Predicting Eye and Hair Color in a Turkish Population Using the HIrisPlex System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Samples and Genotyping
2.3. Statistical Analysis
3. Results and Discussion
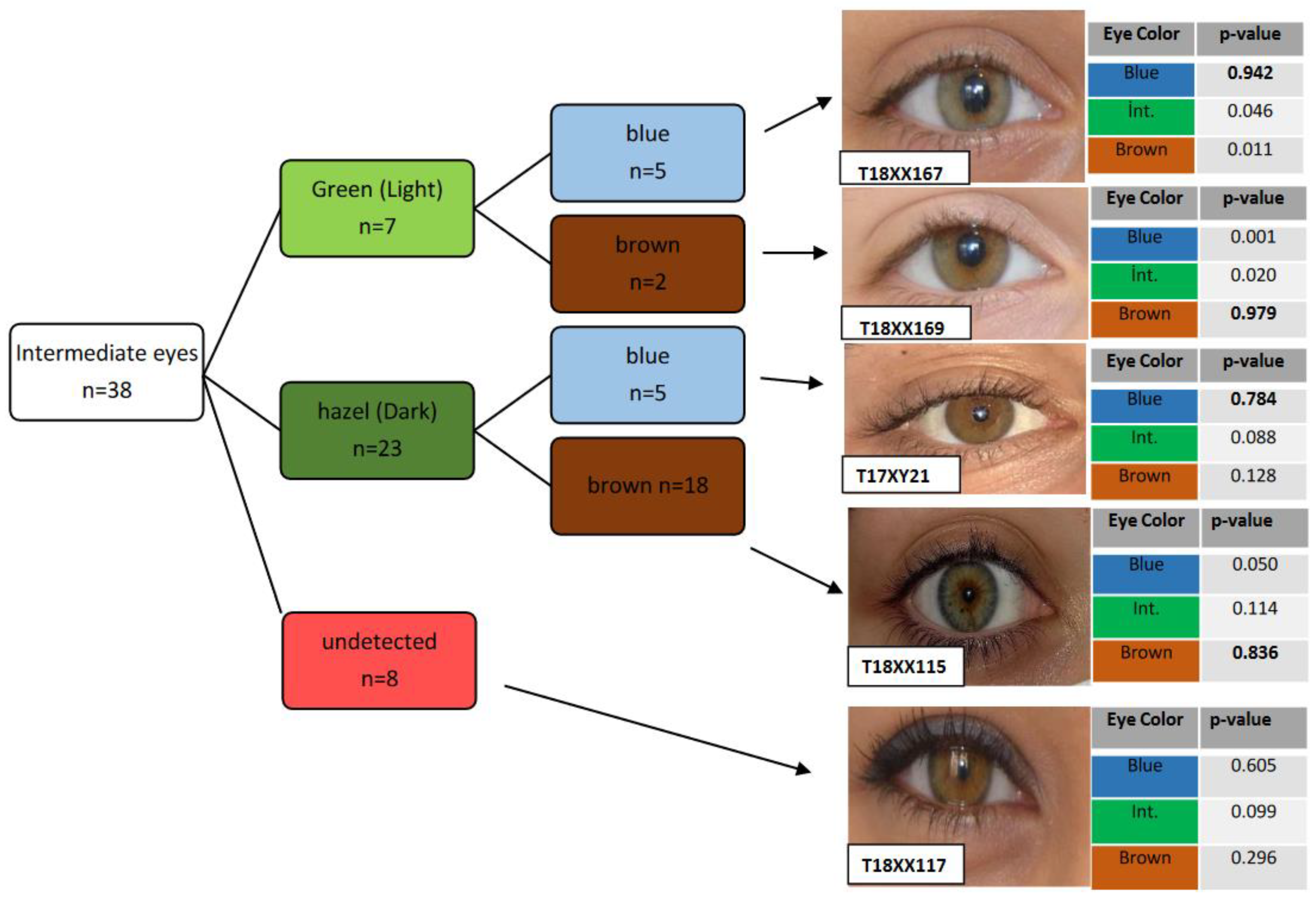
3.1. Eye Color Prediction
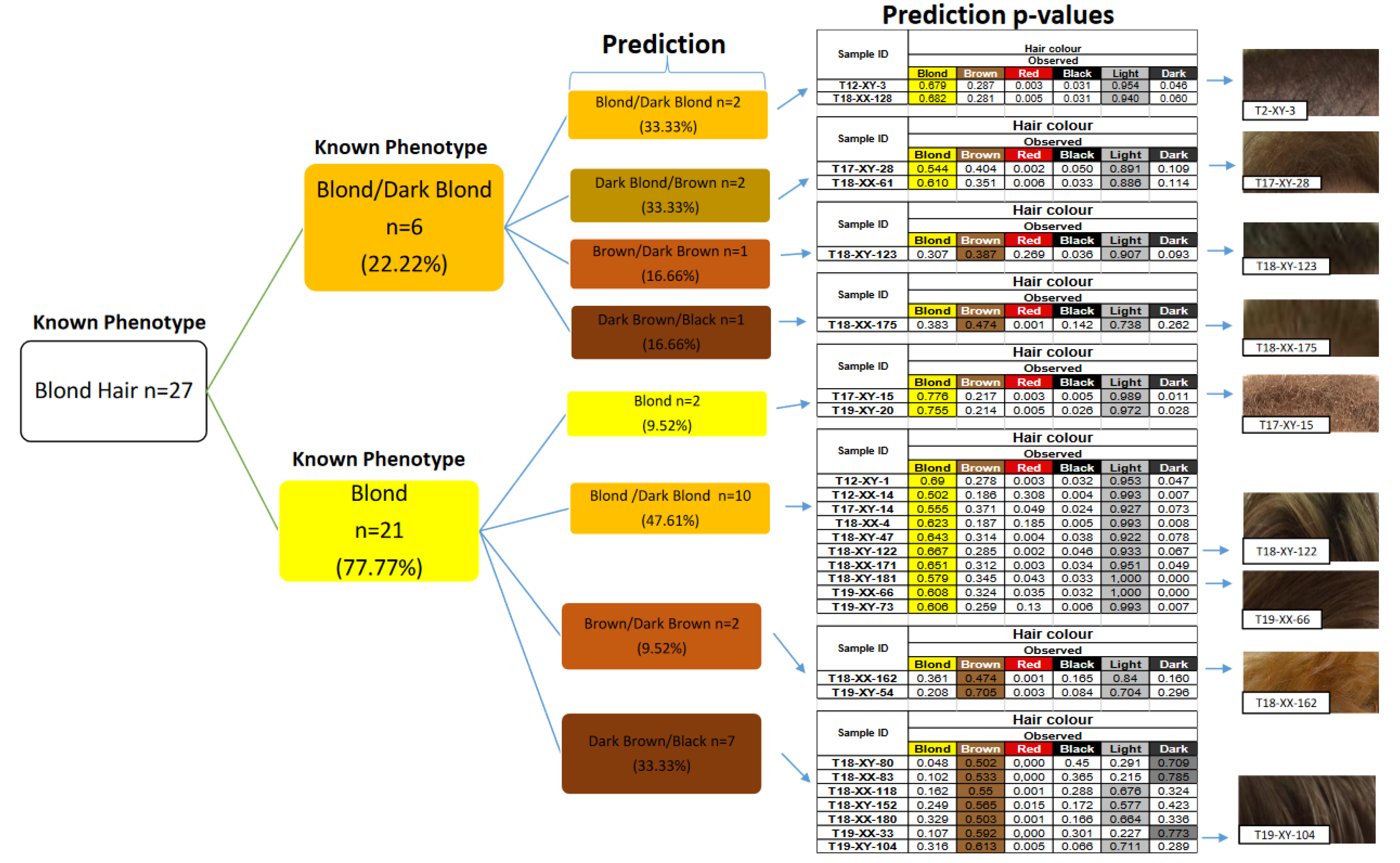
3.2. Hair Color Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EVCs | Externally visible characteristics |
| FDP | Forensic DNA phenotyping |
| MLR | Multinomial logistic regression |
| PISNPs | Phenotype informative SNPs |
| SBE | Single-base extension |
References
- Beck, T.; Hastings, R.K.; Gollapudi, S.; Free, R.C.; Brookes, A.J. GWAS Central: A comprehensive resource for the comparison and interrogation of genome-wide association studies. Eur. J. Hum. Genet. EJHG 2014, 22, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Visser, M.; Duffy, D.L.; Hysi, P.G.; Jacobs, L.C.; Lao, O.; Zhong, K.; Walsh, S.; Chaitanya, L.; Wollstein, A.; et al. Genetics of skin color variation in Europeans: Genome-wide association studies with functional follow-up. Hum. Genet. 2015, 134, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Stranger, B.E.; Stahl, E.A.; Raj, T. Progress and promise of genome-wide association studies for human complex trait genetics. Genetics 2011, 187, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Kayser, M. Forensic DNA Phenotyping: Predicting human appearance from crime scene material for investigative purposes. Forensic Sci. Int. Genet. 2015, 18, 33–48. [Google Scholar] [CrossRef]
- Schneider, P.M.; Prainsack, B.; Kayser, M. The Use of Forensic DNA Phenotyping in Predicting Appearance and Biogeographic Ancestry. Dtsch. Arztebl. Int. 2019, 51–52, 873–880. [Google Scholar] [CrossRef]
- Kayser, M.; de Knijff, P. Improving human forensics through advances in genetics, genomics and molecular biology. Nat. Rev. Genet. 2011, 12, 179–192. [Google Scholar] [CrossRef]
- Kayser, M.; Schneider, P.M. DNA-based prediction of human externally visible characteristics in forensics: Motivations, scientific challenges, and ethical considerations. Forensic Sci. Int. Genet. 2009, 3, 154–161. [Google Scholar] [CrossRef]
