Lack of Association between (AAT)n Polymorphism of the CNR1 Gene Encoding the Cannabinoid Receptor (CB1) and Patient’s Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Assessment of Pain Sensation, Hip Function, and Degree of Disability
2.2.1. The VAS Scale
2.2.2. The Harris Hip Score
2.2.3. Oswestry Disability Questionnaire
2.3. Genotyping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lievense, A.M.; Bierma-Zeinstra, S.M.A.; Verhagen, A.P.; Bernsen, R.M.D.; Verhaar, J.A.N.; Koes, B.W. Influence of sporting activities on the development of osteoarthritis of the hip: A systematic review. Arthritis Rheum. 2003, 49, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y.Q. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998, 41, 1343–1355. [Google Scholar] [CrossRef]
- Grotle, M.; Hagen, K.B.; Natvig, B.; Dahl, F.A.; Kvien, T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol. Clin. N. Am. 2004, 42, 1–9. [Google Scholar] [CrossRef]
- Klimiuk, P.A.; Kuryliszyn-Moskal, A. Choroba zwyrodnieniowa stawów. In Reumatologia; Puszczewicz, M., Ed.; Medical Tribune: Warsaw, Poland, 2012. [Google Scholar]
- Andersen, S.; Thygesen, L.C.; Davidsen, M.; Helweg-Larsen, K. Cumulative years in occupation and the risk of hip or knee oste-oarthritis in men and women: A register-based follow-up study. Occup. Environ. Med. 2012, 69, 325–330. [Google Scholar] [CrossRef]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef]
- Rodriguez-Fontenla, C.; Calaza, M.; Evangelou, E.; Valdes, A.M.; Arden, N.; Blanco, F.J.; Carr, A.; Chapman, K.; Deloukas, P.; Doherty, M.; et al. Assessment of Osteoarthritis Candidate Genes in a Meta-Analysis of Nine Genome-Wide Association Studies. Arthritis Rheum. 2014, 66, 940–949. [Google Scholar] [CrossRef]
- Huang, J.; Ushiyama, T.; Inoue, K.; Kawasaki, T.; Hukuda, S. Vitamin D receptor gene polymorphisms and osteoarthritis of the hand, hip, and knee: A case-control study in Japan. Rheumatology 2000, 39, 79–84. [Google Scholar] [CrossRef]
- Pola, E.; Papaleo, P.; Pola, R.; Gaetani, E.; Tamburelli, F.C.; Aulisa, L.; Logroscino, C.A. Brief report—Interleukin-6 gene poly-morphism and risk of osteoarthritis of the hip: A case-control study. Osteoarthr. Cartil. 2005, 13, 1025–1028. [Google Scholar] [CrossRef]
