Association of Myostatin Gene Polymorphisms with Strength and Muscle Mass in Athletes: A Systematic Review and Meta-Analysis of the MSTN rs1805086 Mutation
Abstract
1. Introduction
1.1. History of Discovery
1.1.1. MSTN Inhibitors
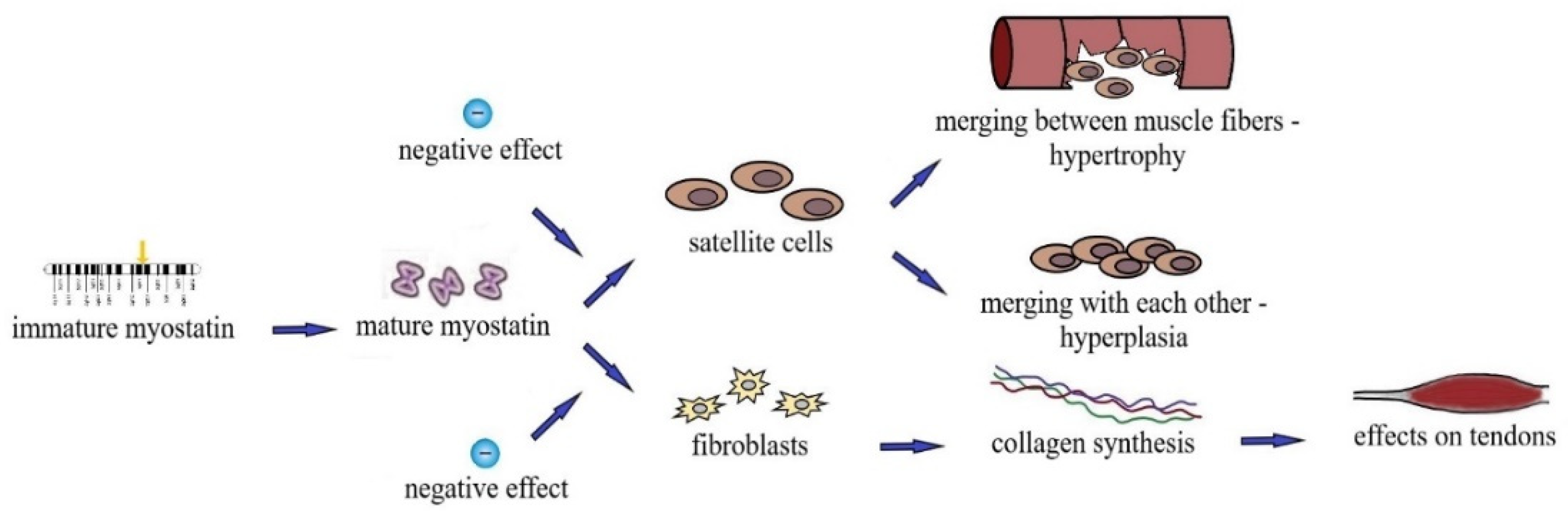
1.1.2. Mechanism of Effect of MSTN on Skeletal Muscle Mass and Strength
1.1.3. Effect of MSTN on Tendons and Bones
1.2. Myostatin Mutations
1.2.1. MSTN Mutation (rs397515373, c.373 + 5 G>A)
1.2.2. MSTN A55T Mutation (rs180565, 163 G>A)
1.2.3. Mutation of MSTN E164K rs35781413 (c.490G>A, p.Glu164Lus)
1.2.4. Mutation of MSTN K153R (rs1805086, p.Lys153Arg, c.458A>G)
1.2.5. MSTN K153R (rs1805086) Polymorphism Frequency
2. Materials and Methods
2.1. META-ANALYSIS
2.1.1. Goal of Research
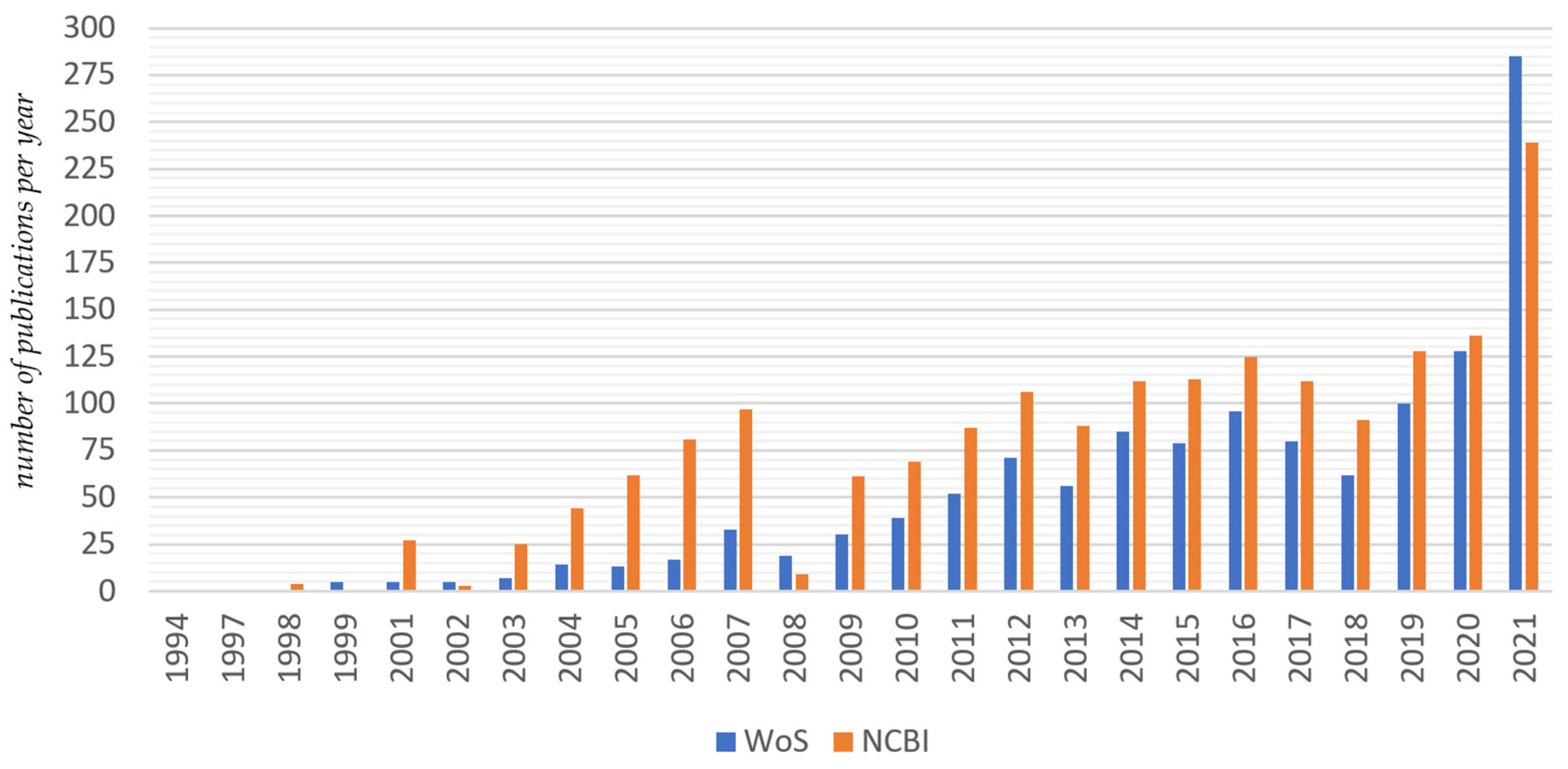
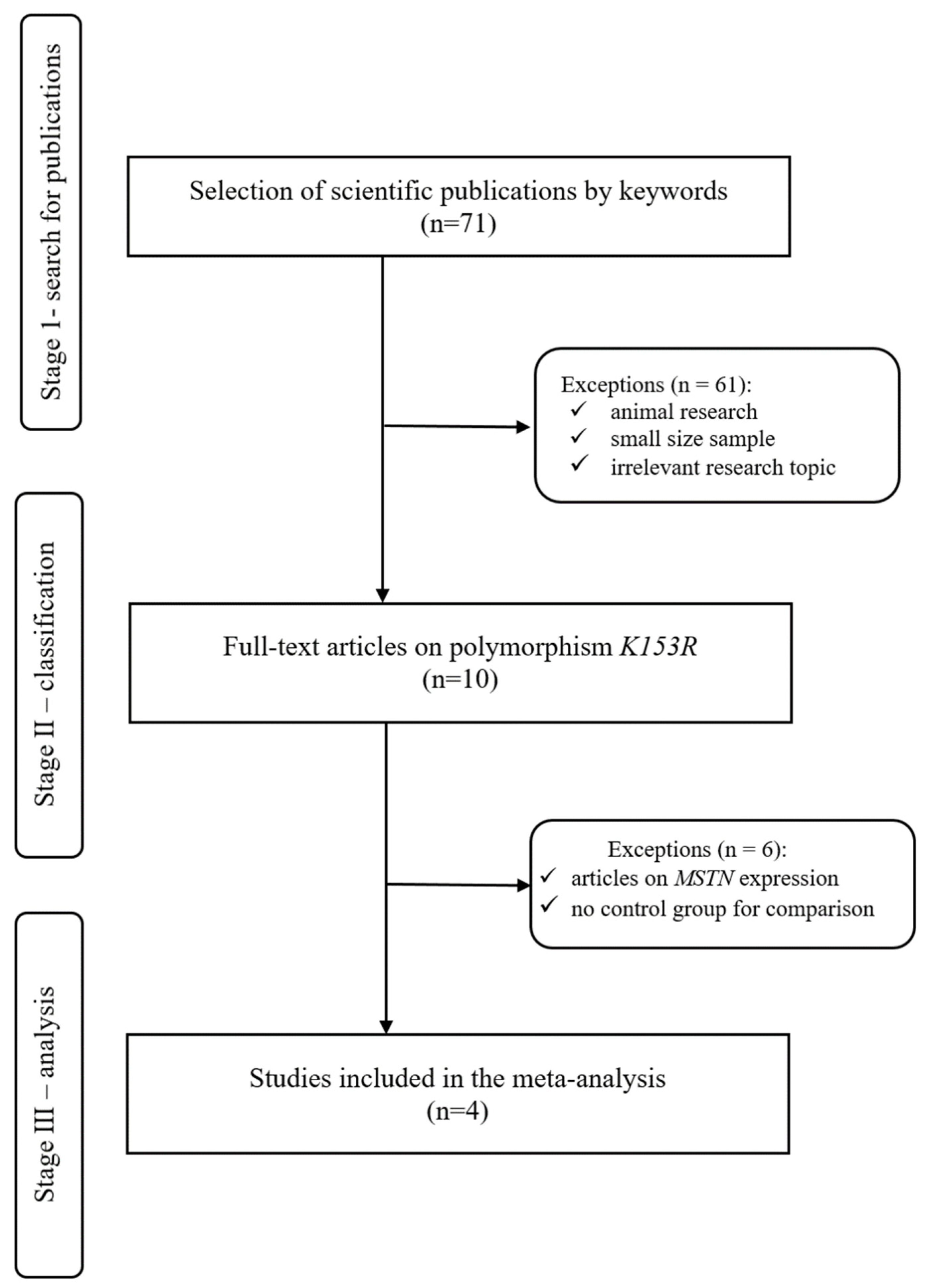
2.1.2. Search for Publications
2.1.3. Inclusion and Exclusion Criteria
- Be published from 1997 to April 2021,
- Sample size should not be less than 10 people, and a control group in the study is mandatory,
- Full text of the study should be available,
- Participants must be adults who are not elderly,
- Subjects had to be healthy at the time of the study,
- No studies should be conducted on animals.
2.2. Data Extraction
2.3. Statistical Analysis
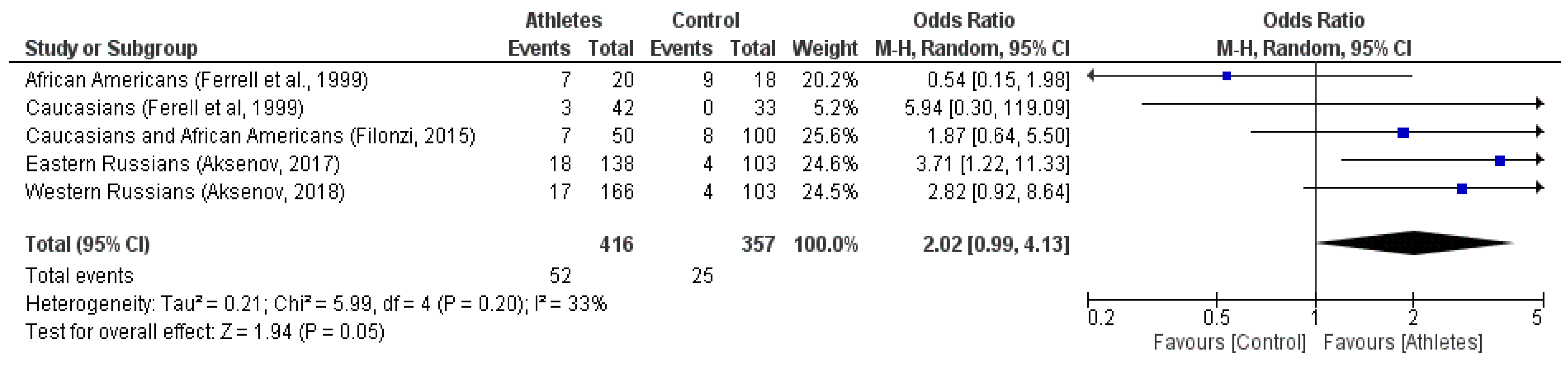
3. Results
Case-Control Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beunen, G.; Thomis, M. Gene powered? Where to go from heritability (H-2) in muscle strength and power? Exerc. Sport Sci. Rev. 2004, 32, 148–154. [Google Scholar] [CrossRef]
- Mangine, G.T.; Hoffman, J.R.; Gonzalez, A.M.; Townsend, J.R.; Wells, A.J.; Jajtner, A.R.; Beyer, K.S.; Boone, C.H.; Miramonti, A.A.; Wang, R.; et al. The effect of training volume and intensity on improvements in muscular strength and size in resistance-trained men. Physiol. Rep. 2015, 3, 17. [Google Scholar] [CrossRef]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Sassi, A.H.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef]
- Yamada, A.K.; Verlengia, R.; Bueno, C.R. Myostatin: Genetic variants, therapy and gene doping. Braz. J. Pharm. Sci. 2012, 48, 369–377. [Google Scholar] [CrossRef][Green Version]
