Multiple Mitochondrial Dysfunction Syndrome Type 3: A Likely Pathogenic Homozygous Variant Affecting a Patient of Cuban Descent and Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Mitochondrial Genome Sequencing
2.2. Leukodystrophy and Leukoencephalopathy Panel
2.3. Whole Exome Sequencing Trio Analysis
2.4. Literature Review
2.5. In Silico Analysis
3. Results
3.1. Clinical Report
3.2. In Silico Analysis
3.3. Literature Review
3.3.1. Clinical Characteristics
3.3.2. Neuroradiographic Findings
3.3.3. Biochemical Findings
3.3.4. Treatments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolar, N.A.; Vanlander, A.V.; Wilbrecht, C.; Van Der Aa, N.; Smet, J.; De Paepe, B.; Vandeweyer, G.; Kooy, F.; Eyskens, F.; De Latter, E.; et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum. Mol. Genet. 2013, 22, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Janer, A.; Levandovskiy, V.; MacKay, N.; Rouault, T.A.; Tong, W.-H.; Ogilvie, I.; Shoubridge, E.A.; Robinson, B.H. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am. J. Hum. Genet. 2011, 89, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassnan, Z.N.; Al-Dosary, M.; Alfadhel, M.; Faqeih, E.A.; Alsagob, M.; Kenana, R.; Almass, R.; Al-Harazi, O.S.; Al-Hindi, H.; Malibari, O.I.; et al. ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J. Med. Genet. 2015, 52, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Hebbar, M.; Srivastava, A.; Kadavigere, R.; Upadhyai, P.; Kanthi, A.; Brandau, O.; Bielas, S.; Girisha, K.M. Homozygous p.(Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. J. Hum. Genet. 2017, 62, 723–727. [Google Scholar] [CrossRef]
- Vögtle, F.-N.; Brändl, B.; Larson, A.; Pendziwiat, M.; Friederich, M.W.; White, S.M.; Basinger, A.; Kücükköse, C.; Muhle, H.; Jähn, J.A.; et al. Mutations in PMPCB Encoding the Catalytic Subunit of the Mitochondrial Presequence Protease Cause Neurodegeneration in Early Childhood. Am. J. Hum. Genet. 2018, 102, 557–573. [Google Scholar] [CrossRef]
- Lebigot, E.; Schiff, M.; Golinelli-Cohen, M.-P. A Review of Multiple Mitochondrial Dysfunction Syndromes, Syndromes Associated with Defective Fe-S Protein Maturation. Biomedicines 2021, 9, 989. [Google Scholar] [CrossRef]
- Camponeschi, F.; Ciofi-Baffoni, S.; Calderone, V.; Banci, L. Molecular Basis of Rare Diseases Associated to the Maturation of Mitochondrial [4Fe-4S]-Containing Proteins. Biomolecules 2022, 12, 1009. [Google Scholar] [CrossRef]
- Cicchillo, R.M.; Tu, L.; Stromberg, J.A.; Hoffart, L.M.; Krebs, C.; Booker, S.J. Escherichia coli quinolinate synthetase does indeed harbor a [4Fe-4S] cluster. J. Am. Chem. Soc. 2005, 127, 7310–7311. [Google Scholar] [CrossRef]