- Meulenbelt, I.; Seymour, A.B.; Nieuwland, M.; Huizinga, T.W.J.; van Duijn, C.M.; Slagboom, P.E. Association of the interleukin-1 gene cluster with radiographic signs of osteoarthritis of the hip. Arthritis Rheum. 2004, 50, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Helgason, H.; Sigurdsson, A.; Norddahl, G.L.; Agustsdottir, A.B.; Reynard, L.N.; Villalvilla, A.; Halldorsson, H.; Jonasdottir, A.; Magnusdottir, A.; et al. Whole-genome sequencing identifies rare genotypes in COMP and CHADL associated with high risk of hip osteoarthritis. Nat. Genet. 2017, 49, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.A.; Garcia-Ibarbia, C.; Gravani, A.; Raine, E.V.A.; Rodriguez-Fontenla, C.; Soto-Hermida, A.; Rego-Perez, I.; Dodd, A.W.; Gómez-Reino, J.J.; Zarrabeitia, M.T.; et al. Common variations in estrogen-related genes are associated with severe large-joint osteoarthritis: A multicenter genetic and functional study. Osteoarthr. Cartil. 2010, 18, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.J.; Berenbaum, F.; Lafeber, F. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Hunter, D.J.; McDougall, J.J.; Keefe, F.J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. N. Am. 2008, 34, 623–643. [Google Scholar] [CrossRef]
- Jóźwiak-Bębenista, M.; Nowak, J.Z. Czy wiemy już wszystko o paracetamolu? najnowsze dane na temat mechanizmu działania, efektów ubocznych i preparatów handlowych. Farmacja Polska 2012, 68, 844–857. [Google Scholar]
- Avouac, J.; Gossec, L.; Dougados, M. Efficacy and safety of opioids for osteoarthritis: A meta-analysis of randomized controlled trials. Osteoarthr. Cartil. 2007, 15, 957–965. [Google Scholar] [CrossRef][Green Version]
- Sibbitt, W.L.; Peisajovich, A.; Michael, A.A.; Park, K.S.; Sibbitt, R.R.; Band, P.A.; Bankhurst, A.D. Does Sonographic Needle Guidance Affect the Clinical Outcome of Intraarticular Injections? J. Rheumatol. 2009, 36, 1892–1902. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Majda, A.; Walas, K.; Gawełek, A. Jakość życia pacjentów z chorobą zwyrodnieniową stawów biodrowych. Problemy Pielęgniarstwa 2013, 21, 29–37. [Google Scholar]
- Buvanendran, A.; Kroin, J.S. Multimodal analgesia for controlling acute postoperative pain. Curr. Opin. Anesthesiol. 2009, 22, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, L.; Brandsborg, B.; Lucht, U.; Jensen, T.S.; Kehlet, H. Chronic pain following total hip arthroplasty: A nationwide questionnaire study. Acta Anaesthesiol. Scand. 2006, 50, 495–500. [Google Scholar] [CrossRef]
- Leźnicka, K.; Kurzawski, M.; Cięszczyk, P.; Safranow, K.; Malinowski, D.; Brzeziańska-Lasota, E.; Zmijewski, P. Genetic poly-morphism of catechol-O-methyltransferase (COMT rs4680:G>A) and µ-opioid receptor (OPRM1 rs1799971:A>G) in relation to pain perception in combat athletes. Biol. Sport 2017, 34, 295–301. [Google Scholar] [CrossRef]