- Dalbo, V.J.; Roberts, M.D.; Sunderland, K.L.; Poole, C.N.; Stout, J.R.; Beck, T.W.; Bemben, M.; Kerksick, C.M. Acute Loading and Aging Effects on Myostatin Pathway Biomarkers in Human Skeletal Muscle After Three Sequential Bouts of Resistance Exercise. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2011, 66, 855–865. [Google Scholar] [CrossRef][Green Version]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sport. Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Chen, P.-J.; Xiao, W.-H. Signaling pathways controlling skeletal muscle mass. Acta Physiol. Sin. 2019, 49, 671–679. [Google Scholar]
- Roth, S.M.; Martel, G.F.; Ferrell, R.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Myostatin gene expression is reduced in humans with heavy resistance strength training: A brief communication. Exp. Biol. Med. 2003, 228, 706–709. [Google Scholar] [CrossRef]
- Shishkin, S.S. Miostatin i nekotorye drugie biohimicheskie faktory, reguliruyushchie rost myshechnyh tkanej u cheloveka i ryada vysshih pozvonochnyh. Uspekhi Biol. Him. 2004, 44, 209–262. [Google Scholar]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Kollias, H.D.; McDermott, J.C. Transforming growth factor-β and myostatin signaling in skeletal muscle. J. Appl. Physiol. 2008, 104, 579–587. [Google Scholar] [CrossRef]
- McFarlane, C.; Hui, G.Z.; Amanda, W.Z.W.; Lau, H.Y.; Lokireddy, S.; Ge, X.J.; Mouly, V.; Butler-Browne, G.; Gluckman, P.D.; Sharma, M.; et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am. J. Physiol.-Cell Physiol. 2011, 301, C195–C203. [Google Scholar] [CrossRef]
- Ferrell, R.E.; Conte, V.; Lawrence, E.C.; Roth, S.M.; Hagberg, J.M.; Hurley, B.F. Frequent sequence variation in the human myostatin (GDF8) gene as a marker for analysis of muscle-related phenotypes. Genomics 1999, 62, 203–207. [Google Scholar] [CrossRef]
- Sergeeva, K.V.; Miroshnikov, A.B.; Smolensky, A.V. Effect of Growth Hormone Administration on the Mass And Strength of Muscles in Healthy Young Adults: A Systematic Review and Meta-Analysis. Hum. Physiol. 2019, 4, 452–460. [Google Scholar] [CrossRef]
- Pan, H.; Ping, X.C.; Zhu, H.J.; Gong, F.Y.; Dong, C.X.; Li, N.S.; Wang, L.J.; Yang, H.B. Association of myostatin gene polymorphisms with obesity in Chinese north Han human subjects. Gene 2012, 494, 237–241. [Google Scholar] [CrossRef]
- Thomis, M.A.; Huygens, W.; Peeters, M.; Vlietinck, R.; Beunen, G.P. Linkage analysis of myostatin-pathway genes in human adiposity: The Leuven Genes for Muscular Strength Project. Med. Sci. Sport. Exerc. 2004, 36, S99. [Google Scholar]
- Gonzalez-Cadavid, N.F.; Bhasin, S. Role of myostatin in metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 451–457. [Google Scholar] [CrossRef]