- Haddrill, P.R. Developments in forensic DNA analysis. Emerg. Top. Life Sci. 2021. [Google Scholar] [CrossRef]
- Grimes, E.A.; Noake, P.J.; Dixon, L.; Urquhart, A. Sequence polymorphism in the human melanocortin 1 receptor gene as an indicator of the red hair phenotype. Forensic Sci. Int. 2001, 122, 124–129. [Google Scholar] [CrossRef]
- Liu, F.; van Duijn, K.; Vingerling, J.R.; Hofman, A.; Uitterlinden, A.G.; Janssens, A.C.; Kayser, M. Eye color and the prediction of complex phenotypes from genotypes. Curr. Biol. CB 2009, 19, R192–R193. [Google Scholar] [CrossRef]
- Walsh, S.; Liu, F.; Ballantyne, K.N.; van Oven, M.; Lao, O.; Kayser, M. IrisPlex: A sensitive DNA tool for accurate prediction of blue and brown eye colour in the absence of ancestry information. Forensic Sci. Int. Genet. 2011, 5, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Freire-Aradas, A.; Ruiz, Y.; Phillips, C.; Maronas, O.; Sochtig, J.; Tato, A.G.; Dios, J.A.; de Cal, M.C.; Silbiger, V.N.; Luchessi, A.D.; et al. Exploring iris colour prediction and ancestry inference in admixed populations of South America. Forensic Sci. Int. Genet. 2014, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, O.; Zorlu, T.; Filoglu, G. Prediction of human eye colour using highly informative phenotype SNPs (PISNPs). Aust. J. Forensic Sci. 2018, 52, 27–37. [Google Scholar] [CrossRef]
- Salvoro, C.; Faccinetto, C.; Zucchelli, L.; Porto, M.; Marino, A.; Occhi, G.; de Los Campos, G.; Vazza, G. Performance of four models for eye color prediction in an Italian population sample. Forensic Sci. Int. Genet. 2019, 40, 192–200. [Google Scholar] [CrossRef]
- Walsh, S.; Chaitanya, L.; Clarisse, L.; Wirken, L.; Draus-Barini, J.; Kovatsi, L.; Maeda, H.; Ishikawa, T.; Sijen, T.; de Knijff, P.; et al. Developmental validation of the HIrisPlex system: DNA-based eye and hair colour prediction for forensic and anthropological usage. Forensic Sci. Int. Genet. 2014, 9, 150–161. [Google Scholar] [CrossRef]
