for depression in Alzheimer`s disease patients. Curr. Alzheimer Res. 2013, 10, 72–81. [Google Scholar] [CrossRef]
- Babiloni, C.; Del Percio, C.; Caroli, A.; Salvatore, E.; Nicolai, E.; Marzano, N.; Lizio, R.; Cavedo, E.; Landau, S.; Chen, K.; et al. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer’s disease: An EEG-PET study. Neurobiol. Aging 2016, 48, 122–134. [Google Scholar] [CrossRef]
- Howells, F.M.; Temmingh, H.S.; Hsieh, J.H.; Van Dijen, A.V.; Baldwin, D.S.; Stein, D. Electroencephalographic delta/α frequency activity differentiates psychotic disorders: A study of schizophrenia, bipolar disorder and methamphetamine-induced psychotic disorder. Transl. Psychiatry 2018, 8, 75. [Google Scholar] [CrossRef]
- Moretti, D.; Frisoni, G.; Fracassi, C.; Pievani, M.; Geroldi, C.; Binetti, G.; Rossini, P.; Zanetti, O. MCI patients’ EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol. Aging 2011, 32, 563–571. [Google Scholar] [CrossRef]
- Ponomareva, N.; Korovaitseva, G.; Rogaev, E. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol. Aging 2008, 29, 819–827. [Google Scholar] [CrossRef]
- Huang, C.; Wahlund, L.-O.; Dierks, T.; Julin, P.; Winblad, B.; Jelic, V. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clin. Neurophysiol. 2000, 111, 1961–1967. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Niciu, M.J.; Mathews, D.C.; Richards, E.M.; Zarate, C.A. Neurobiology of Anxious Depression: A Review. Depress. Anxiety 2013, 30, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Lesch, K.-P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Sheikh, H.I.; Kryski, K.R.; Kotelnikova, Y.; Hayden, E.P.; Singh, S.M. Catechol-O-Methyltransferase gene (val158met) polymorphisms and anxious symptoms in early childhood: The roles of hypothalamus-pituitary-adrenal axis reactivity and life stress. Neurosci. Lett. 2017, 659, 86–91. [Google Scholar] [CrossRef]
- Peskind, E.R.; Wilkinson, C.W.; Petrie, E.C.; Schellenberg, G.D.; Raskind, M.A. Increased CSF cortisol in AD is a function of APOE genotype. Neurology 2001, 56, 1094–1098. [Google Scholar] [CrossRef]
- Mota, N.P.; Han, S.; Harpaz-Rotem, I.; Maruff, P.; Krystal, J.H.; Southwick, S.M.; Gelernter, J.; Pietrzak, R.H. Apolipoprotein E gene polymorphism, trauma burden, and posttraumatic stress symptoms in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. Depress. Anxiety 2018, 35, 168–177. [Google Scholar] [CrossRef]
- Binder, E.B.; Bradley, R.G.; Liu, W.; Epstein, M.P.; Deveau, T.C.; Mercer, K.B.; Tang, Y.; Charles, F.; Heim, C.M.; Nemeroff, C.B.; et al. NIH Public Access. JAMA 2008, 299, 1291–1305. [Google Scholar] [CrossRef] [Green Version]
- Blair, L.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.; O’Leary, J.C.; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [Green Version]
- Malone, S.M.; Burwell, S.J.; Vaidyanathan, U.; Miller, M.B.; McGue, M.; Iacono, W.G. Activity: A Genome-Wide Association Study. Psychophysiology 2014, 51, 1225–1245. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, B.; O’Neil, K.; Berwise, C.; Stevens, M.; Calhoun, V.D.; Clementz, B.A.; Tamminga, C.A.; Sweeney, J.A.; Keshavan, M.S.; Pearlson, G.D. Resting State Electroencephalogram Oscillatory Abnormalities in Schizophrenia and Psychotic Bipolar Patients and Their Relatives from the Bipolar and Schizophrenia Network on Intermediate Phenotypes Study. Biol. Psychiatry 2014, 76, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Enoch, M.-A.; Shen, P.-H.; Ducci, F.; Yuan, Q.; Liu, J.; White, K.V.; Albaugh, B.; Hodgkinson, C.A.; Goldman, D. Common Genetic Origins for EEG, Alcoholism and Anxiety: The Role of CRH-BP. PLoS ONE 2008, 3, e3620. [Google Scholar] [CrossRef] [Green Version]
- Porjesz, B.; Rangaswamy, M. Neurophysiological Endophenotypes, CNS Disinhibition, and Risk for Alcohol Dependence and Related Disorders. Sci. World J. 2007, 7, 131–141. [Google Scholar] [CrossRef]