- Beilschmidt, L.K.; Puccio, H.M. Mammalian Fe-S cluster biogenesis and its implication in disease. Biochimie 2014, 100, 48–60. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zhang, Z.; Zhou, L.; Kong, W.; Jiang, Y.; Wang, J.; Xiao, J.; Wu, Y. Genotypic Spectrum and Natural History of Cavitating Leukoencephalopathies in Childhood. Pediatr. Neurol. 2019, 94, 38–47. [Google Scholar] [CrossRef]
- Cherian, A.; Yalapalli, M.K.; P, D.K.; Vijayaraghavan, A.; Sundaram, S. Teaching NeuroImage: IBA57 Mutation–Associated Infantile Cavitating Leukoencephalopathy. Neurology 2022, 98, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Liu, X.; Ni, R.; Liu, T.; Cao, Y.; Wu, J.; Tian, W.; Luan, X.; Cao, L. Novel IBA57 mutations in two chinese patients and literature review of multiple mitochondrial dysfunction syndrome. Metab. Brain Dis. 2022, 37, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Aoki, R.; Nagano, N.; Takano, C.; Seimiya, A.; Kato, R.; Ogawa, E.; Ishige, M.; Okazaki, Y.; Murayama, K.; et al. Unique and abnormal subependymal pseudocysts in a newborn with mitochondrial disease. Sci. Prog. 2021, 104, 368504211011873. [Google Scholar] [CrossRef]
- Hu, C.; Li, X.; Zhao, L.; Shi, Y.; Zhou, S.; Wu, B.; Wang, Y. Clinical and molecular characterization of pediatric mitochondrial disorders in south of China. Eur. J. Med. Genet. 2020, 63, 103898. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, K.; Miyatake, S.; Zerem, A.; Lev, R.; Blumkin, L.; Yokochi, K.; Fujita, A.; Imagawa, E.; Iwama, K.; Nakashima, M.; et al. Expanding the phenotype of IBA57 mutations: Related leukodystrophy can remain asymptomatic. J. Hum. Genet. 2018, 63, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.S.; Sonam, K.; Chiplunkar, S.; Govindaraj, P.; Nagappa, M.; Vekhande, C.C.; Aravinda, H.R.; Ponmalar, J.J.; Mahadevan, A.; Gayathri, N.; et al. Mitochondrial leukoencephalopathies: A border zone between acquired and inherited white matter disorders in children? Mult. Scler. Relat. Disord. 2018, 20, 84–92. [Google Scholar] [CrossRef]
- Ishiyama, A.; Sakai, C.; Matsushima, Y.; Noguchi, S.; Mitsuhashi, S.; Endo, Y.; Hayashi, Y.K.; Saito, Y.; Nakagawa, E.; Komaki, H.; et al. IBA57 mutations abrogate iron-sulfur cluster assembly leading to cavitating leukoencephalopathy. Neurol. Genet. 2017, 3, e184. [Google Scholar] [CrossRef]
