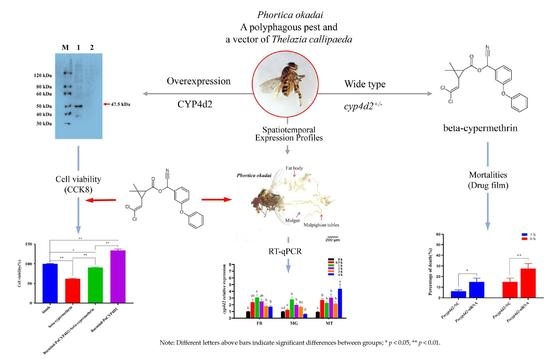
Identification and Functional Characterization of CYP4D2 Putatively Associated with β-Cypermethrin Detoxification in Phortica okadai
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Total RNA Extraction, Sequencing, and CYP450 Gene Identification
2.3. Cloning of the Upregulated CYP450 Genes
2.4. Bioinformatics Analysis
2.5. Expression Profiles of Three CYP450 Genes
2.6. Functional Expression of Pocyp4d2 in S2
2.7. Functional Analysis of Pocyp4d2 by RNAi
2.8. Data Analysis
3. Results
3.1. Identification of Upregulated CYP450 Genes by RNA Sequencing in P. okadai
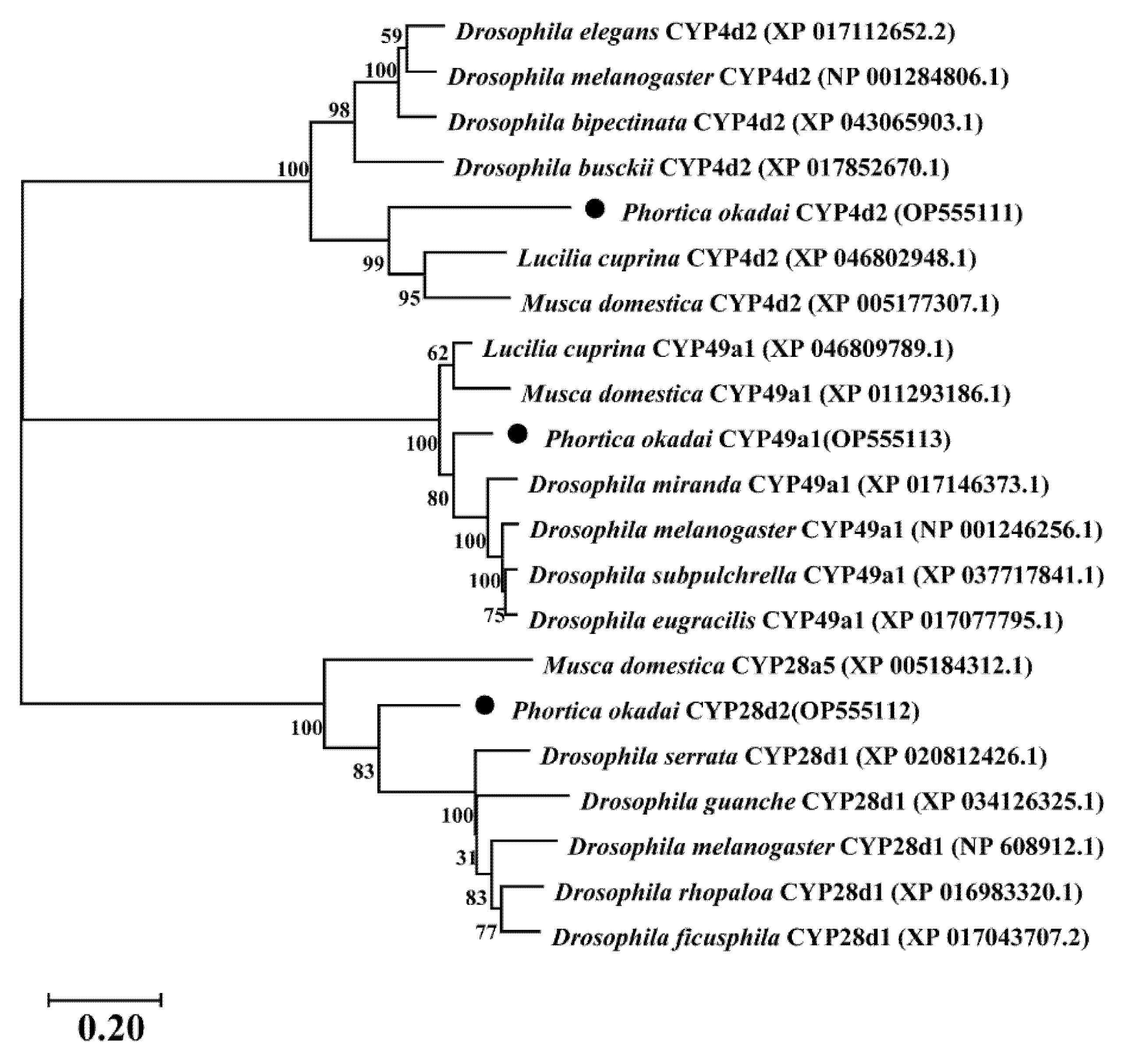
3.2. Cloning and Bioinformatic Analysis of Pocyp4d2, Pocyp49a1, and Pocyp28d2
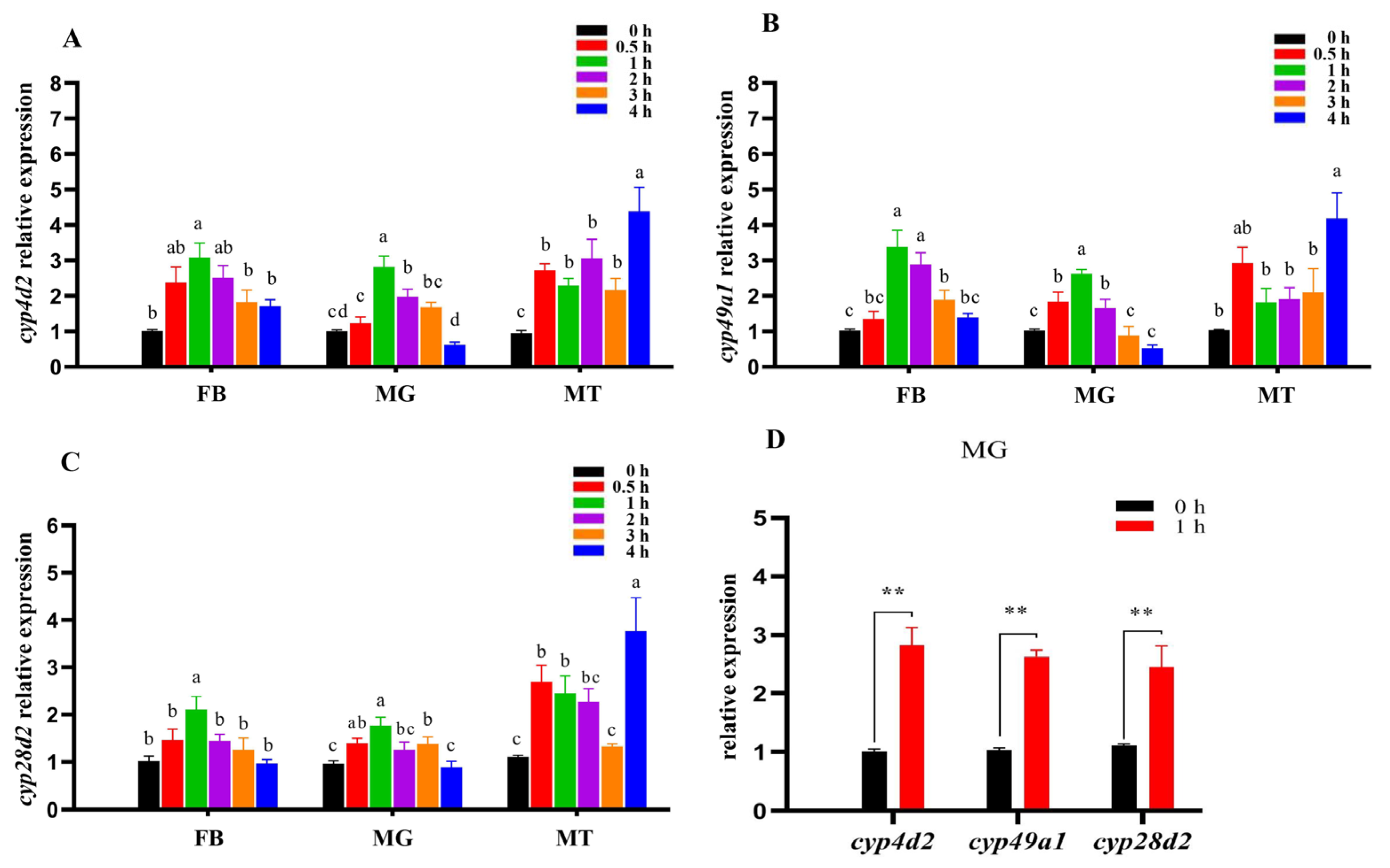
3.3. Expression Profiles of Pocyp4d2, Pocyp49a1, and Pocyp28d2 following β-Cypermethrin Exposure
3.4. Functional Expression of Pocyp4d2 in S2 Cells
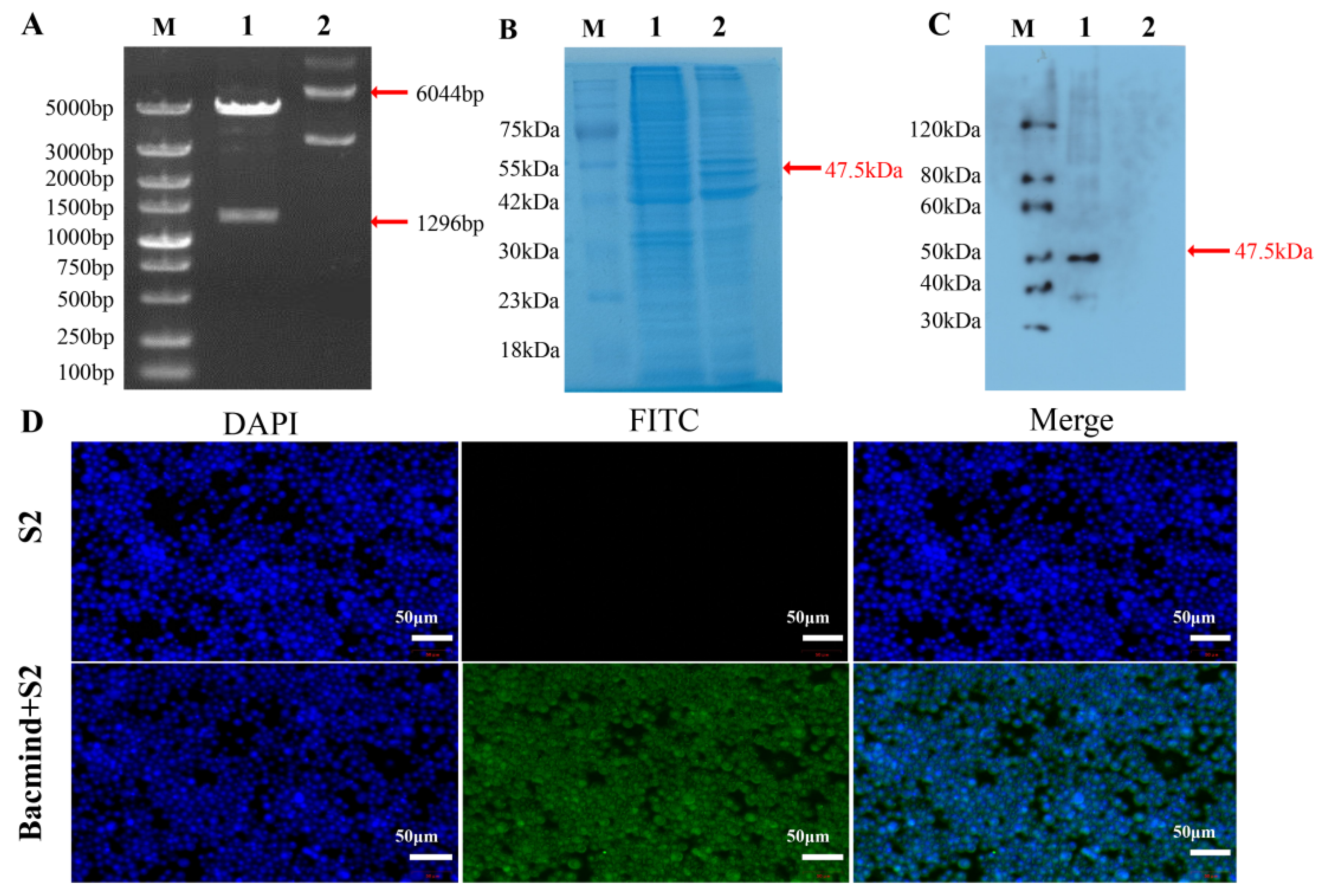
3.4.1. Identification of the Recombinant Plasmid pFastBac1-Pocyp4d2 and the PoCYP4D2 Protein
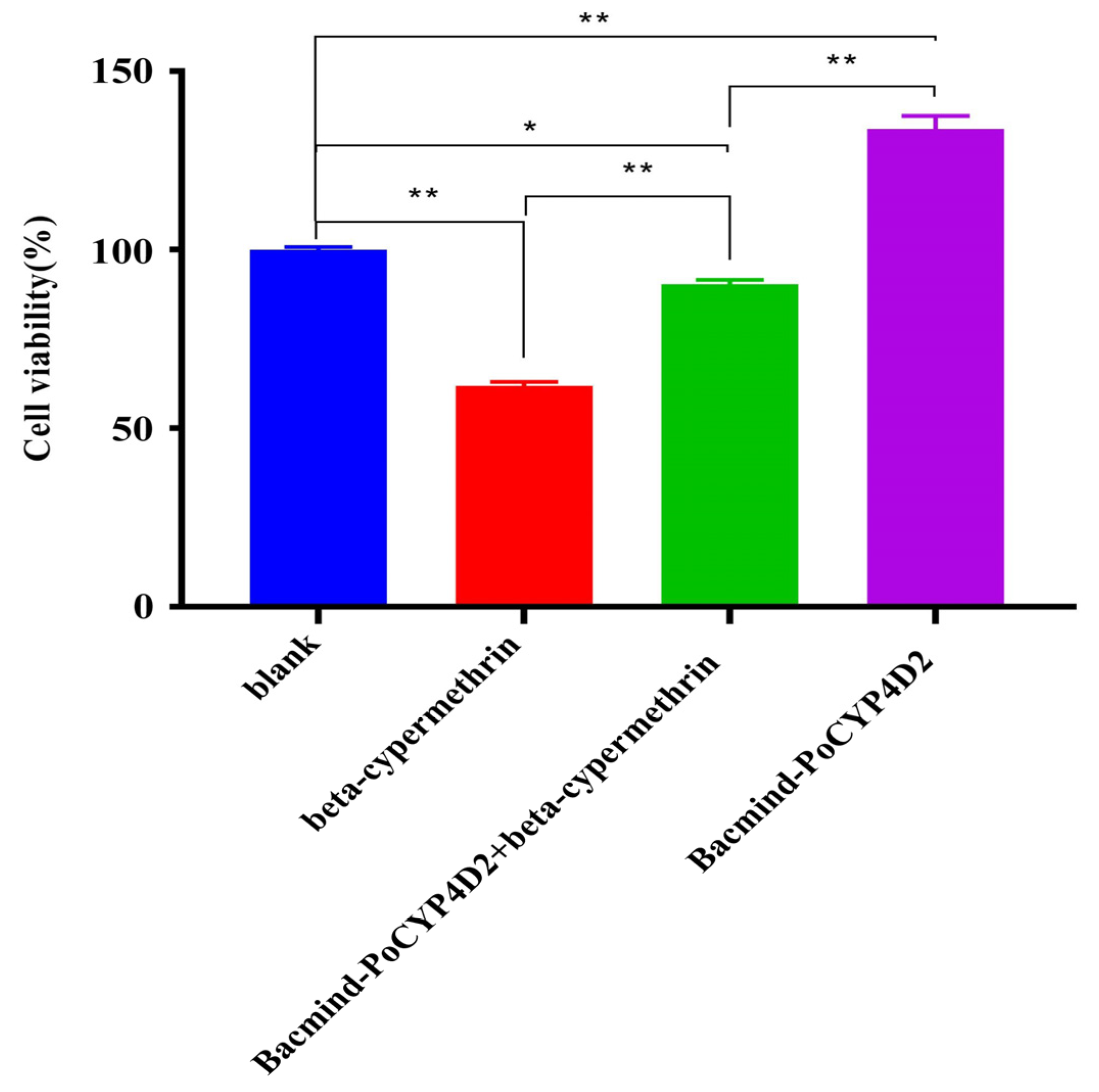
3.4.2. Metabolic Detoxification Function of the Recombinant Protein PoCYP4D2 in S2 Cells
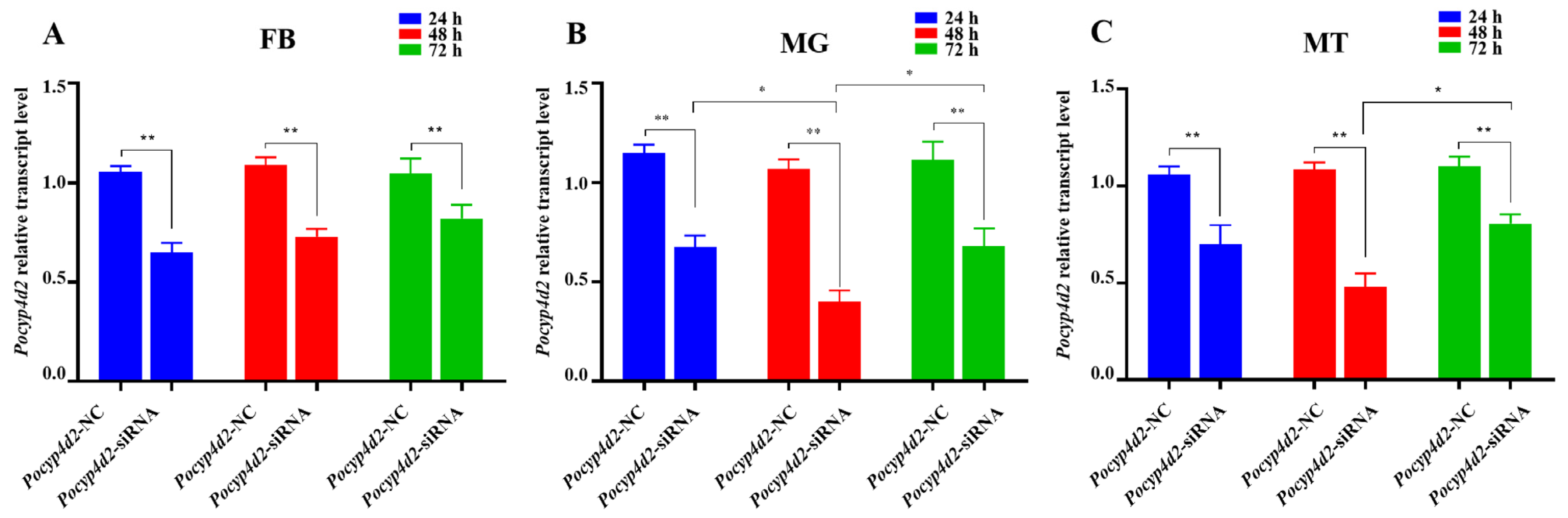
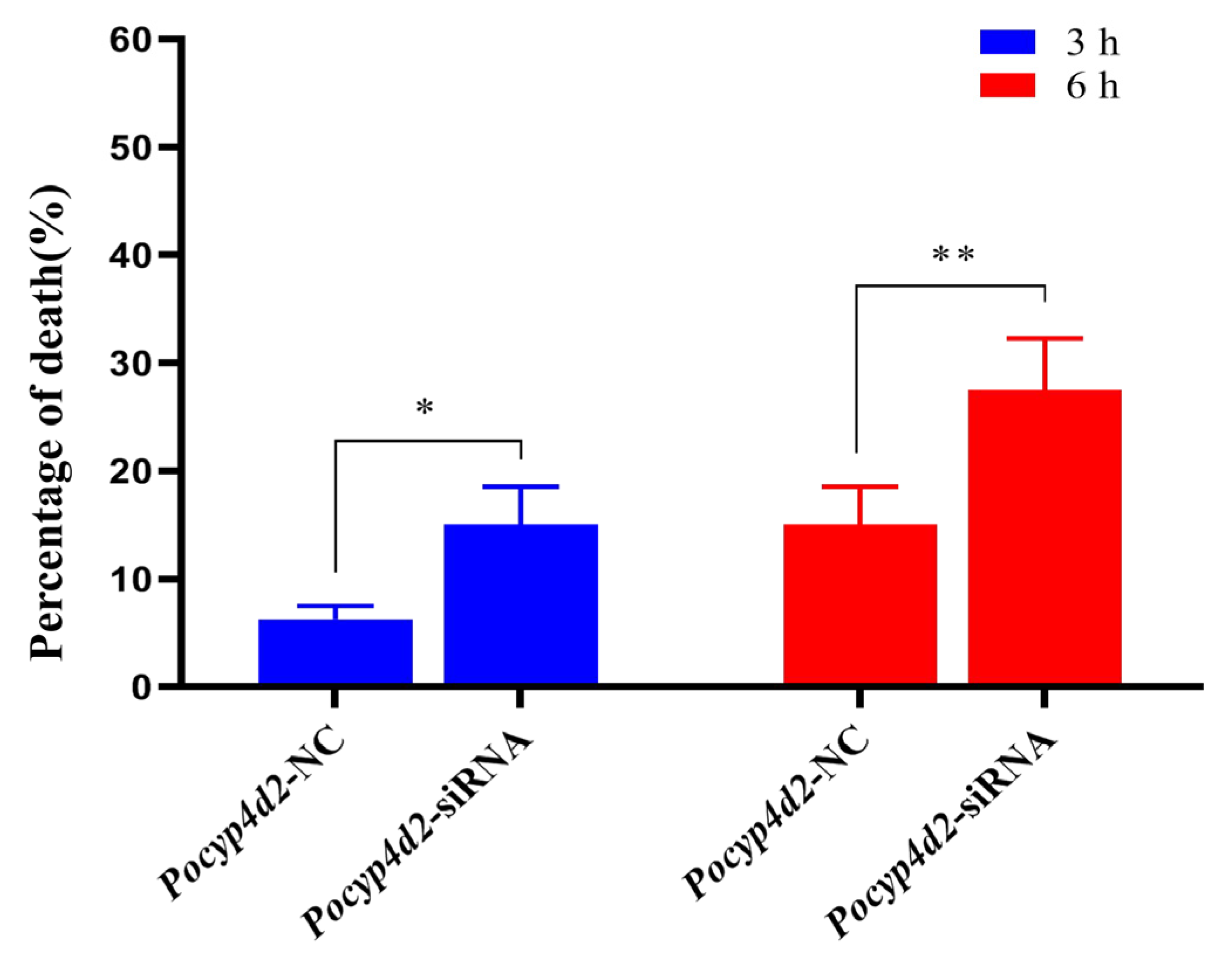
3.5. Functional Analysis of Pocyp4d2 by siRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Li, D.; Yin, C.; Tang, H.; Luo, B.; Yan, R.; Shen, Y.; Liu, H. Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts. Pathogens 2022, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Bernardini, I.; Lia, R.P.; Mendoza-Roldan, J.A.; Beugnet, F.; Pombi, M.; Otranto, D. Phortica oldenbergi (Diptera: Drosophilidae): A new potential vector of the zoonotic Thelazia callipaeda eyeworm. Acta Trop. 2022, 233, 106565. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Testini, G.; Lia, R.P. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: Evidence for pathogen transmission by a male arthropod vector. Int. J. Parasitol. 2006, 36, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Moroni, B.; Mendoza-Roldan, J.A.; Perrucci, S.; Cavicchio, P.; Cordon, R.; Cianfanelli, C.; Lia, R.P.; Rossi, L.; Otranto, D. Wild carnivores and Thelazia callipaeda zoonotic eyeworms: A focus on wolves. Int. J. Parasitol. Parasites Wildl. 2022, 17, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cabanova, V.; Miterpakova, M.; Oravec, M.; Hurnikova, Z.; Jerg, S.; Nemcikova, G.; Červenská, M. Nematode Thelazia callipaeda is spreading across Europe. The first survey of red foxes from Slovakia. Acta Parasitol. 2018, 63, 160–166. [Google Scholar] [CrossRef]
- Liu, S.N.; Xu, F.F.; Chen, W.Q.; Jiang, P.; Cui, J.; Wang, Z.Q.; Zhang, X. A Case of Human Thelaziasis and Review of Chinese Cases. Acta Parasitol. 2020, 65, 783–786. [Google Scholar] [CrossRef]
- González, M.A.; Bravo-Barriga, D.; Alarcón-Elbal, P.M.; Álvarez-Calero, J.M.; Quero, C.; Ferraguti, M.; López, S. Development of Novel Management Tools for Phortica variegata (Diptera: Drosophilidae), Vector of the Oriental Eyeworm, Thelazia callipaeda (Spirurida: Thelaziidae), in Europe. J. Med. Entomol. 2022, 59, 328–336. [Google Scholar] [CrossRef]