- Han, D.S.; Huang, C.H.; Chen, S.Y.; Yang, W.S. Serum reference value of two potential doping candidates-myostatin and insulin-like growth factor-I in the healthy young male. J. Int. Soc. Sport. Nutr. 2017, 14, 2. [Google Scholar] [CrossRef]
- Lakshman, K.M.; Bhasin, S.; Corcoran, C.; Collins-Racie, L.A.; Tchistiakova, L.; Forlow, S.B.; Ledger, K.S.; Burczynski, M.E.; Dorner, A.J.; LaVallie, E.R. Measurement of myostatin concentrations in human serum: Circulating concentrations in young and older men and effects of testosterone administration. Mol. Cell. Endocrinol. 2009, 302, 26–32. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Rodriguez-Romo, G.; Santiago, C.; Bustamante-Ara, N.; Yvert, T.; Gomez-Gallego, F.; Rexach, J.A.S.; Ruiz, J.R.; Lucia, A. The K153R variant in the myostatin gene and sarcopenia at the end of the human lifespan. Age 2010, 32, 405–409. [Google Scholar] [CrossRef][Green Version]
- Sharma, M.; McFarlane, C.; Kambadur, R.; Kukreti, H.; Bonala, S.; Srinivasan, S. Myostatin: Expanding horizons. IUBMB Life 2015, 67, 589–600. [Google Scholar] [CrossRef]
- Feder, D.; Rugollini, M.; Santomauro, A.; Oliveira, L.P.; Lioi, V.P.; dos Santos, R.; Ferreira, L.G.; Nunes, M.T.; Carvalho, M.H.; Delgado, P.O.; et al. Erythropoietin reduces the expression of myostatin in mdx dystrophic mice. Braz. J. Med. Biol. Res. 2014, 47, 966–971. [Google Scholar] [CrossRef][Green Version]
- Gentile, M.A.; Nantermet, P.V.; Vogel, R.L.; Phillips, R.; Holder, D.; Hodor, P.; Cheng, C.; Dai, H.Y.; Freedman, L.P.; Ray, W.J. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of β-catenin. J. Mol. Endocrinol. 2010, 44, 55–73. [Google Scholar] [CrossRef]
- Kim, J.S.; Cross, J.M.; Bamman, M.M. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1110–E1119. [Google Scholar] [CrossRef]
- Lach-Trifilieff, E.; Minetti, G.C.; Sheppard, K.; Ibebunjo, C.; Feige, J.N.; Hartmann, S.; Brachat, S.; Rivet, H.; Koelbing, C.; Morvan, F.; et al. An Antibody Blocking Activin Type II Receptors Induces Strong Skeletal Muscle Hypertrophy and Protects from Atrophy. Mol. Cell. Biol. 2014, 34, 606–618. [Google Scholar] [CrossRef]
- Jespersen, J.G.; Nedergaard, A.; Andersen, L.L.; Schjerling, P.; Andersen, J.L. Myostatin expression during human muscle hypertrophy and subsequent atrophy: Increased myostatin with detraining. Scand. J. Med. Sci. Sport. 2011, 21, 215–223. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef]