- Walsh, S.; Liu, F.; Wollstein, A.; Kovatsi, L.; Ralf, A.; Kosiniak-Kamysz, A.; Branicki, W.; Kayser, M. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Sci. Int. Genet. 2013, 7, 98–115. [Google Scholar] [CrossRef]
- Walsh, S.; Kayser, M. Practical Guide to the HIrisPlex System: Simultaneous Prediction of Eye and Hair Color from DNA. Methods Mol. Biol. 2016, 1420, 213–231. [Google Scholar]
- Tavaci, İ.; Şimşek, S.Z.; Sapan, V.; Arslan, C.; AsiciogLu, F.; FilogluLu, G.; Bülbül, Ö. Optimization and Validation of HIrisPlex Panel for Predicting of the Eye and Hair Color. Turk. Klin. J. Forensic Med. Forensic Sci. 2021, 18, 10–20. [Google Scholar] [CrossRef]
- Frudakis, T.; Terravainen, T.; Thomas, M. Multilocus OCA2 genotypes specify human iris colors. Hum. Genet. 2007, 122, 311–326. [Google Scholar] [CrossRef]
- Branicki, W.; Brudnik, U.; Draus-Barini, J.; Kupiec, T.; Wojas-Pelc, A. Association of the SLC45A2 gene with physiological human hair colour variation. J. Hum. Genet. 2008, 53, 966–971. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int. J. Leg. Med. 2007, 121, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kayser, M.; Palstra, R.J. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012, 22, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Phillips, C.; Gomez-Tato, A.; Alvarez-Dios, J.; Casares de Cal, M.; Cruz, R.; Maronas, O.; Sochtig, J.; Fondevila, M.; Rodriguez-Cid, M.J.; et al. Further development of forensic eye color predictive tests. Forensic Sci. Int. Genet. 2013, 7, 28–40. [Google Scholar] [CrossRef]
- Kukla-Bartoszek, M.; Teisseyre, P.; Pospiech, E.; Karlowska-Pik, J.; Zielinski, P.; Wozniak, A.; Boron, M.; Dabrowski, M.; Zubanska, M.; Jarosz, A.; et al. Searching for improvements in predicting human eye colour from DNA. Int. J. Leg. Med. 2021, 135, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, L.; Walsh, S.; Andersen, J.D.; Ansell, R.; Ballantyne, K.; Ballard, D.; Banemann, R.; Bauer, C.M.; Bento, A.M.; Brisighelli, F.; et al. Collaborative EDNAP exercise on the IrisPlex system for DNA-based prediction of human eye colour. Forensic Sci. Int. Genet. 2014, 11, 241–251. [Google Scholar] [CrossRef]
