Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells
Abstract
1. Introduction
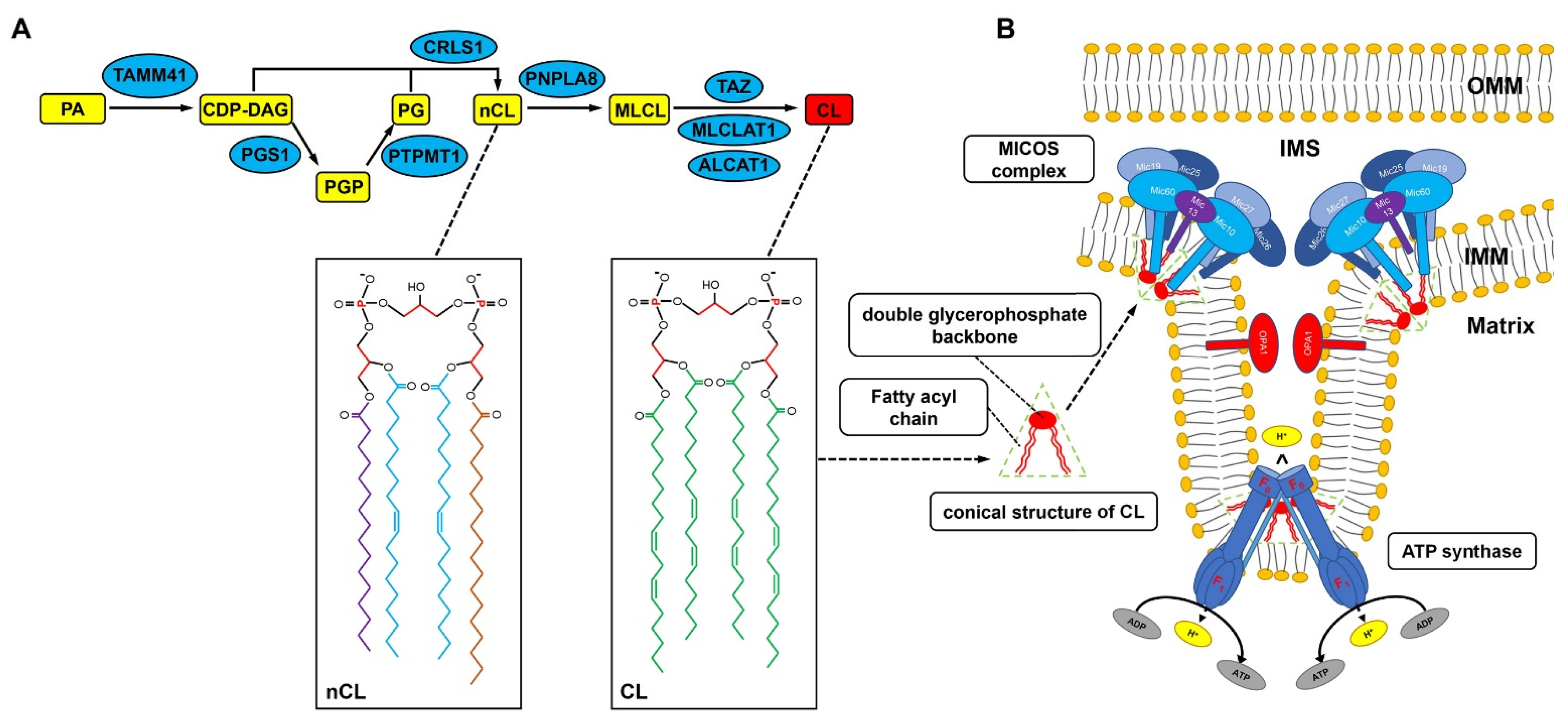
2. CL Biosynthesis and Remodeling
3. CL and Mitochondrial Cristae Formation
4. CL Regulates Mitochondrial Function
5. CL Deficiency and Diseases
6. CL and Cardiomyopathy
7. CL in Neuronal Diseases
8. CL in Immune Cells
9. CL and Metabolic Disorders
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, L.; Tso, A.; Wang, S.; Fang, X.; Ouyang, K.; Han, Z. Mitochondrial Chaperones and Proteases in Cardiomyocytes and Heart Failure. Front. Mol. Biosci. 2021, 8, 630332. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Lu, Y.W.; Claypool, S.M. Disorders of phospholipid metabolism: An emerging class of mitochondrial disease due to defects in nuclear genes. Front. Genet. 2015, 6, 3. [Google Scholar] [CrossRef]
- Hatch, G.M. Cell biology of cardiac mitochondrial phospholipids. Biochem. Cell Biol. 2004, 82, 99–112. [Google Scholar] [CrossRef]
- Elías-Wolff, F.; Lindén, M.; Lyubartsev, A.P.; Brandt, E.G. Curvature sensing by cardiolipin in simulated buckled membranes. Soft Matter 2019, 15, 792–802. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Di Venosa, N.; Federici, A.; Ruggiero, F.M. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: Involvement of reactive oxygen species and cardiolipin. Circ. Res. 2004, 94, 53–59. [Google Scholar] [CrossRef]
- Jalmar, O.; François-Moutal, L.; García-Sáez, A.J.; Perry, M.; Granjon, T.; Gonzalvez, F.; Gottlieb, E.; Ayala-Sanmartin, J.; Klösgen, B.; Schwille, P.; et al. Caspase-8 binding to cardiolipin in giant unilamellar vesicles provides a functional docking platform for bid. PLoS ONE 2013, 8, e55250. [Google Scholar] [CrossRef]