- Lebigot, E.; Gaignard, P.; Dorboz, I.; Slama, A.; Rio, M.; de Lonlay, P.; Héron, B.; Sabourdy, F.; Boespflug-Tanguy, O.; Cardoso, A.; et al. Impact of mutations within the [Fe-S] cluster or the lipoic acid biosynthesis pathways on mitochondrial protein expression profiles in fibroblasts from patients. Mol. Genet. Metab. 2017, 122, 85–94. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Zhang, Z.; Zhou, L.; Jiang, Y.; Wang, J.; Xiao, J.; Wu, Y. Phenotypic spectrum of mutations in IBA57, a candidate gene for cavitating leukoencephalopathy. Clin. Genet. 2018, 93, 235–241. [Google Scholar] [CrossRef]
- Torraco, A.; Ardissone, A.; Invernizzi, F.; Rizza, T.; Fiermonte, G.; Niceta, M.; Zanetti, N.; Martinelli, D.; Vozza, A.; Verrigni, D.; et al. Novel mutations in IBA57 are associated with leukodystrophy and variable clinical phenotypes. J. Neurol. 2017, 264, 102–111. [Google Scholar] [CrossRef]
- Debray, F.-G.; Stümpfig, C.; Vanlander, A.V.; Dideberg, V.; Josse, C.; Caberg, J.-H.; Boemer, F.; Bours, V.; Stevens, R.; Seneca, S.; et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes fatal infantile leukodystrophy. J. Inherit. Metab. Dis. 2015, 38, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Lossos, A.; Stümpfig, C.; Stevanin, G.; Gaussen, M.; Zimmerman, B.-E.; Mundwiller, E.; Asulin, M.; Chamma, L.; Sheffer, R.; Misk, A.; et al. Fe/S protein assembly gene IBA57 mutation causes hereditary spastic paraplegia. Neurology 2015, 84, 659. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Smeitink, J.A.M.; Mariman, E.C.M.; Loeffen, J.; Plecko, B.; Trijbels, F.J.M.; Stöckler-Ipsiroglu, S.; van den Heuvel, L. Mutant NDUFV1 subunit of mitochondrial complex I causes leukodystrophy and myoclonic epilepsy. Nat. Genet. 1999, 21, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Bibat, G.; Lin, D.; Burger, P.; Barker, P.; Rosemberg, S.; Braverman, N.; Arroyo, H.; Dowling, M.; Hamosh, A.; et al. Progressive cavitating leukoencephalopathy: A novel childhood disease. Ann. Neurol. 2005, 58, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gourdoupis, S.; Nasta, V.; Calderone, V.; Ciofi-Baffoni, S.; Banci, L. IBA57 Recruits ISCA2 to Form a [2Fe-2S] Cluster-Mediated Complex. J. Am. Chem. Soc. 2018, 140, 14401–14412. [Google Scholar] [CrossRef]
- Gourdoupis, S.; Nasta, V.; Ciofi-Baffoni, S.; Banci, L.; Calderone, V. In-house high-energy-remote SAD phasing using the magic triangle: How to tackle the P1 low symmetry using multiple orientations of the same crystal of human IBA57 to increase the multiplicity. Acta Crystallogr. D Struct. Biol. 2019, 75, 317–324. [Google Scholar] [CrossRef]