- Fotakis, E.A.; Mastrantonio, V.; Grigoraki, L.; Porretta, D.; Puggioli, A.; Chaskopoulou, A.; Osório, H.; Weill, M.; Bellini, R.; Urbanelli, S.; et al. Identification and detection of a novel point mutation in the Chitin Synthase gene of Culex pipiens associated with diflubenzuron resistance. PLoS Negl. Trop. Dis. 2020, 14, e0008284. [Google Scholar] [CrossRef]
- Chouaïbou, M.S.; Fodjo, B.K.; Fokou, G.; Allassane, O.F.; Koudou, B.G.; David, J.P.; Antonio-Nkondjio, C.; Ranson, H.; Bonfoh, B. Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Côte d’Ivoire. Malar. J. 2016, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey, P.V.; Justin, K.D.; Michael, C.W.; Christopher, D.C.; Michael, B.H. Permethrin Susceptibility for the Vector Culex tarsalis and a Nuisance Mosquito Aedes vexans in an Area Endemic for West Nile Virus. Biomed. Res. Int. 2018, 2018, 2014764. [Google Scholar]
- Chen, J.; Aimanova, K.G.; Gill, S.S. Aedes cadherin receptor that mediates Bacillus thuringiensis Cry11A toxicity is essential for mosquito development. PLoS Negl. Trop. Dis. 2020, 14, e0007948. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.H.; Zhang, Y.B.; Shu, J.P.; Wu, H.; Wang, H.J. Methodology. RNA-sequencing analysis of fungi-induced transcripts from the bamboo wireworm Melanotus cribricollis (Coleoptera: Elateridae) larvae. PLoS ONE 2018, 13, e0191187. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.J.; Leong, Y.Q.; Bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: A review. Parasit. Vectors 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, X.; Lan, M.; Yuan, Y.; Guo, Z.; Tang, P.; Li, M.; Liao, X.; Zhu, J.; Li, Z.; et al. De novo transcriptome analysis and identification of genes associated with immunity, detoxification and energy metabolism from the fat body of the tephritid gall fly, Procecidochares utilis. PLoS ONE 2019, 14, e0226039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, e8773. [Google Scholar] [CrossRef]
