Transcriptomic Study of Spermatogenesis in the Testis of Hu Sheep and Tibetan Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Preparation and Data Analysis of Testicular Tissue Sections
2.4. RNA Extraction, cDNA Library Preparation, and RNA-Seq
2.5. Gene Expression Quantification and Identification of Differentially Expressed Genes (DEGs)
2.6. Functional Enrichment Analysis of DEGs
2.7. RT-qPCR Analysis for the Validation of RNA-Seq Data
3. Results
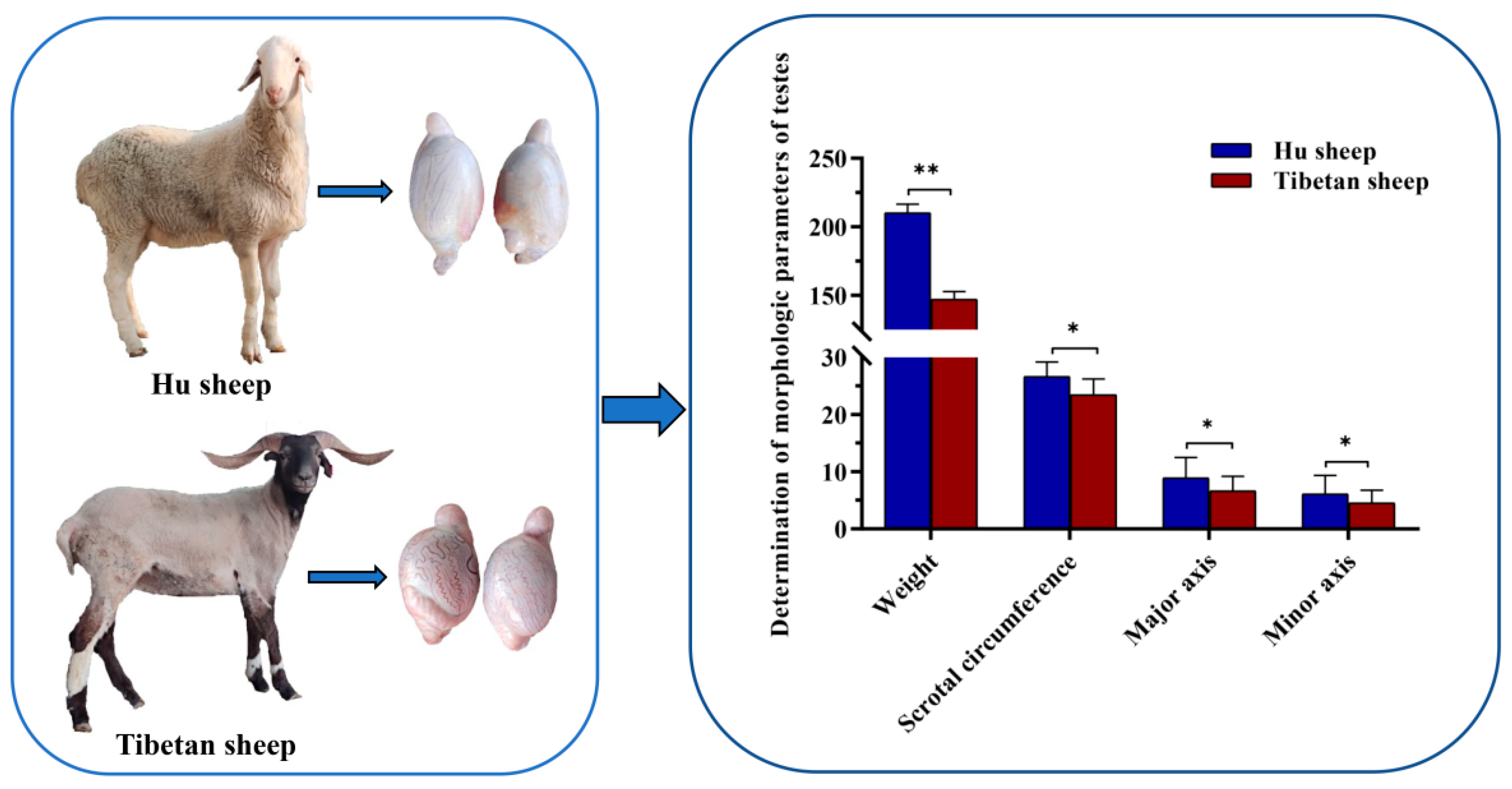
3.1. Morphologic Parameters of Testis
3.2. Histological Observation of Testis
3.3. Summary of the RNA-Seq Data
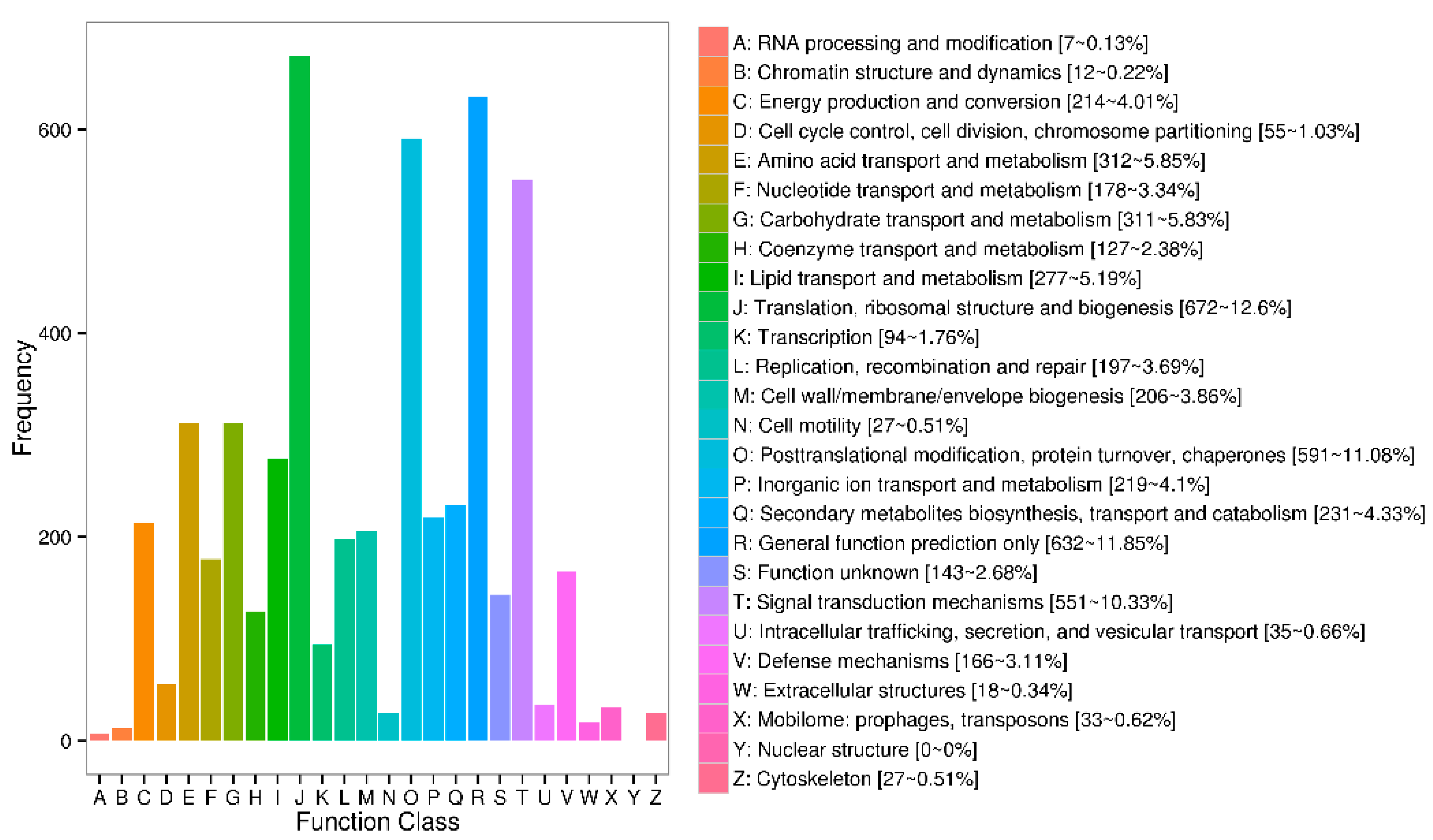
3.4. Functional Categories of the Identified Genes
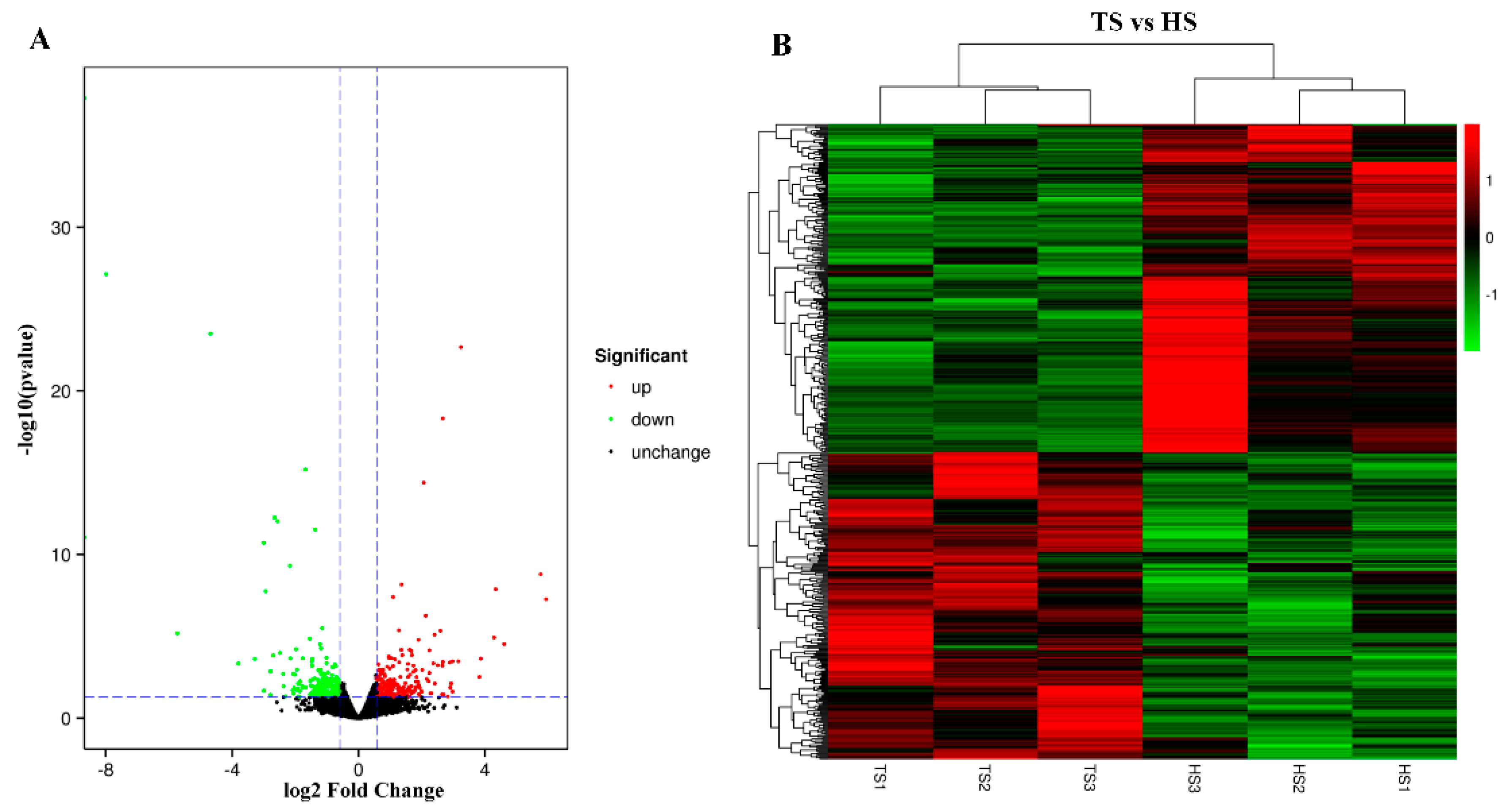
3.5. Analysis of Differentially Expressed Genes
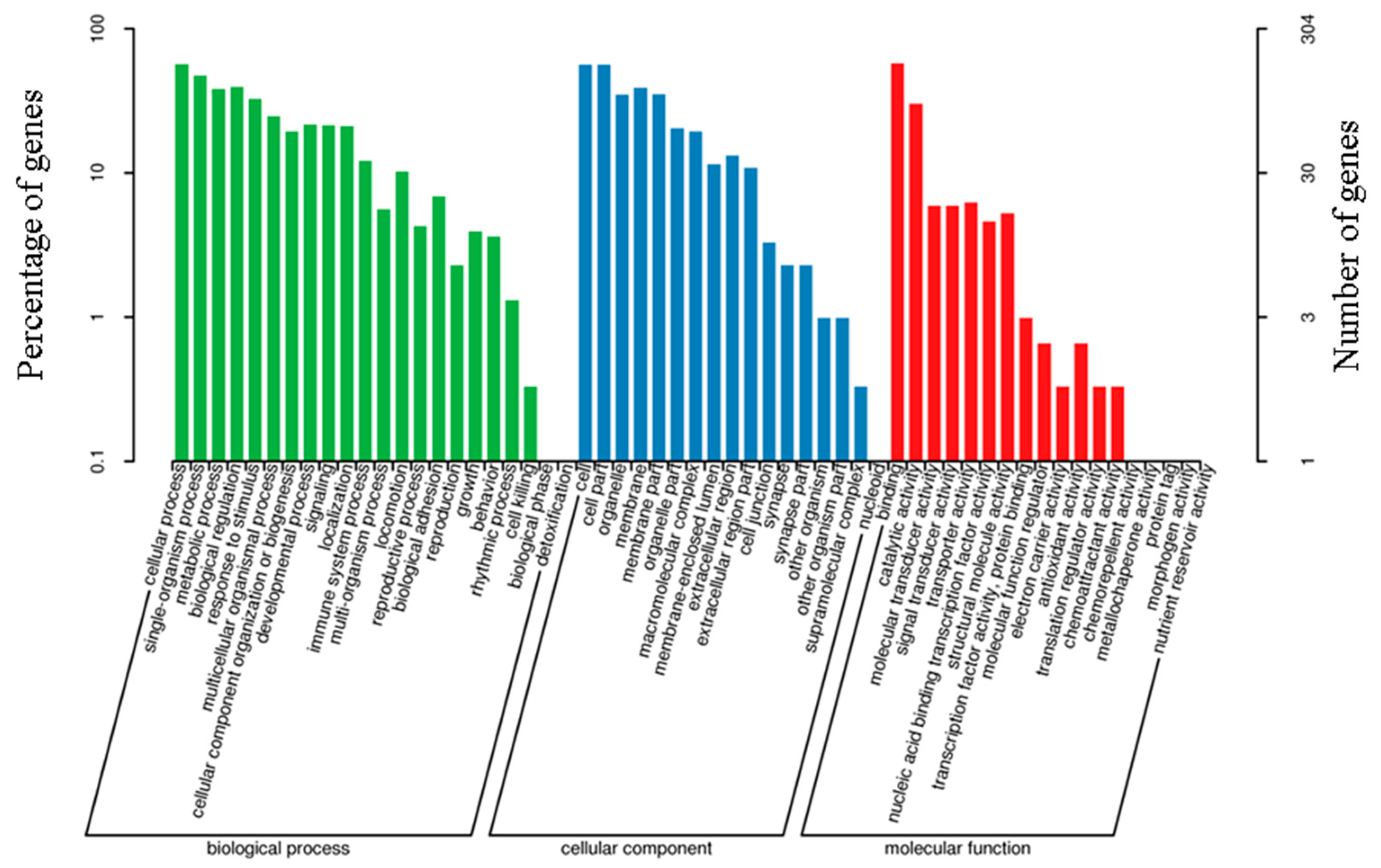
3.6. GO Enrichment Analysis of DEGs
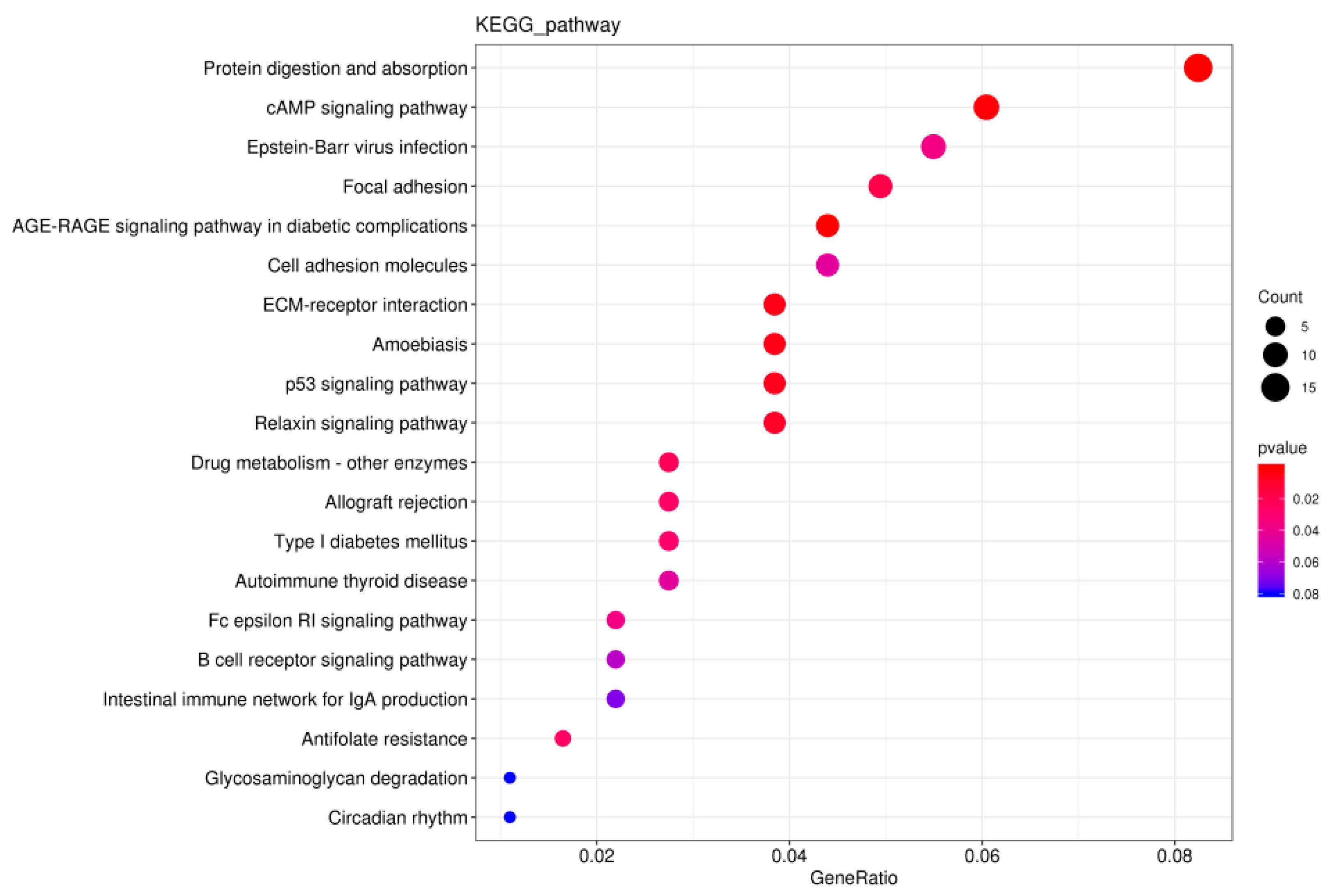
3.7. KEGG Pathway Enrichment Analysis of DEGs
3.8. Protein–Protein Interaction Network Analysis
3.9. Validation of RNA-Seq Data by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tornero, C.; Balasse, M.; Molist, M.; Saa, M. Seasonal reproductive patterns of early domestic sheep at Tell Halula (PPNB, Middle Euphrates Valley): Evidence from sequential oxygen isotope analyses of tooth enamel. J. Archaeol. Sci. Rep. 2016, 6, 810–818. [Google Scholar] [CrossRef]
- Bei, L.I.; He, X.; Zhao, Y.; Bai, D.; Manglai, D. Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse. BMC Genet. 2020, 21, 46. [Google Scholar]