- Woo, J.H.; Kim, H.; Kim, J.H.; Kim, J.G. Cannabinoid receptor gene polymorphisms and bone mineral density in Korean post-menopausal women. Menopause 2015, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; Essig, J.; Erxlebe, E.; Bab, I.; Kubisch, C.; et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005, 14, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Luongo, L.; Novellis, V.; Rossi, F.; Maione, S. The Role of Cannabinoid Receptors in the Descending Modulation of Pain. Pharmaceuticals 2010, 3, 2661–2673. [Google Scholar] [CrossRef]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-serotonin systems interaction in health and disease. Prog. Brain Res. 2021, 259, 83–134. [Google Scholar] [CrossRef]
- Straiker, A.; Wagner-Miller, J.; Hutchens, J.; Mackie, K. Differential signaling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neurons. Br. J. Pharmacol. 2012, 165, 2660–2671. [Google Scholar] [CrossRef]
- Ryberg, E.; Vu, H.K.; Larsson, N.; Groblewski, T.; Hjorth, S.; Elebring, T.; Sjögren, S.; Greasley, P.J. Identification and characterisation of a novel splice variant of the human CB1receptor. FEBS Lett. 2005, 579, 259–264. [Google Scholar] [CrossRef]
- Xiao, J.C.; Jewell, J.P.; Lin, L.S.; Hagmann, W.K.; Fong, T.M.; Shen, C.P. Similar in vitro pharmacology of human cannabinoid CB1 receptor variants expressed in CHO cells. Brain Res. 2008, 1238, 36–43. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Kelly, M.E.M.; Denovan-Wright, E.M. The dynamic nature of type 1 cannabinoid receptor (CB1) gene transcription. Br. J. Pharmacol. 2012, 167, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.W.; Ishiguro, H.; Ohtsuki, T.; Hess, J.; Carillo, F.; Walther, D.; Onaivi, E.S.; Arinami, T.; Uhl, G.R. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol. Psychiatry 2004, 9, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Bozzali, M.; Bari, M.; Mori, F.; Studer, V.; Motta, C.; Buttari, F.; Cercignani, M.; Gravina, P.; Mastrangelo, N.; et al. Association between a Genetic Variant of Type-1 Cannabinoid Receptor and Inflammatory Neurodegeneration in Multiple Sclerosis. PLoS ONE 2013, 8, e82848. [Google Scholar] [CrossRef]
- Proudnikov, D.; Kroslak, T.; Sipe, J.C.; Randesi, M.; Li, D.; Hamon, S.; Ho, A.; Ott, J.; Kreek, M.J. Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: Impact of long repeats of CNR1. Pharmacogen. J. 2010, 10, 232–242. [Google Scholar] [CrossRef]