- Ponomareva, N.V.; Andreeva, T.V.; Protasova, M.S.; Shagam, L.I.; Malina, D.D.; Goltsov, A.Y.; Fokin, V.F.; Illarioshkin, S.N.; Rogaev, E.I. Quantitative EEG during normal aging: Association with the Alzheimer’s disease genetic risk variant in PICALM gene. Neurobiol. Aging 2017, 51, 177.e1–177.e8. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34, S186–S195. [Google Scholar] [CrossRef]
- Lavebratt, C.; Åberg, E.; Sjöholm, L.K.; Forsell, Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J. Affect. Disord. 2010, 125, 249–255. [Google Scholar] [CrossRef]
- Grad, I.; Picard, D. The glucocorticoid responses are shaped by molecular chaperones. Mol. Cell. Endocrinol. 2007, 275, 2–12. [Google Scholar] [CrossRef]
- Magee, J.A.; Chang, L.-W.; Stormo, G.D.; Milbrandt, J. Direct, Androgen Receptor-Mediated Regulation of the FKBP5 Gene via a Distal Enhancer Element. Endocrinology 2006, 147, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Reul, J.M.; Collins, A.; Saliba, R.S.; Mifsud, K.; Carter, S.D.; Gutierrez-Mecinas, M.; Qian, X.; Linthorst, A.C. Glucocorticoids, epigenetic control and stress resilience. Neurobiol. Stress 2015, 1, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Klengel, T.; Binder, E.B. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron 2015, 86, 1343–1357. [Google Scholar] [CrossRef] [Green Version]
- Golenkina, S.A.; Gol’Tsov, A.I.; Kuznetsova, I.L.; Grigorenko, A.P.; Andreeva, T.V.; Reshetov, D.A.; Kunizheva, S.S.; Shagam, L.I.; Morozova, I.I.; Goldenkova-Pavlova, I.V.; et al. Analysis of clusterin gene (CLU/APOJ) polymorphism in Alzheimer’s disease patients and in normal cohorts from Russian populations. Mol. Biol. 2010, 44, 620–626. [Google Scholar] [CrossRef] [Green Version]
- John, E.R.; Ahn, H.; Prichep, L.; Trepetin, M.; Brown, D.; Kaye, H. Developmental Equations for the Electroencephalogram. Science 1980, 210, 1255–1258. [Google Scholar] [CrossRef]
| Group | Genotype Frequency | Allele Frequency | |||
|---|---|---|---|---|---|
| CC | CT | TT | C | T | |
| ND | 0.582 | 0.353 | 0.065 | 0.759 | 0.241 |
| Nona+ | 0.580 | 0.370 | 0.050 | 0.765 | 0.235 |
| Patients with AD (total) | 0.556 | 0.367 | 0.077 | 0.740 | 0.260 |
| Patients with AD without depression | 0.617 | 0.290 | 0.093 | 0.762 | 0.238 |
| Patients with AD with depression | 0.495 | 0.434 | 0.071 | 0.712 | 0.288 |
| Group | Number | Age Mean ± SD (Age Range) | ||||
|---|---|---|---|---|---|---|
| Total | Females | Males | Total | Females | Males | |
| ND | 479 | 214 | 265 | 62.07 ± 18.03 (19–89) | 58.23 ± 16.77 (19–89) | 65.17 ± 18.45 (19–88) |
| Nona+ | 100 | 76 | 24 | 97.00 ± 4.49 (90–107) | 97.30 ± 4.48 (90–107) | 97.00 ± 4.60 (90–105) |
| AD | 221 | 156 | 65 | 67.00 ± 8.75 (41–87) | 66.82 ± 9.12 (41–87) | 68.00 ± 7.64 (52–84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, I.L.; Ponomareva, N.V.; Alemastseva, E.A.; Manakhov, A.D.; Andreeva, T.V.; Gusev, F.E.; Rogaev, E.I. The Interactive Effect of Genetic and Epigenetic Variations in FKBP5 and ApoE Genes on Anxiety and Brain EEG Parameters. Genes 2022, 13, 164. https://doi.org/10.3390/genes13020164
Kuznetsova IL, Ponomareva NV, Alemastseva EA, Manakhov AD, Andreeva TV, Gusev FE, Rogaev EI. The Interactive Effect of Genetic and Epigenetic Variations in FKBP5 and ApoE Genes on Anxiety and Brain EEG Parameters. Genes. 2022; 13(2):164. https://doi.org/10.3390/genes13020164
Chicago/Turabian StyleKuznetsova, Irina L., Natalya V. Ponomareva, Ekaterina A. Alemastseva, Andrey D. Manakhov, Tatyana V. Andreeva, Fedor E. Gusev, and Evgeny I. Rogaev. 2022. "The Interactive Effect of Genetic and Epigenetic Variations in FKBP5 and ApoE Genes on Anxiety and Brain EEG Parameters" Genes 13, no. 2: 164. https://doi.org/10.3390/genes13020164
APA StyleKuznetsova, I. L., Ponomareva, N. V., Alemastseva, E. A., Manakhov, A. D., Andreeva, T. V., Gusev, F. E., & Rogaev, E. I. (2022). The Interactive Effect of Genetic and Epigenetic Variations in FKBP5 and ApoE Genes on Anxiety and Brain EEG Parameters. Genes, 13(2), 164. https://doi.org/10.3390/genes13020164