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Sun, L.; Zhao, J.; Xiang, L.; Cheng, X.; Li, J.; Jia, C.; Jiang, H. Histological analysis and identification of spermatogenesis-related genes in 2-, 6-, and 12-month-old sheep testes. Naturwissenschaften 2017, 104, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Lan, S.; Jia, R.X.; Yan, G.Y.; Wang, L.Z.; Nie, H.T.; Lei, Z.H.; Wang, F. Age-associated and tissue-specific expression of osteopontin in male Hu sheep reproductive tract. Tissue Cell 2016, 48, 496–502. [Google Scholar] [CrossRef]
- Nie, H.; Zheng, M.; Wang, Z.; Xu, Q.; Yan, X. Transcriptomic analysis provides insights into candidate genes and molecular pathways involved in growth of Manila clam Ruditapes philippinarum. Funct. Integr. Genom. 2021, 21, 341–353. [Google Scholar] [CrossRef]
- Amelia, C.; Antonio, F.; Monica, R.; Sabrina, E.; Alfredo, C. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017, 18, 1652. [Google Scholar]
- White-Cooper, H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010, 139, 11–21. [Google Scholar] [CrossRef]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Xie, J.; Wang, W.; Li, W. Identification of transcriptome differences in goat ovaries at the follicular phase and the luteal phase using an RNA-Seq method. Theriogenology 2020, 158, 239–249. [Google Scholar] [CrossRef]
- Benoit, G.; Jean-Louis, D.; Florence, J.; Anne, L.; Guillemette, M.; Marie-Jose, M.; Sandrine, S.; Jean-Luc, G. The Adult Boar Testicular and Epididymal Transcriptome. Biol. Reprod. 2008, 10, 175–176. [Google Scholar]
- Andrea, F.; Damian, S.; Sune, F.; Michael, K.; Milan, S.; Alexander, R.; Lin, J.; Pablo, M.; Peer, B.; Christian, V.M. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2012, 41, D808–D815. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Meng, H.; Guo, X.; Li, X.; Meng, F. Differential gene expression profiles in peripheral blood in Northeast Chinese Han people with acute myocardial infarction. Genet. Mol. Biol. 2018, 41, 59–66. [Google Scholar] [CrossRef]