- Benyamina, A.; Kebir, O.; Blecha, L.; Reynaud, M.; Krebs, M.O. CNR1 gene polymorphisms in addictive disorders: A systematic review and a meta-analysis. Addict. Biol. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and Pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Misiołek, H.; Mayzner-Zawadzka, E.; Dobrogowski, J.; Wordliczek, J. Zalecenia 2011 postępowania w bólu ostrym i pooperacyjnym. Ból 2011, 12, 9–33. [Google Scholar]
- Kościelna, P.; Pogorzała, A.M. Badanie funkcjonalne stawu biodrowego w przypadku zmian zwyrodnieniowych. In Innowacyjność i Tradycja w Fizjoterapii; Borowicz, A.M., Ed.; Wyższa Szkoła Edukacji i Terapii w Poznaniu: Poznań, Poland, 2017; pp. 51–70. [Google Scholar]
- Dawson, E. Identification of a polymorphic triplet repeat marker for the brain cannabinoid receptor gene: Use in linkage and association studies. Psychiatr. Genet. 1995, 5, 850. [Google Scholar] [CrossRef]
- Comings, D.E.; Muhleman, D.; Gade, R.; Johnson, P.; Verde, R.; Saucier, G.; MacMurray, J. Cannabinoid receptor gene (CNR1): Association with i.v. drug use. Mol. Psychiatry. 1997, 2, 161–168. [Google Scholar] [CrossRef]
- Schlitt, P.; Freedman, M.; Tan, T.L.; Minori, J.; Schroeder, J.T.; Parvizi, J. Predicting outcomes of total joint arthroplasty using the distress and risk assessment method. Hip Int. 2020, 30, 276–280. [Google Scholar] [CrossRef]
- Li, H.; Zeng, W.N.; Ding, Z.C.; Yuan, M.C.; Cai, Y.R.; Zhou, Z.K. Duloxetine reduces pain after Total hip arthroplasty: A prospective, randomized controlled study. BMC Musculoskelet. Disord. 2021, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Buttari, F.; Studer, V.; Motta, C.; Gravina, P.; Castelli, M.; Mantovani, V.; De Chiara, V.; Musella, A.; Fiore, S.; et al. The (AAT)(n) repeat of the cannabinoid CB1 receptor gene influences disease progression in relapsing multiple sclerosis. Mult. Scler. 2011, 17, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Medeiros, L.; Souza, A.; Silva, J. Fibromyalgia: A Review of Related Polymorphisms and Clinical Relevance. An. Acad. Bras. Cienc. 2021, 93 (Suppl. 4), e20210618. [Google Scholar] [CrossRef]
- Gerra, M.; González-Villar, A.; Arendt-Nielsen, L.; Pedersen, I.; Triñanes, Y.; Donnini, C.; Manfredini, M.; Walther, D.; Moeller, G.L.; Pidal-Miranda, M.; et al. A family-based study to identify genetic biomarkers of fibromyalgia: Consideration of patients’ subgroups. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 130), 144–152. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.A.; Fernandez-Quiroga, K.A.; Morales-San, C.P.D.; Balderas-Rentería, I.; González-Santiago, O. No asso-ciation between G1359A CB1 polymorphisms and pain in young northeastern Mexicans. Pharmacogenomics 2018, 19, 1251–1258. [Google Scholar] [CrossRef]