- Liska, D.J. The detoxification enzyme systems. Altern. Med. Rev. 1998, 3, 187–198. [Google Scholar] [PubMed]
- Zhen, C.; Tan, Y.; Miao, L.; Wu, J.; Gao, X. Overexpression of cytochrome P450s in a lambda-cyhalothrin resistant population of Apolygus lucorum (Meyer-Dür). PLoS ONE 2018, 13, e0198671. [Google Scholar] [CrossRef] [PubMed]
- Grant, F.; Jeremy, N.M.; Cam, D. The ABCB Multidrug Resistance Proteins Do Not Contribute to Ivermectin Detoxification in the Colorado Potato Beetle, Leptinotarsa decemlineata (Say). Insects 2020, 11, 135. [Google Scholar]
- Zhou, Y.; Fu, W.B.; Si, F.L.; Yan, Z.T.; Zhang, Y.J.; He, Q.Y.; Chen, B. UDP-glycosyltransferase genes and their association and mutations associated with pyrethroid resistance in Anopheles sinensis (Diptera: Culicidae). Malar. J. 2019, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.G.; Zhang, L.F.; Wang, L.J.; Zheng, M.H.; Liu, H. The zoophilic fruitfly Amiota okadai in Zunyi City: Flies capture, morphology identification and laboratory breeding. J. Med. Pest Control. 2017, 33, 765–766+770. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 7, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.F.; Dai, Z.H.; Feng, T.Z.; Zhou, L.; Tang, W.L.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J. Study on The Expression Characteristics of CYP450 Genes in Phortica Okadai Following Treatment with β-Cypermethrin; Zunyi Medical University: Zunyi, China, 2019; pp. 21–28. [Google Scholar]