- Walker, R.G.; Poggioli, T.; Katsimpardi, L.; Buchanan, S.M.; Oh, J.; Wattrus, S.; Heidecker, B.; Fong, Y.W.; Rubin, L.L.; Ganz, P.; et al. Biochemistry and Biology of GDF11 and Myostatin Similarities, Differences, and Questions for Future Investigation. Circ. Res. 2016, 118, 1125–1141. [Google Scholar] [CrossRef]
- Hill, J.J.; Qiu, Y.C.; Hewick, R.M.; Wolfman, N.M. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: A novel protein with protease inhibitor and follistatin domains. Mol. Endocrinol. 2003, 17, 1144–1154. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Chen, X.L.; Chen, D.W. Myostatin: A novel insight into its role in metabolism, signal pathways, and expression regulation. Cell. Signal. 2011, 23, 1441–1446. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential Amino Acids Increase MicroRNA-499,-208b, and-23a and Downregulate Myostatin and Myocyte Enhancer Factor 2C mRNA Expression in Human Skeletal Muscle. J. Nutr. 2009, 139, 2279–2284. [Google Scholar] [CrossRef]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Rabinovich, M.; Kassem, E.; Eliakim, A. Frequency of the MSTN Lys(K)-153Arg(R) polymorphism among track & field athletes and swimmers. Growth Horm. IGF Res. 2015, 25, 196–200. [Google Scholar] [CrossRef]
- Fuku, N.; Alis, R.; Yvert, T.; Zempo, H.; Naito, H.; Abe, Y.; Arai, Y.; Murakami, H.; Miyachi, M.; Pareja-Galeano, H.; et al. Muscle-Related Polymorphisms (MSTN rs1805086 and ACTN3 rs1815739) Are Not Associated with Exceptional Longevity in Japanese Centenarians. PLoS ONE 2016, 11, 0166605. [Google Scholar] [CrossRef]
- Joulia-Ekaza, D.; Cabello, G. The myostatin gene: Physiology and pharmacological relevance. Curr. Opin. Pharmacol. 2007, 7, 310–315. [Google Scholar] [CrossRef]
- Kostyunina, D.S.; Ivanova, A.D.; Smirnova, O.V. Myostatin: 20 years later. Hum. Physiol. 2018, 1, 99–114. [Google Scholar]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Szlama, G.; Trexler, M.; Buday, L.; Patthy, L. K153R polymorphism in myostatin gene increases the rate of promyostatin activation by furin. FEBS Lett. 2015, 589, 295–301. [Google Scholar] [CrossRef]
- Walsh, F.S.; Celeste, A.J. Myostatin: A modulator of skeletal-muscle stem cells. Biochem. Soc. Trans. 2005, 33, 1513–1517. [Google Scholar] [CrossRef]
- Zhang, Z.L.; He, J.W.; Qin, Y.J.; Hu, Y.Q.; Li, M.; Zhang, H.; Hu, W.W.; Liu, Y.J.; Gu, J.M. Association between myostatin gene polymorphisms and peak BMD variation in Chinese nuclear families. Osteoporos. Int. 2008, 19, 39–47. [Google Scholar] [CrossRef]