- Ramesh, D.; D’Agata, A.; Starkweather, A.; Young, E. Contribution of endocannabinoid gene expression and genotype on low back pain susceptibility and chronicity. Clin. J. Pain. 2018, 34, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Nishizawa, D.; Hasegawa, J.; Fukuda, K.; Ichinohe, T.; Nagashima, M.; Hayashida, M.; Ikeda, K. Short Tandem Repeat Variation in the CNR1 Gene Associated With Analgesic Requirements of Opioids in Postoperative Pain Management. Front. Genet. 2022, 13, 815089. [Google Scholar] [CrossRef]
- Camilleri, M.; Kolar, G.; Vazquez-Roque, M.; Carlson, P.; Burton, D.; Zinsmeister, A. Cannabinoid receptor 1 gene and irritable bowel syndrome: Phenotype and quantitative traits. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G553–G560. [Google Scholar] [CrossRef]
- Azim, S.; Nicholson, J.; Rebecchi, M.J.; Galbavy, W.; Feng, T.; Reinsel, R.; Volkow, N.D.; Benveniste, H.; Kaczocha, M. Endocanna-binoids and acute pain after total knee arthroplasty. Pain 2015, 156, 341–347. [Google Scholar] [CrossRef]
- Hickernell, T.R.; Lakra, A.; Berg, A.; Cooper, H.J.; Geller, J.A.; Shah, R.P. Should Cannabinoids Be Added to Multimodal Pain Regimens After Total Hip and Knee Arthroplasty? J. Arthroplasty 2018, 33, 3637–3641. [Google Scholar] [CrossRef]
- Jennings, J.M.; Angerame, M.R.; Eschen, C.L.; Phocas, A.J.; Dennis, D.A. Cannabis Use Does Not Affect Outcomes After Total Knee Arthroplasty. J. Arthroplasty 2019, 34, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Runner, R.P.; Luu, A.N.; Nassif, N.A.; Scudday, T.S.; Patel, J.J.; Barnett, S.L.; Gorab, R.S. Use of Tetrahydrocannabinol and Cannabidiol Products in the Perioperative Period Around Primary Unilateral Total Hip and Knee Arthroplasty. J. Arthroplasty 2020, 35, S138–S143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, D. The Pattern of microRNA Binding Site Distribution. Genes 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Patanwala, A.E.; Norwood, C.; Steiner, H.; Morrison, D.; Li, M.; Walsh, K.; Martinez, M.; Baker, S.E.; Snyder, E.M.; Karnes, J.H. Psy-chological and genetic predictors of pain tolerance. Clin. Transl. Sci. 2019, 12, 189–195. [Google Scholar] [CrossRef]
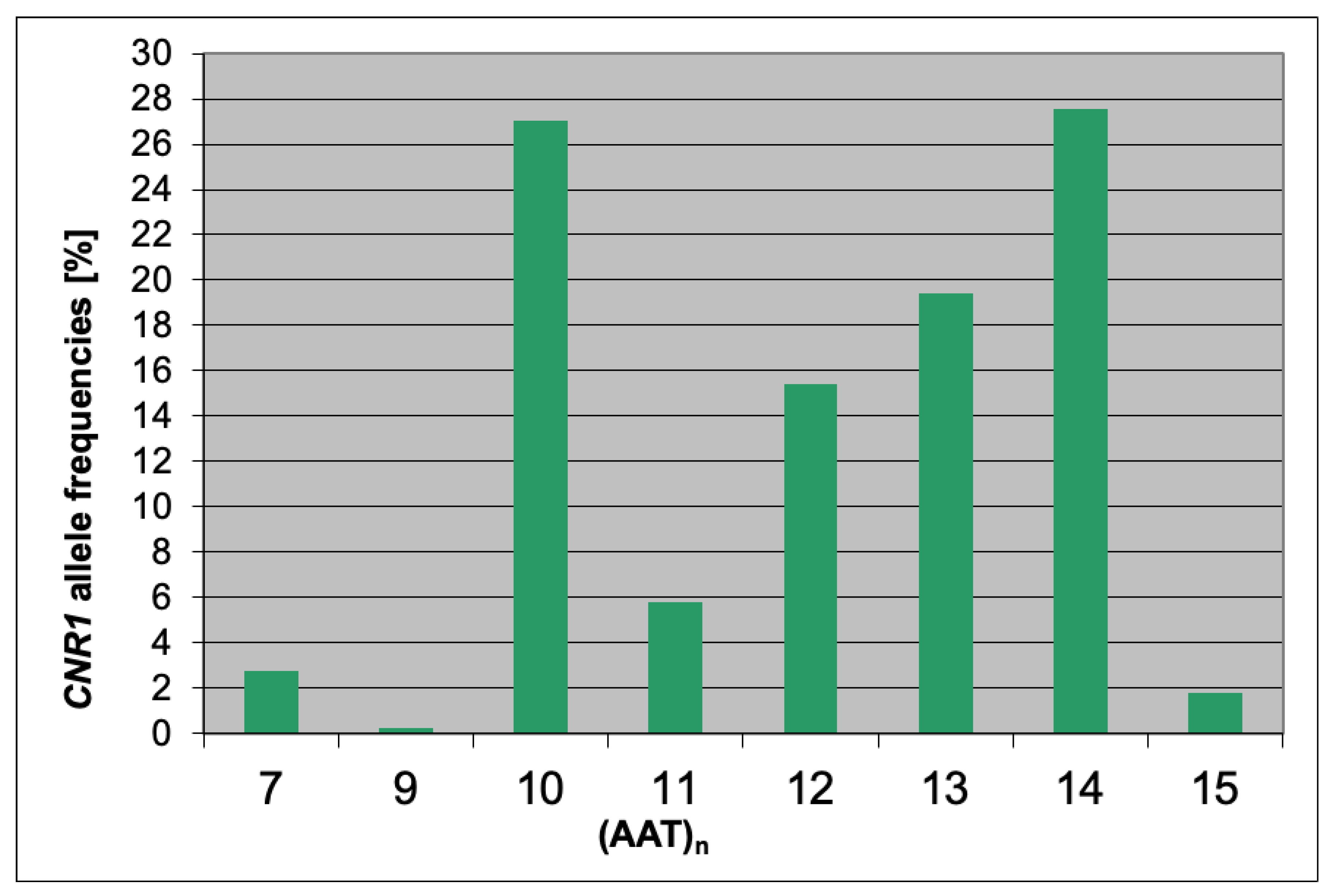
| Scale | Mean ± SD | Median | Lower Quartile | Upper Quartile | (Min–Max) |
|---|---|---|---|---|---|
| VAS (after 1.5 month) | 3.0 ± 1.1 | 3 | 2 | 4 | (0–7) |
| VAS (after 6 months) | 1.5 ± 1.7 | 1 | 0 | 2 | (0–9) |