- Ruijter, J.M.; Barnewall, R.J.; Marsh, I.B.; Szentirmay, A.N.; Quinn, J.C.; van Houdt, R.; Gunst, Q.D.; van den Hoff, M.J.B. Efficiency Correction Is Required for Accurate Quantitative PCR Analysis and Reporting. Clin. Chem. 2021, 67, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Wei, J.; Wen, Y.; He, N.; Tang, L.; Lin, D.; Lin, J. A first report of Thelazia callipaeda infection in Phortica okadai and wildlife in national nature reserves in China. Parasit. Vectors 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Marino, V.; Gálvez, R.; Colella, V.; Sarquis, J.; Checa, R.; Montoya, A.; Barrera, J.P.; Domínguez, S.; Lia, R.P.; Otranto, D.; et al. Detection of Thelazia callipaeda in Phortica variegata and spread of canine thelaziosis to new areas in Spain. Parasit. Vectors 2018, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Paim, R.M.M.; Pessoa, G.C.D.; Nascimento, B.W.L.; Nascimento, A.M.D.; Pinheiro, L.C.; Koerich, L.B.; Diotaiuti, L.; Araujo, R.N.; Sant’Anna, M.R.V.; Gontijo, N.F.; et al. Effect of salivary CYP4EM1 and CYP4EM2 gene silencing on the life span of Chagas disease vector Rhodnius prolixus (Hemiptera, Reduviidae) exposed to sublethal dose of deltamethrin. Insect Mol. Biol. 2022, 31, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.W.; He, Z.B.; Yan, Z.T.; Si, F.L.; Zhou, Y.; Chen, B. Genome-wide and expression-profiling analyses suggest the main cytochrome P450 genes related to pyrethroid resistance in the malaria vector, Anopheles sinensis (Diptera Culicidae). Pest Manag. Sci. 2018, 74, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Rajak, P.; Dutta, M.; Khatun, S.; Mandi, M.; Roy, S. Exploring hazards of acute exposure of Acephate in Drosophila melanogaster and search for Lascorbic acid mediated defense in it. J. Hazard. Mater. 