- Elkasrawy, M.N.; Hamrick, M.W. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact. 2010, 10, 56–63. [Google Scholar]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between transforming growth factor-β 1, myostatin, and decorin—Implications for skeletal muscle fibrosis. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar] [CrossRef]
- Guo, W.; Flanagan, J.; Jasuja, R.; Kirkland, J.; Jiang, L.; Bhasin, S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J. Biol. Chem. 2008, 283, 9136–9145. [Google Scholar] [CrossRef]
- Artaza, J.N.; Bhasin, S.; Magee, T.R.; Reisz-Porszasz, S.; Shen, R.Q.; Groome, N.P.; Fareez, M.M.; Gonzalez-Cadavid, N.F. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology 2005, 146, 3547–3557. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Arounleut, P.; Kellum, E.; Cain, M.; Immel, D.; Liang, L.F. Recombinant Myostatin (GDF-8) Propeptide Enhances the Repair and Regeneration of Both Muscle and Bone in a Model of Deep Penetrant Musculoskeletal Injury. J. Trauma-Inj. Infect. Crit. Care 2010, 69, 579–583. [Google Scholar] [CrossRef]
- Thomis, M.A.I.; Huygens, W.; Heuninckx, S.; Chagnon, M.; Maes, H.H.M.; Claessens, A.L.; Vlietinck, R.; Bouchard, C.; Beunen, G.P. Exploration of myostatin polymorphisms and the angiotensin-converting enzyme insertion/deletion genotype in responses of human muscle to strength training. Eur. J. Appl. Physiol. 2004, 92, 267–274. [Google Scholar] [CrossRef]
- Santiago, C.; Ruiz, J.R.; Rodriguez-Romo, G.; Fiuza-Luces, C.; Yvert, T.; Gonzalez-Freire, M.; Gomez-Gallego, F.; Moran, M.; Lucia, A. The K153R Polymorphism in the Myostatin Gene and Muscle Power Phenotypes in Young, Non-Athletic Men. PLoS ONE 2011, 6, e16323. [Google Scholar] [CrossRef]
- Garatachea, N.; Pinos, T.; Camara, Y.; Rodriguez-Romo, G.; Emanuele, E.; Ricevuti, G.; Venturini, L.; Santos-Lozano, A.; Santiago-Dorrego, C.; Fiuza-Luces, C.; et al. Association of the K153R polymorphism in the myostatin gene and extreme longevity. Age 2013, 35, 2445–2454. [Google Scholar] [CrossRef][Green Version]
- Volkov, N.I.; Nesen, E.N.; Osipenko, A.A.; Korsun, S.N. Biohimiya Myshechnoj Deyatel’nosti; Olimpijskaya Literatura: Kiev, Ukraine, 2000; pp. 286–485. [Google Scholar]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. 2004. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Corsi, A.M.; Ferrucci, L.; Gozzini, A.; Tanini, A.; Brandi, M.L. Myostatin polymorphisms and age-related sarcopenia in the Italian population. J. Am. Geriatr. Soc. 2002, 50, 1463. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.J.; Tan, S.C.; Chew, P.L.; Liu, L.H.; Wang, L.; Wen, L.; Ma, L.H. The A55T and K153R polymorphisms of MSTN gene are associated with the strength training-induced muscle hypertrophy among Han Chinese men. J. Sport Sci. 2014, 32, 883–891. [Google Scholar] [CrossRef]