- Ran, M.; Chen, B.; Wu, M.; Liu, X.; He, C.; Yang, A.; Li, Z.; Xiang, Y.; Li, Z.; Zhang, S. Integrated analysis of miRNA and mRNA expression profiles in development of porcine testes. RSC Adv. 2015, 5, 63439–63449. [Google Scholar] [CrossRef]
- Yao, J.; Chiba, T.; Sakai, J.; Hirose, K.; Yamamoto, M.; Hada, A.; Kuramoto, K.; Higuchi, K.; Mori, M. Mouse testis transcriptome revealed using serial analysis of gene expression. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2004, 15, 433–451. [Google Scholar] [CrossRef]
- Chen, C.S.; Alonso, J.L.; Ostuni, E.; Whitesides, G.M.; Ingber, D.E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003, 307, 355–361. [Google Scholar] [CrossRef]
- Siu, M.K.; Wong, C.H.; Lee, W.M.; Cheng, C.Y. Sertoli-Germ Cell Anchoring Junction Dynamics in the Testis Are Regulated by an Interplay of Lipid and Protein Kinases. J. Biol. Chem. 2005, 280, 25029–25047. [Google Scholar] [CrossRef]
- Nakajima, M.; Welch, D.R.; Belloni, P.N.; Nicolson, G.L. Degradation of Basement Membrane Type IV Collagen and Lung Subendothelial Matrix by Rat Mammary Adenocarcinoma Cell Clones of Differing Metastatic Potentials. Cancer Res. 1987, 47, 4869–4876. [Google Scholar]
- Koch, M.; Laub, F.; Zhou, P.; Hahn, R.A.; Tanaka, S.; Burgeson, R.E.; Gerecke, D.R.; Ramirez, F.; Gordon, M.K. Collagen XXIV, a vertebrate fibrillar collagen with structural features of invertebrate collagens: Selective expression in developing cornea and bone. J. Biol. Chem. 2003, 278, 43236–43244. [Google Scholar] [CrossRef]