| Scale | Mean ± SD | Median | Lower Quartile | Upper Quartile | (Min–Max) |
|---|---|---|---|---|---|
| HHS (after 1 week) | 34.9 ± 12.7 | 37 | 26 | 43 | (0–70) |
| HHS (after 1.5 month) | 69.8 ± 11.7 | 71 | 64 | 77 | (17–96) |
| HHS (after 6 months) | 86.8 ± 11.5 | 90 | 83 | 95 | (32–99) |
| Δ HHS (1.5 month—1 week) | 34.9 ± 15.1 | 33.5 | 26 | 44 | (−14–78) |
| Δ HHS (6 months—1 week) | 51.9 ± 14.4 | 52 | 43 | 61 | (10–94) |
| Scale | Mean ± SD | Median | Lower Quartile | Upper Quartile | (Min–Max) |
|---|---|---|---|---|---|
| ODI (after 1 week) | 52.2 ± 16.1 | 50 | 40 | 64 | (18–96) |
| ODI (after 1.5 month) | 18.2 ± 10.9 | 16 | 12 | 22 | (0–89) |
| ODI (after 6 months) | 7.1 ± 10.1 | 4 | 0 | 9 | (0–58) |
| Δ ODI (1.5 month—1 week) | −34.0 ± 17.7 | −30.5 | −46 | −23 | (−82–13) |
| R%Δ ODI (1.5 month—1 week) | −62.7 ± 20.9 | −64.7 | −77 | −53 | (−100–50) |
| Δ ODI (6 months—1 week) | −45.1 ± 18.2 | −44 | −58 | −33 | (−95–28) |
| R%Δ ODI (6 months—1 week) | −85.5 ± 25.5 | −93.9 | −100 | −80 | (−100–156) |
| Genotypes | Female | Male | pa | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| S-S | 11 | 8.59% | 9 | 12.86% | 0.51 |
| S-L | 54 | 42.19% | 25 | 35.71% | |
| L-L | 63 | 49.22% | 36 | 51.43% | |
| Genotypes | Female | Male | pa | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| S-S | 11 | 8.59% | 9 | 12.86% | 0.51 |
| S-L | 54 | 42.19% | 25 | 35.71% | |
| L-L | 63 | 49.22% | 36 | 51.43% | |
| Scale | Genotypes | S-S vs. S-L + L-L | S-S + S-L vs. L-L | S-S vs. L-L | S-S vs. S-L | L-L vs. S-L | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S-S (n = 20) | S-L (n = 79) | L-L (n = 99) | S-S + S-L (n = 99) | S-L + L-L (n = 178) | p & | |||||
| Mean ± SD | ||||||||||
| VAS (after 1.5 month) | 2.8 ± 1.1 | 3.1 ± 1.1 | 3.0 ± 1.0 | 3.0 ± 1.1 | 3.0 ± 1.1 | 0.21 | 0.56 | 0.19 | 0.27 | 0.88 |
| VAS (after 6 months) | 1.0 ± 1.0 | 1.4 ± 1.7 | 1.6 ± 1.8 | 1.3 ± 1.5 | 1.5 ± 1.7 | 0.34 | 0.29 | 0.26 | 0.52 | 0.44 |
| Scale | Genotypes | S-S vs. S-L + L-L | S-S + S-L vs. L-L | S-S vs. L-L | S-S vs. S-L | L-L vs. S-L | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S-S (n = 20) | S-L (n = 79) | L-L (n = 99) | S-S + S-L (n = 99) | S-L + L-L (n = 178) | p & | |||||
| Mean ± SD | ||||||||||
| HHS (after 1 week) | 37.2 ± 9.8 | 35.4 ± 12.4 | 34.0 ± 13.4 | 35.8 ± 11.9 | 34.6 ± 12.9 | 0.38 | 0.36 | 0.31 | 0.55 | 0.50 |
| HHS (after 1.5 month) | 71.3 ± 11.7 | 70.2 ± 10.1 | 69.2 ± 12.9 | 70.4 ± 10.4 | 69.7 ± 11.7 | 0.34 | 0.88 | 0.41 | 0.30 | 0.87 |
| HHS (after 6 months) | 90.7 ± 4.5 | 87.3 ± 10.1 | 85.7 ± 13.2 | 88.0 ± 9.4 | 86.4 ± 11.9 | 0.24 | 0.55 | 0.25 | 0.27 | 0.81 |