- Nasta, V.; Da Vela, S.; Gourdoupis, S.; Ciofi-Baffoni, S.; Svergun, D.I.; Banci, L. Structural properties of [2Fe-2S] ISCA2-IBA57: A complex of the mitochondrial iron-sulfur cluster assembly machinery. Sci. Rep. 2019, 9, 18986. [Google Scholar] [CrossRef]
- Tavtigian, S.V.; Harrison, S.M.; Boucher, K.M.; Biesecker, L.G. Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum. Mutat. 2020, 41, 1734–1737. [Google Scholar] [CrossRef]
- Vinkler, C.; Lev, D.; Kalish, H.; Watemberg, N.; Yanoov-Sharav, M.; Leshinsky-Silver, E.; Lerman-Sagie, T. Familial optic atrophy with white matter changes. Am. J. Med. Genet. A 2003, 121a, 263–265. [Google Scholar] [CrossRef]
- Phoenix, C.; Schaefer, A.; Elson, J.; Morava, E.; Bugiani, M.; Uziel, G.; Smeitink, J.; Turnbull, D.; McFarland, R. A scale to monitor progression and treatment of mitochondrial disease in children. Neuromuscul. Disord. 2006, 16, 814–820. [Google Scholar] [CrossRef]



| Evidence of Pathogenicity | Category | Evidence | Score |
|---|---|---|---|
| Moderate | PM1 | Hotspot of length 17 amino acid residues contains 7 pathogenic variants (2 frameshift, 2 nonsense, and 3 missense), 3 variants of uncertain significance (missense), and no benign variants | 2 |
| PM2 | Variant not found in gnomAD genomes | 2 | |
| PM3 | Detected in trans with a pathogenic variant (Sato et al., 2021) | 2 | |
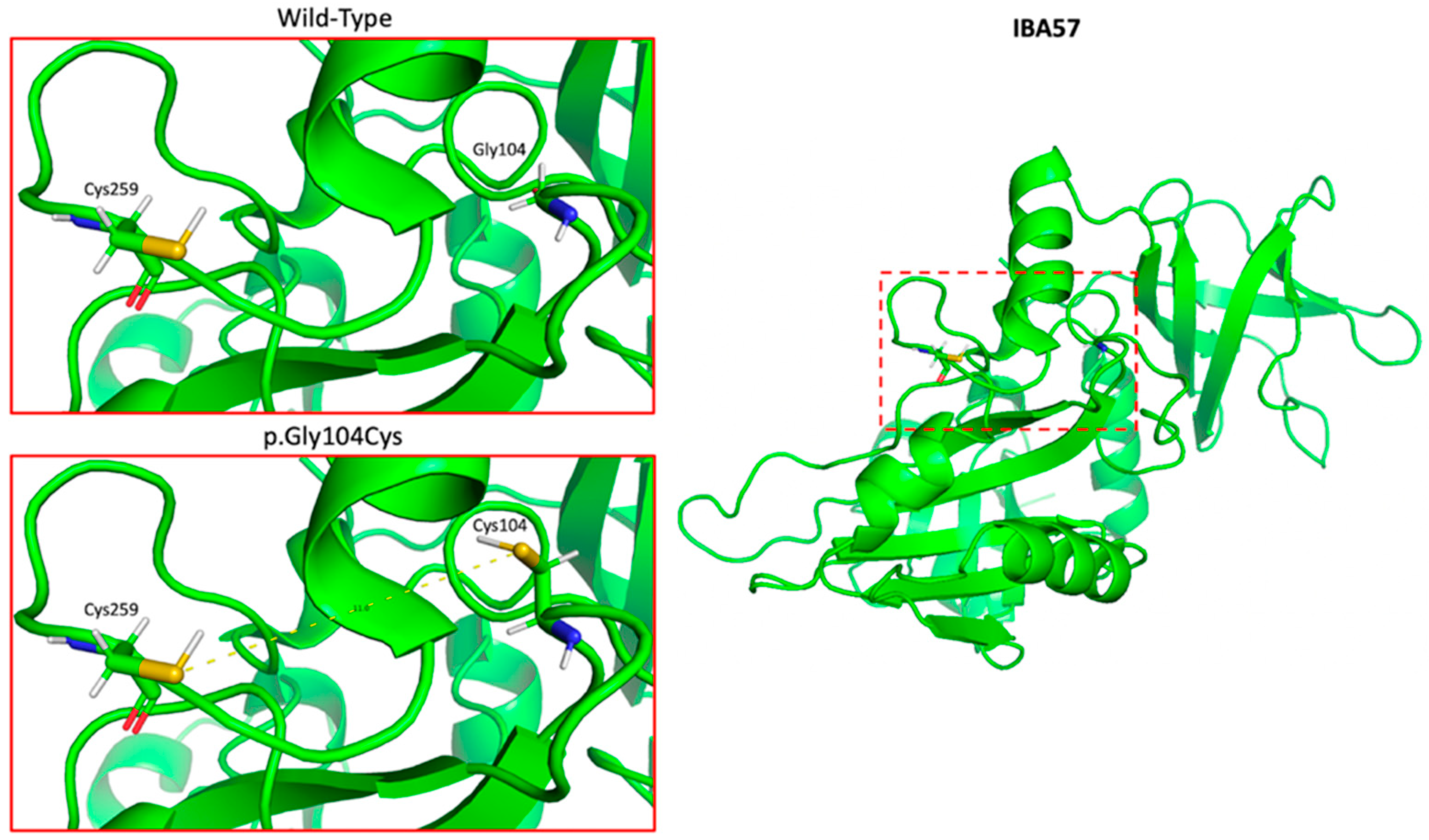
| Supporting | PP3 | Multiple lines of computational evidence support a deleterious effect on the gene In addition to PyMOL model and MutationTaster used in our manuscript, BayesDel_addAF gives the variant a score of 0.546, which is between 0.421 and 0.625, thus predicting strong pathogenicity | 1 |
| PP4 | Patient’s phenotype is highly specific for multiple mitochondrial dysfunctions syndrome-3 (MMDS3), as demonstrated by our review of the literature | 1 | |
| Total | 8 |
| Presenting Sign | % (n/N) | Age (Months) [Range] |
|---|---|---|
| Psychomotor Regression | 81.8 (27/33) | - |
| Decreased Energy | 12.1 (4/33) | - |
| Hypotonia at Birth | 9.1 (3/33) | - |
| Visual Impairment | 6.1 (2/33) | - |
| Hemiparesis | 3 (1/33) | - |
| Sex | ||
| Female | 54.9 (28/51) | - |
| Male | 45.1 (23/51) | - |
| Neurological Features | ||
| Impaired Motor Function | 97.6 (40/41) | - |
| Hypotonia | 91.7 (11/12) | - |
| Spasticity | 84.6 (11/13) | - |
| Spastic Tetraparesis | 50 (5/10) | - |
| Intellectual Disability | 80 (12/15) | - |
| Dysphagia | 80 (4/5) | - |
| Muscle Weakness | 71.4 (5/7) | - |
| Limb Contractures | 60 (3/5) | - |
| Nystagmus | 60 (3/5) | - |
| Epilepsy | 35 (7/20) | - |
| Ophthalmologic Features | ||
| Visual Impairment | 46.7 (7/15) | - |