- Chen, G.; Liu, D.; Tadokoro, M.; Hirochika, R.; Ohgushi, H.; Tanaka, J.; Tateishi, T. Chondrogenic differentiation of human mesenchymal stem cells cultured in a cobweb-like biodegradable scaffold. Biochem. Biophys. Res. Commun. 2004, 322, 50–55. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Feng, L.; Zhang, X.; Geng, Y.; Dym, M. Expression of Col1a1, Col1a2 and procollagen I in germ cells of immature and adult mouse testis. Reproduction 2005, 130, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Fakoya, F. Reticulin fibres in the tunica albuginea and peritubular tissue of seminiferous tubules of adult male Wistar rats. Acta Histochem. 2002, 104, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Vera, J.; Wolkenhauer, O. The systems biology of mitochondrial fission and fusion and implications for disease and aging. Biogerontology 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Cribbs, J.T.; Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. Embo Rep. 2007, 8, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Fröjdman, K.; Harley, V.R.; Pelliniemi, L.J. Sox9 protein in rat Sertoli cells is age and stage dependent. Histochem. Cell Biol. 2000, 113, 31–36. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Genetic Control of Testis Development. Sex. Dev. 2013, 7, 21–32. [Google Scholar] [CrossRef]
- Beverdam, A.; Svingen, T.; Bagheri-Fam, S.; Bernard, P.; Mcclive, P.; Robson, M.; Khojasteh, M.B.; Salehi, M.; Sinclair, A.H.; Harley, V.R. Sox9-dependent expression of Gstm6 in Sertoli cells during testis development in mice. Reproduction 2009, 137, 481–486. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jeyasuria, P.; Parker, K.L.; Koopman, P. The Transcription Factors Steroidogenic Factor-1 and SOX9 Regulate Expression of Vanin-1 during Mouse Testis Development. J. Biol. Chem. 2005, 280, 5917–5923. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Georg, I.; Scherthan, H.; Lécureuil, C.; Guillou, F.; Wegner, M.; Scherer, G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev. Biol. 2009, 327, 301–312. [Google Scholar] [CrossRef]
- Rao, M.; Xia, W.; Yang, J.; Hu, L.X.; Hu, S.F.; Lei, H.; Wu, Y.Q.; Zhu, C.H. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology 2016, 4, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodriguez, J. A matter of death and life: The significance of germ cell death during spermatogenesis. Int. J. Androl. 2010, 21, 236–248. [Google Scholar] [CrossRef]
- Panda, P.K.; Naik, P.P.; Meher, B.R.; Das, D.N.; Bhutia, S.K. PUMA dependent mitophagy by Abrus agglutinin contributes to apoptosis through ceramide generation. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2018, 1865, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fu, Q.; Pan, H.; Chen, F.; Zhao, X.; Wang, H.; Zhang, P.; Huang, F.; Lu, Y.; Zhang, M. Spermatogenesis-associated proteins at different developmental stages of buffalo testicular seminiferous tubules identified by comparative proteomic analysis. Proteomics 2016, 16, 2005–2018. [Google Scholar] [CrossRef]
- Youn, J.; Cha, S.; Park, C.; Yang, K.; Kim, J.; Koong, M.; Kang, I.; Song, I.; Han, S. Predictive value of sperm motility characteristics assessed by computer-assisted sperm analysis in intrauterine insemination with superovulation in couples with unexplained infertility. Clin. Exp. Reprod. Med. 2011, 38, 47–52. [Google Scholar] [CrossRef]