- Kostek, M.A.; Angelopoulos, T.J.; Clarkson, P.M.; Gordon, P.M.; Moyna, N.M.; Visich, P.S.; Zoeller, R.F.; Price, T.B.; Seip, R.L.; Thompson, P.D.; et al. Myostatin and Follistatin Polymorphisms Interact with Muscle Phenotypes and Ethnicity. Med. Sci. Sport Exerc. 2009, 41, 1063–1071. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Nigam, P.; Misra, A.; Guleria, R.; Luthra, K.; Jain, S.K.; Pasha, M.A.Q. Association of the Myostatin Gene with Obesity, Abdominal Obesity and Low Lean Body Mass and in Non-Diabetic Asian Indians in North India. PLoS ONE 2012, 7, e40977. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Lee, J. Myostatin A55T Genotype is Associated with Strength Recovery Following Exercise-Induced Muscle Damage. Int. J. Environ. Res. Public Health 2020, 17, 4900. [Google Scholar] [CrossRef]
- Usac, G.; Eroglu, O.; Zileli, R. The Evaluation of RS1805086 and RS1805065 Polymorphisms in MSTN Gene and Anthropometric Properties of National and Amateur Arm Wrestlers. Int. J. Morphol. 2020, 38, 1148–1154. [Google Scholar] [CrossRef]
- Grealy, R.; Herruer, J.; Smith, C.L.E.; Hiller, D.; Haseler, L.J.; Griffiths, L.R. Evaluation of a 7-Gene Genetic Profile for Athletic Endurance Phenotype in Ironman Championship Triathletes. PLoS ONE 2015, 10, e0145171. [Google Scholar] [CrossRef]
- Fernandez-Santander, A.; Valveny, N.; Harich, N.; Kandil, M.; Luna, F.; Martin, M.A.; Rubio, J.C.; Lucia, A.; Gaibar, M. Polymorphisms influencing muscle phenotypes in North-African and Spanish populations. Ann. Hum. Biol. 2012, 39, 166–169. [Google Scholar] [CrossRef]
- Juffer, P.; Furrer, R.; Gonzalez-Freire, M.; Santiago, C.; Verde, Z.; Serratosa, L.; Morate, F.J.; Rubio, J.C.; Martin, M.A.; Ruiz, J.R.; et al. Genotype Distributions in Top-level Soccer Players: A Role for ACE? Int. J. Sport Med. 2009, 30, 387–392. [Google Scholar] [CrossRef]
- Aksenov, M.O.; Andryushchenko, L.B. Myostatin gene role in strength building process. Teor. Prakt. Fiz. Kult. 2018, 4, 71–73. [Google Scholar]
- Filonzi, L.; Franchini, N.; Vaghi, M.; Chiesa, S.; Nonnis Marzano, F. The potential role of myostatin and neurotransmission genes in elite sport performances. J. Biosci. 2015, 40, 531–537. [Google Scholar] [CrossRef]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Eliakim, A. The combined frequency of IGF and myostatin polymorphism among track & field athletes and swimmers. Growth Horm. IGF Res. 2017, 32, 29–32. [Google Scholar] [CrossRef]