| Biochemical Findings | ||
| Elevated CSF Lactate | 72.7 (8/11) | - |
| Elevated Serum Glycine | 60 (6/10) | - |
| Elevated Serum Lactate | 53.8 (7/13) | - |
| Other | - | |
| Feeding Difficulty | 100 (6/6) | - |
| Apneic Episodes/Respiratory Failure | 100 (6/6) | - |
| Natural History | ||
| Normal Development Prior to Presentation | 67.9 (19/28) | - |
| Median Age Of Symptom Onset, N = 52 | - | 9 (0–22) |
| Median Age at Last Evaluation, N = 30 | - | 27 (0–348) |
| Median Age of Death, N = 10 | - | 7 (0–27) |
| Preceding Infection/Vaccine | 56 (14/25) | - |
| Stable/Improvement | 27.3 (6/22) | - |
| Paroxysmal Worsening | 22.7 (5/22) | - |
| Progressive Worsening | 50 (11/22) | - |
| MRI Findings | |
|---|---|
| White Matter Lesion Location | % (n/N) |
| Periventricular | 83.3 (20/24) |
| Parieto-occipital White Matter | 66.7 (14/21) |
| Corpus Callosum | 62.5 (15/24) |
| Splenium | 38.5 (5/13) |
| Spinal Cord | 41.7 (5/12) |
| Frontal White Matter | 30 (3/10) |
| Brainstem | 28.6 (4/14) |
| Cerebellum | 25.9 (7/27) |
| Internal Capsule | 22.7 (5/22) |
| Temporal White Matter | 19 (4/21) |
| Thalamus | 10 (1/10) |
| Optic Nerves | 10 (1/10) |
| Other | |
| Lactate Peak on MRS | 100 (9/9) |
| Presence of Cavitations | 81.5 (22/27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, S.H.; Camponeschi, F.; de Joya, E.; Borjas-Mendoza, P.; Tekin, M.; Thorson, W. Multiple Mitochondrial Dysfunction Syndrome Type 3: A Likely Pathogenic Homozygous Variant Affecting a Patient of Cuban Descent and Literature Review. Genes 2022, 13, 2044. https://doi.org/10.3390/genes13112044
Lang SH, Camponeschi F, de Joya E, Borjas-Mendoza P, Tekin M, Thorson W. Multiple Mitochondrial Dysfunction Syndrome Type 3: A Likely Pathogenic Homozygous Variant Affecting a Patient of Cuban Descent and Literature Review. Genes. 2022; 13(11):2044. https://doi.org/10.3390/genes13112044
Chicago/Turabian StyleLang, Steven H., Francesca Camponeschi, Evan de Joya, Paulo Borjas-Mendoza, Mustafa Tekin, and Willa Thorson. 2022. "Multiple Mitochondrial Dysfunction Syndrome Type 3: A Likely Pathogenic Homozygous Variant Affecting a Patient of Cuban Descent and Literature Review" Genes 13, no. 11: 2044. https://doi.org/10.3390/genes13112044
APA StyleLang, S. H., Camponeschi, F., de Joya, E., Borjas-Mendoza, P., Tekin, M., & Thorson, W. (2022). Multiple Mitochondrial Dysfunction Syndrome Type 3: A Likely Pathogenic Homozygous Variant Affecting a Patient of Cuban Descent and Literature Review. Genes, 13(11), 2044. https://doi.org/10.3390/genes13112044