- Francis, H.; Hargrove, L. Knockout of Histidine Decarboxylase (HDC) Reduces Hepatic Fibrosis in Bile Duct Ligated (BDL) Mice. FASEB J. 2015, 29, 53–56. [Google Scholar] [CrossRef]
- Mondillo, C.; Varela, M.L.; Abiuso AM, B.; Vázquez, R. Potential negative effects of anti-histamines on male reproductive function. Reprod. Off. J. Soc. Study Fertil. 2018, 155, R221–R227. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A.; Bartke, A.; Amador, A.; Began, T. Histamine affects testicular steroid production in the golden hamster. Endocrinology 1989, 125, 2212–2214. [Google Scholar] [CrossRef]
- Mondillo, C.; Falus, A.; Pignataro, O.; Pap, E. Prolonged Histamine Deficiency in Histidine Decarboxylase Gene Knockout Mice Affects Leydig Cell Function. J. Androl. 2007, 28, 86–91. [Google Scholar] [CrossRef]
- Wei, J.R.; Dong, J.; Li, L. Cancer-associated fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor growth and drug resistance in lung adenocarcinoma. Aging 2020, 12, 13220. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.Q.; Zhang, S.; Cao, R.; Zhao, J.; Sun, Z.J.; Zou, W. Augmented expression of gamma-glutamyl transferase 5 (GGT5) impairs testicular steroidogenesis by deregulating local oxidative stress. Cell Tissue Res. 2016, 366, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Piotrkowski, B.; Monzon, C.M.; Pagotto, R.M.; Reche, C.G.; Besio, M.; Cymeryng, C.; Pignataro, O.P. Effects of heme oxygenase isozymes on Leydig cells steroidogenesis. J. Endocrinol. 2009, 203, 155–165. [Google Scholar] [CrossRef] [PubMed]




| Breeds | Birthplace | Number | Sex | Body Weight | Age at Slaughter |