| Δ HHS (1.5 month—1 week) | 34.1 ± 14.8 | 34.8 ± 14.1 | 35.3 ± 16.1 | 34.6 ± 14.2 | 35.1 ± 15.2 | 0.99 | 0.79 | 0.92 | 0.92 | 0.79 |
| Δ HHS (6 months—1 week) | 53.5 ± 9.2 | 51.8 ± 14.4 | 51.7 ± 15.3 | 52.2 ± 13.5 | 51.8 ± 14.9 | 0.51 | 0.91 | 0.63 | 0.43 | 0.74 |
| Scale | Genotypes | S-S vs. S-L + L-L | S-S + S-L vs. L-L | S-S vs. L-L | S-S vs. S-L | L-L vs. S-L | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S-S (n = 20) | S-L (n = 79) | L-L (n = 99) | S-S + S-L (n = 99) | S-L + L-L (n = 178) | p & | |||||
| Mean ± SD | ||||||||||
| ODI (after 1 week) | 54.5 ± 15.0 | 52.0 ± 16.6 | 51.9 ± 16.1 | 52.5 ± 16.2 | 51.9 ± 16.2 | 0.40 | 0.94 | 0.46 | 0.37 | 0.83 |
| ODI (after 1.5 month) | 15.7 ± 8.0 | 18.2 ± 10.4 | 18.7 ± 11.8 | 17.7 ± 10.0 | 18.5 ± 11.2 | 0.21 | 0.88 | 0.25 | 0.23 | 0.76 |
| ODI (after 6 months) | 3.9 ± 3.7 | 6.7 ± 9.4 | 8.0 ± 11.4 | 6.1 ± 8.6 | 7.4 ± 10.6 | 0.41 | 0.39 | 0.32 | 0.59 | 0.55 |
| Δ ODI (1.5 month—1 week) | −38.8 ± 17.1 | −33.7 ± 17.6 | −33.2 ± 17.9 | −34.7 ± 17.5 | −33.4 ± 17.7 | 0.16 | 0.71 | 0.20 | 0.17 | 0.93 |
| R%Δ ODI (1.5 month—1 week) | −69.4 ± 16.4 | −62.7 ± 18.5 | −61.4 ± 23.3 | −64.0 ± 18.2 | −62.0 ± 21.3 | 0.11 | 0.72 | 0.14 | 0.11 | 0.86 |
| Δ ODI (6 months—1 week) | −50.6 ± 15.8 | −45.3 ± 18.2 | −43.8 ± 18.6 | −46.4 ± 17.8 | −44.5 ± 18.4 | 0.11 | 0.55 | 0.12 | 0.14 | 0.96 |
| R%Δ ODI (6 months—1 week) | −91.9 ± 9.7 | −86.9 ± 18.2 | −83.1 ± 31.8 | −87.9 ± 16.9 | −84.8 ± 26.6 | 0.33 | 0.45 | 0.33 | 0.40 | 0.63 |
| Scale/Questionnaire | Number of AAT Repeats in the Shorter Allele | |
|---|---|---|
| Rs | p | |
| VAS (after 1.5 month) | 0.03 | 0.63 |
| VAS (after 6 months) | 0.10 | 0.16 |
| HHS (after 1 week) | −0.04 | 0.59 |
| HHS (after 1.5 month) | 0.01 | 0.92 |
| HHS (after 6 months) | −0.04 | 0.61 |
| Δ HHS (1.5 month—1 week) | 0.02 | 0.83 |
| Δ HHS (6 months—1 week) | −0.01 | 0.92 |
| ODI (after 1 week) | −0.04 | 0.56 |
| ODI (after 1.5 month) | −0.01 | 0.87 |
| ODI (after 6 months) | 0.04 | 0.54 |
| Δ ODI (1.5 month—1 week) | 0.03 | 0.63 |
| R%Δ ODI (1.5 month—1 week) | 0.01 | 0.89 |
| Δ ODI (6 months—1 week) | 0.07 | 0.36 |
| R%Δ ODI (6 months—1 week) | 0.04 | 0.54 |
| Scale/Questionnaire | Number of AAT Repeats in the Longer Allele | |
|---|---|---|
| Rs | p | |
| VAS (after 1.5 month) | 0.08 | 0.27 |
| VAS (after 6 months) | 0.10 | 0.18 |
| HHS (after 1 week) | −0.11 | 0.11 |
| HHS (after 1.5 month) | −0.03 | 0.70 |
| HHS (after 6 months) | −0.08 | 0.27 |
| Δ HHS (1.5 month—1 week) | 0.04 | 0.54 |