2017, 321, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Zhu, S.K.; Baerson, S.R.; Xin, X.W.; Li, J.; Su, Y.J.; Zeng, R.S. Identification of a novel cytochrome P450 CYP321B1 gene from tobacco cutworm (Spodoptera litura) and RNA interference to evaluate its role in commonly used insecticides. Insect Sci. 2016, 24, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, J.; Wu, H.; Zhang, H.; Zhang, J.; Ma, E. Knockdown of cytochrome P450 CYP6 family genes increases susceptibility to carbamates and pyrethroids in the migratory locust, Locusta migratoria. Chemosphere 2019, 223, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Terhzaz, S.; Cabrero, P.; Brinzer, R.A.; Halberg, K.A.; Dow, J.A.; Davies, S.A. A novel role of Drosophila cytochrome P450-4e3 in permethrin insecticide tolerance. Insect Biochem. Mol. Biol. 2015, 67, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.N.B.; Kalogeridi, M.; Vontas, J.; Denecke, S. Using tissue specific P450 expression in Drosophila melanogaster larvae to understand the spatial distribution of pesticide metabolism in feeding assays. Insect Mol. Biol. 2022, 31, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, Y.; Hu, J.; Wang, Y.; Zhao, Z.; Ma, R.; Gao, L.; Guo, Y. Identification and Characterization of CYP6 Family Genes from the Oriental Fruit Moth (Grapholita molesta) and Their Responses to Insecticides. Insects 2022, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Maibeche, C.M.; Jacquin, J.E. cDNA claning of biotrans formation enzymes belonging to the cytochrome P450 family in the antenase of the noctuid moth