- Dembinski, G.M.; Picard, C.J. Evaluation of the IrisPlex DNA-based eye color prediction assay in a United States population. Forensic Sci. Int. Genet. 2014, 9, 111–117. [Google Scholar] [CrossRef]
- Kastelic, V.; Pospiech, E.; Draus-Barini, J.; Branicki, W.; Drobnic, K. Prediction of eye color in the Slovenian population using the IrisPlex SNPs. Croat. Med. J. 2013, 54, 381–386. [Google Scholar] [CrossRef]
- Purps, J.; Geppert, M.; Nagy, M.; Roewer, L. Evaluation of the IrisPlex eye colour prediction tool in a German population sample. Forensic Sci. Int. Genet. Suppl. Ser. 2011, 3, e202–e203. [Google Scholar] [CrossRef]
- Salvo, N.M.; Janssen, K.; Kirsebom, M.K.; Meyer, O.S.; Berg, T.; Olsen, G.H. Predicting eye and hair colour in a Norwegian population using Verogen’s ForenSeq DNA signature prep kit. Forensic Sci. Int. Genet. 2022, 56, 102620. [Google Scholar] [CrossRef]
- Parra, E.J. Human pigmentation variation: Evolution, genetic basis, and implications for public health. Am. J. Phys. Anthropol. 2007, 45, 85–105. [Google Scholar] [CrossRef]
- Junker, K.; Staadig, A.; Sidstedt, M.; Tillmar, A.; Hedman, J. Phenotype prediction accuracy—A Swedish perspective. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 384–386. [Google Scholar] [CrossRef]
- Carratto, T.M.T.; Marcorin, L.; do Valle-Silva, G.; de Oliveira, M.L.G.; Donadi, E.A.; Simoes, A.L.; Castelli, E.C.; Mendes-Junior, C.T. Prediction of eye and hair pigmentation phenotypes using the HIrisPlex system in a Brazilian admixed population sample. Int. J. Leg. Med. 2021, 135, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Balanovska, E.; Lukianova, E.; Kagazezheva, J.; Maurer, A.; Leybova, N.; Agdzhoyan, A.; Gorin, I.; Petrushenko, V.; Zhabagin, M.; Pylev, V.; et al. Optimizing the genetic prediction of the eye and hair color for North Eurasian populations. BMC Genom. 2020, 21, 527. [Google Scholar] [CrossRef] [PubMed]
- Atwood, L.; Raymond, J.; Sears, A.; Bell, M.; Daniel, R. From Identification to Intelligence: An Assessment of the Suitability of Forensic DNA Phenotyping Service Providers for Use in Australian Law Enforcement Casework. Front. Genet. 2020, 11, 568701. [Google Scholar] [CrossRef] [PubMed]