- Khanal, P.; He, L.X.; Herbert, A.J.; Stebbings, G.K.; Onambele-Pearson, G.L.; Degens, H.; Morse, C.I.; Thomis, M.; Williams, A.G. The Association of Multiple Gene Variants with Ageing Skeletal Muscle Phenotypes in Elderly Women. Genes 2020, 11, 1459. [Google Scholar] [CrossRef]
- Peng, L.N.; Lee, W.J.; Liu, L.K.; Lin, M.H.; Chen, L.K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2018, 9, 635–642. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ-Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Seibert, M.J.; Xue, Q.L.; Fried, L.P.; Walston, J.D. Polymorphic variation in the human myostatin (GDF-8) gene and association with strength measures in the Women’s Health and Aging Study II cohort. J. Am. Geriatr. Soc. 2001, 49, 1093–1096. [Google Scholar] [CrossRef]
- Tosun Tasar, P.; Sahin, S.; Karaman, E.; Oz, A.; Ulusoy, M.G.; Duman, S.; Berdeli, A.; Akcicek, F. Myostatin Gene Polymorphism in an Elderly Sarcopenic Turkish Population. Genet. Test. Mol. Biomark. 2015, 19, 457–460. [Google Scholar] [CrossRef]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef]
- McNally, E.M. Powerful genes—Myostatin regulation of human muscle mass. N. Engl. J. Med. 2004, 350, 2642–2644. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, A.; Diel, P. The growth factor myostatin, a key regulator in skeletal muscle growth and homeostasis. Int. J. Sports Med. 2005, 26, 83–89. [Google Scholar] [CrossRef]
- Aksenov, M.O. Teoretiko-Metodicheskie Osnovy Postroeniya Trenirovochnogo Processa v Tyazheloatleticheskih Vidah Sporta s Uchetom Geneticheskih Osobennostej. Ph.D. Thesis, Pedagogicheskih Nauk, Ulan-Ude, Russia, 2017; 407. [Google Scholar]
- Aksenov, M.O. Fundamentals of the Training Process in Weightlifting Sports Taking into Account Genetic Features; Publishing House of the Buryat State University: Ulan-Ude, Russia, 2018; 300p. [Google Scholar]
- Aksenov, M.O. Theoretical and Methodological Foundations of Building the Training Process in Weightlifting Sports, Taking into Account Genetic Characteristics; Publishing House of the Buryat State University: Ulan-Ude, Russia, 2017; 407p. [Google Scholar]
- Ivey, F.M.; Roth, S.M.; Ferrell, R.E.; Tracy, B.L.; Lemmer, J.T.; Hurlbut, D.E.; Martel, G.F.; Siegel, E.L.; Fozard, J.L.; Metter, E.J.; et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2000, 55, 641–648. [Google Scholar] [CrossRef]