|---|---|---|---|---|---|
| Hu sheep | Minqin, Gansu | 3 | male | 63.23 ± 2.30 | 360 ± 5 d |
| Tibetan sheep | Linxia, Gansu | 3 | male | 46.58 ± 4.17 | 360 ± 5 d |
| Gene Name | Primer Sequences (5′–3′) | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| FOXP2 | F: GAGATTGCCCCAAACTACGAG R: GCAAATGTCCGTGTAAACCAG | 132 | 54 |
| GGT5 | F: AGCTTCCTGCACAGCCCGTTC R: ACCTTCCTTGGCGATGTCCTCC | 182 | 58 |
| HKDC1 | F: AGAAGAAATTACCTCTTGGCCTA R: CTCGCCTTAAACTTCTTGGTC | 134 | 49 |
| IL5RA | F: GTGAATTTAACTTGCACCACA R: GACATTCTTCAATCCGAGAGC | 137 | 51 |
| SH2D4B | F: CTTCCTTAACTGCAAGCCAGA R: CCATCATTGGCAGTCTACCTC | 130 | 51 |
| BCL2 | F: TGAGGCTTATGAACATTCCAGT R: TTCTTCCTCCCACCCCTGCAA | 121 | 51 |
| COL1A1 | F: CGCTCCTTGTGTAACTGCAT R: TTCACATGAGTCCCCATCCAC | 281 | 57 |
| COL1A2 | F: GCCTATCCTTGATATTGCACCT R: CTTTTGCCCACAATTTAAGCAAG | 220 | 53 |
| MMP2 | F: CATCTGGCGAACAGTGACACC R: AAGAACACAGCCTTCTCCTCC | 129 | 57 |
| SOX9 | F: TGTCTAAATTCATCTGCTCCC R: TGAGCCTAAATAGACTCTGC | 271 | 56 |
| β-actin | F: TGATGATCGCAGAAAGAACCC R: CTCGCTTTGAAGGTTTCCAGT | 133 | 52 |
| Parameters | HS | TS |
|---|---|---|
| Diameter of seminiferous tubules (µm) | 152.26 ± 19.64 a | 126.35 ± 11.64 b |
| Cross-sectional area of seminiferous Tubule (µm2) | 16,716.71 ± 3408.55 a | 13,482.91 ± 1780.51 b |
| Seminiferous epithelium thickness (µm) | 38.82 ± 5.13 a | 34.42 ± 6.23 b |
| Spermatogonia area (µm2) | 13.47 ± 2.80 a | 11.51 ± 2.572 b |
| Primary spermatocyte area (µm2) | 29.27 ± 5.89 a | 25.59 ± 3.20 b |
| Sperm cell diameter area (µm2) | 9.91 ± 2.25 | 8.78 ± 1.52 |
| Number of spermatogonias | 37.78 ± 5.27 a | 32.08 ± 4.90 b |
| Number of primary spermatocytes | 26.46 ± 4.23 a | 22.96 ± 5.27 b |