| Predicted % (Bold Numbers Indicates Prediction Success Percentage) | Summary Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIrisPlex (Eye Color) | Blue | Brown | Intermediate | Undetected | Samples | AUC a | Sensitivity % | Specificity % | PPV % b | NPV % c |
| Blue | 100 | 0 | ND | 0 | 20 | 0.66 | 100 | 92.24 | 66.66 | 100 |
| Brown | 0 | 95.60 | ND | 4.39 | 91 | 0.88 | 95.60 | 65.51 | 81.30 | 90.47 |
| Intermediate | 26.31 | 52.63 | ND * | 21.05 | 38 | 0.59 | 0 | 100 | ND | 74.49 |
| Predicted % (Bold Numbers Indicates Prediction Success Percentage) | Summary Statistics % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIrisPlex (Hair Color) | Black | Brown | Blond | Red | Samples | AUC a | Sensitivity % | Specificity % | PPV % b | NPV % c |
| Black | 95.23 | 4.76 | 0 | 0 | 21 | 0.96 | 95.23 | 98.43 | 90.90 | 99.21 |
| Brown | 2.06 | 96.90 | 1.03 | 0 | 97 | 0.89 | 96.90 | 76.92 | 88.67 | 93.02 |
| Blond | 0 | 40.74 | 59.25 | 0 | 27 | 0.90 | 59.25 | 98.36 | 88.88 | 91.60 |
| Red | 0 | 0 | 25.00 | 75.00 | 4 | 0.55 | 75.00 | 100 | 100 | 99.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari O, I.; Simsek, S.Z.; Filoglu, G.; Bulbul, O. Predicting Eye and Hair Color in a Turkish Population Using the HIrisPlex System. Genes 2022, 13, 2094. https://doi.org/10.3390/genes13112094
Sari O I, Simsek SZ, Filoglu G, Bulbul O. Predicting Eye and Hair Color in a Turkish Population Using the HIrisPlex System. Genes. 2022; 13(11):2094. https://doi.org/10.3390/genes13112094
Chicago/Turabian StyleSari O, Ilksen, Sumeyye Zulal Simsek, Gonul Filoglu, and Ozlem Bulbul. 2022. "Predicting Eye and Hair Color in a Turkish Population Using the HIrisPlex System" Genes 13, no. 11: 2094. https://doi.org/10.3390/genes13112094
APA StyleSari O, I., Simsek, S. Z., Filoglu, G., & Bulbul, O. (2022). Predicting Eye and Hair Color in a Turkish Population Using the HIrisPlex System. Genes, 13(11), 2094. https://doi.org/10.3390/genes13112094