Mamestra Brassice. Inset Mol. Biol. 2012, 11, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Yuan, Y.F.; Wu, L.; Chen, M. Effects of host plants on the feeding behavior and detoxification enzyme activities in Hyphantria cunea (Lepidoptera: Arctiidae) larvae. Acta Entomol. Sin. 2018, 61, 232–239. [Google Scholar]
- Pan, Z.Y.; Mo, X.N.; Meng, X.; Chen, M. Effects of chlorogenic acid on the growth and development and detoxification-related(Lepidoptera: Arctiidae) larvae. Acta Entomol. Sin. 2020, 63, 1081–1090. [Google Scholar]
- Hembrom, P.S.; Jose, J.; Grace, T. Detection of Variation in Expression of Insecticide Resistance Gene Cyp4d2 involved in Detoxification of Insecticides in Drosophila melanogaster. Res. J. Pharm. Technol. 2019, 12, 5315–5319. [Google Scholar] [CrossRef]
- Dai, W.T.; Li, J.; Ban, L.P. Genome-Wide Selective Signature Analysis Revealed Insecticide Resistance Mechanisms in Cydia pomonella. Insects 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, T.; Feng, X.; Li, M.; Liu, S.; Liu, N. Multiple cytochrome P450 genes: Conferring high levels of permethrin resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2021, 11, 9041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fan, X.; Gao, Y.; Yang, J.; Qian, J.; Fan, D. Identification of two novel P450 genes and their responses to deltamethrin in the cabbage moth, Mamestra brassicae Linnaeus. Pestic. Biochem. Physiol. 2016, 141, 76–83. [Google Scholar] [CrossRef]