| Δ HHS (6 months—1 week) | −0.001 | 0.99 |
| ODI (after 1 week) | −0.07 | 0.36 |
| ODI (after 1.5 month) | −0.01 | 0.89 |
| ODI (after 6 months) | −0.01 | 0.94 |
| Δ ODI (1.5 month—1 week) | 0.08 | 0.24 |
| R%Δ ODI (1.5 month—1 week) | 0.07 | 0.35 |
| Δ ODI (6 months—1 week) | 0.12 | 0.10 |
| R%Δ ODI (6 months—1 week) | 0.01 | 0.85 |
| Scale/Questionnaire | Average Number of AATs from both Alleles a | |
|---|---|---|
| Rs | p | |
| VAS (after 1.5 month) | 0.06 | 0.41 |
| VAS (after 6 months) | 0.12 | 0.099 |
| HHS (after 1 week) | −0.08 | 0.24 |
| HHS (after 1.5 month) | −0.01 | 0.91 |
| HHS (after 6 months) | −0.08 | 0.25 |
| Δ HHS (1.5 month—1 week) | 0.03 | 0.64 |
| Δ HHS (6 months—1 week) | −0.01 | 0.84 |
| ODI (after 1 week) | −0.06 | 0.38 |
| ODI (after 1.5 month) | −0.01 | 0.91 |
| ODI (after 6 months) | 0.03 | 0.66 |
| Δ ODI (1.5 month—1 week) | 0.07 | 0.34 |
| R%Δ ODI (1.5 month—1 week) | 0.04 | 0.56 |
| Δ ODI (6 months—1 week) | 0.10 | 0.15 |
| R%Δ ODI (6 months—1 week) | 0.04 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machoy-Mokrzyńska, A.; Rać, M.; Jurewicz, A.; Dziedziejko, V.; Safranow, K.; Kurzawski, M.; Boroń, A.; Stefaniak, A.; Leźnicka, K.; Bohatyrewicz, A.; et al. Lack of Association between (AAT)n Polymorphism of the CNR1 Gene Encoding the Cannabinoid Receptor (CB1) and Patient’s Quality of Life. Genes 2022, 13, 2046. https://doi.org/10.3390/genes13112046
Machoy-Mokrzyńska A, Rać M, Jurewicz A, Dziedziejko V, Safranow K, Kurzawski M, Boroń A, Stefaniak A, Leźnicka K, Bohatyrewicz A, et al. Lack of Association between (AAT)n Polymorphism of the CNR1 Gene Encoding the Cannabinoid Receptor (CB1) and Patient’s Quality of Life. Genes. 2022; 13(11):2046. https://doi.org/10.3390/genes13112046
Chicago/Turabian StyleMachoy-Mokrzyńska, Anna, Monika Rać, Alina Jurewicz, Violetta Dziedziejko, Krzysztof Safranow, Mateusz Kurzawski, Agnieszka Boroń, Arkadiusz Stefaniak, Katarzyna Leźnicka, Andrzej Bohatyrewicz, and et al. 2022. "Lack of Association between (AAT)n Polymorphism of the CNR1 Gene Encoding the Cannabinoid Receptor (CB1) and Patient’s Quality of Life" Genes 13, no. 11: 2046. https://doi.org/10.3390/genes13112046
APA StyleMachoy-Mokrzyńska, A., Rać, M., Jurewicz, A., Dziedziejko, V., Safranow, K., Kurzawski, M., Boroń, A., Stefaniak, A., Leźnicka, K., Bohatyrewicz, A., & Białecka, M. (2022). Lack of Association between (AAT)n Polymorphism of the CNR1 Gene Encoding the Cannabinoid Receptor (CB1) and Patient’s Quality of Life. Genes, 13(11), 2046. https://doi.org/10.3390/genes13112046