| Group | Athletes | Control | χ2 P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | Genotype | n | ||||||
| KK | KR | RR | KK | KR | RR | ||||
| Caucasus natives [13] | 39 | 3 | 0 | 42 | 33 | 0 | 0 | 33 | - |
| African–Americans [13] | 13 | 7 | 0 | 20 | 9 | 6 | 3 | 18 | 0.157 |
| Caucasus natives, African–Americans, and Maori [60] | 43 | 7 | 0 | 50 | 92 | 6 | 2 | 100 | 0.166 |
| Eastern Russians [71] | 120 | 4 | 14 | 138 | 99 | 4 | 0 | 103 | 0.004 * |
| Western Russians [72] | 149 | 16 | 1 | 166 | 99 | 4 | 0 | 103 | 0.155 |
| Generalized data | 364 | 37 | 15 | 416 | 332 | 20 | 5 | 357 | 0.030 * |
| Group | Athletes | Control | χ2 P | link | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | Genotype | n | |||||||
| KK | KR/RR | KK | KR/RR | |||||||
| Caucasus natives | 39 | 3 | (7.1%) | 42 | 33 | 0 | (-) | 33 | 0.118 | [13] |
| African–Americans | 13 | 7 | (35.0%) | 20 | 9 | 9 | (50.0%) | 18 | 0.700 | [13] |
| Caucasus natives, African–Americans, and Maori | 43 | 7 | (14.0%) | 50 | 92 | 8 | (8.0%) | 100 | 0.249 | [60] |
| Eastern Russians | 120 | 18 | (13.0%) | 138 | 99 | 4 | (3.9%) | 103 | 0.015 * | [71] |
| Western Russians | 149 | 17 | (10.2%) | 166 | 99 | 4 | (3.9%) | 103 | 0.059 | [72] |
| Generalized data | 364 | 52 | (12.5%) | 416 | 332 | 25 | (7.9%) | 357 | 0.011 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewski, M.; Aksenov, M.O. Association of Myostatin Gene Polymorphisms with Strength and Muscle Mass in Athletes: A Systematic Review and Meta-Analysis of the MSTN rs1805086 Mutation. Genes 2022, 13, 2055. https://doi.org/10.3390/genes13112055
Kruszewski M, Aksenov MO. Association of Myostatin Gene Polymorphisms with Strength and Muscle Mass in Athletes: A Systematic Review and Meta-Analysis of the MSTN rs1805086 Mutation. Genes. 2022; 13(11):2055. https://doi.org/10.3390/genes13112055
Chicago/Turabian StyleKruszewski, Marek, and Maksim Olegovich Aksenov. 2022. "Association of Myostatin Gene Polymorphisms with Strength and Muscle Mass in Athletes: A Systematic Review and Meta-Analysis of the MSTN rs1805086 Mutation" Genes 13, no. 11: 2055. https://doi.org/10.3390/genes13112055
APA StyleKruszewski, M., & Aksenov, M. O. (2022). Association of Myostatin Gene Polymorphisms with Strength and Muscle Mass in Athletes: A Systematic Review and Meta-Analysis of the MSTN rs1805086 Mutation. Genes, 13(11), 2055. https://doi.org/10.3390/genes13112055