- Elzaki, M.E.A.; Miah, M.A.; Peng, Y.; Zhang, H.; Jiang, L.; Wu, M.; Han, Z. Deltamethrin is metabolized by CYP6FU1, a cytochrome P450 associated with pyrethroid resistance, in Laodelphax striatellus. Pest Manag. Sci. 2018, 74, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ni, M.; Zhang, H.; Wang, B.; Xu, K.; Tian, J.; Hu, J.; Shen, W.; Li, B. Expression profile analysis of silkworm P450 family genes after phoxim induction. Pestic. Biochem. Physiol. 2014, 122, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Foster, D.J.; Davey, A.K.; Muhlhausler, B.S. Rosiglitazone Metabolism in Human Liver Microsomes Using a Substrate Depletion Method. Drugs R&D 2017, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tang, H.; Xie, Z.; Li, D.; Yin, C.; Luo, B.; Yan, R.; Sun, W.; Liu, H. Identification and Functional Characterization of CYP4D2 Putatively Associated with β-Cypermethrin Detoxification in Phortica okadai. Genes 2022, 13, 2338. https://doi.org/10.3390/genes13122338
Wang L, Tang H, Xie Z, Li D, Yin C, Luo B, Yan R, Sun W, Liu H. Identification and Functional Characterization of CYP4D2 Putatively Associated with β-Cypermethrin Detoxification in Phortica okadai. Genes. 2022; 13(12):2338. https://doi.org/10.3390/genes13122338
Chicago/Turabian StyleWang, Lingjun, Hongri Tang, Zhimei Xie, Di Li, Changzhu Yin, Bo Luo, Rong Yan, Wei Sun, and Hui Liu. 2022. "Identification and Functional Characterization of CYP4D2 Putatively Associated with β-Cypermethrin Detoxification in Phortica okadai" Genes 13, no. 12: 2338. https://doi.org/10.3390/genes13122338
APA StyleWang, L., Tang, H., Xie, Z., Li, D., Yin, C., Luo, B., Yan, R., Sun, W., & Liu, H. (2022). Identification and Functional Characterization of CYP4D2 Putatively Associated with β-Cypermethrin Detoxification in Phortica okadai. Genes, 13(12), 2338. https://doi.org/10.3390/genes13122338