| Number of spermatides | 30.02 ± 5.14 a | 24.96 ± 4.78 b |
| Number of Sertoli cells | 22.83 ± 3.72 a | 15.62 ± 2.73 b |
| Items | HS1 | HS2 | HS3 | TS1 | TS2 | TS3 |
|---|---|---|---|---|---|---|
| Raw reads | 43,732,762 | 44,297,084 | 44,120,746 | 42,299,378 | 45,277,764 | 46,281,832 |
| Clean reads | 21,866,381 | 22,148,542 | 22,060,373 | 21,149,689 | 22,638,882 | 23,140,916 |
| Q20 (%) | 98.42 | 98.16 | 97.48 | 98.29 | 97.41 | 97.38 |
| Q30 (%) | 95.09 | 94.71 | 93.22 | 94.89 | 93.08 | 92.96 |
| GCcontent (%) | 50.01 | 51.14 | 50.60 | 50.20 | 50.32 | 49.61 |
| Mapped Reads (%) | 97.45 | 95.97 | 96.02 | 96.93 | 96.02 | 96.02 |
| Uniq Mapped Reads (%) | 94.99 | 93.61 | 93.56 | 94.47 | 93.45 | 93.49 |
| Multipl Mapped Reads (%) | 2.46 | 2.36 | 2.46 | 2.46 | 2.57 | 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Yang, Y.; Yan, Z.; Liu, M.; Wang, X. Transcriptomic Study of Spermatogenesis in the Testis of Hu Sheep and Tibetan Sheep. Genes 2022, 13, 2212. https://doi.org/10.3390/genes13122212
Fu X, Yang Y, Yan Z, Liu M, Wang X. Transcriptomic Study of Spermatogenesis in the Testis of Hu Sheep and Tibetan Sheep. Genes. 2022; 13(12):2212. https://doi.org/10.3390/genes13122212
Chicago/Turabian StyleFu, Xiaoyu, Yanan Yang, Zunqiang Yan, Miaomiao Liu, and Xinrong Wang. 2022. "Transcriptomic Study of Spermatogenesis in the Testis of Hu Sheep and Tibetan Sheep" Genes 13, no. 12: 2212. https://doi.org/10.3390/genes13122212
APA StyleFu, X., Yang, Y., Yan, Z., Liu, M., & Wang, X. (2022). Transcriptomic Study of Spermatogenesis in the Testis of Hu Sheep and Tibetan Sheep. Genes, 13(12), 2212. https://doi.org/10.3390/genes13122212