- Miranda-Díaz, A.G.; Cardona-Muñoz, E.G.; Pacheco-Moisés, F.P. The Role of Cardiolipin and Mitochondrial Damage in Kidney Transplant. Oxidative Med. Cell. Longev. 2019, 2019, 3836186. [Google Scholar] [CrossRef] [PubMed]
- Ikon, N.; Ryan, R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, S.; Wang, H.; Wang, L.; Zhang, J.; Gu, Y.; Tan, C.; Dhanani, M.; Wever, E.; Wang, X.; et al. PTPMT1 Is Required for Embryonic Cardiac Cardiolipin Biosynthesis to Regulate Mitochondrial Morphogenesis and Heart Development. Circulation 2021, 144, 403–406. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Z.; Zhu, M.; Shen, Y.; Leon, L.J.; Chi, L.; Spinozzi, S.; Tan, C.; Gu, Y.; Nguyen, A.; et al. Cardiolipin Remodeling Defects Impair Mitochondrial Architecture and Function in a Murine Model of Barth Syndrome Cardiomyopathy. Circ. Heart Fail. 2021, 14, e008289. [Google Scholar] [CrossRef]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef]
- Malhotra, K.; Modak, A.; Nangia, S.; Daman, T.H.; Gunsel, U.; Robinson, V.L.; Mokranjac, D.; May, E.R.; Alder, N.N. Cardiolipin mediates membrane and channel interactions of the mitochondrial TIM23 protein import complex receptor Tim50. Sci. Adv. 2017, 3, e1700532. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Bao, Y.; Mitchell-Silbaugh, K.; Veevers, J.; Fang, X. Barth Syndrome Cardiomyopathy: An Update. Genes 2022, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Falabella, M.; Vernon, H.J.; Hanna, M.G.; Claypool, S.M.; Pitceathly, R.D.S. Cardiolipin, Mitochondria, and Neurological Disease. Trends Endocrinol. Metab. 2021, 32, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Pelegrin, P. Cardiolipin in Immune Signaling and Cell Death. Trends Cell Biol. 2020, 30, 892–903. [Google Scholar] [CrossRef]
- Ren, M.; Phoon, C.K.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- El-Hafidi, M.; Correa, F.; Zazueta, C. Mitochondrial dysfunction in metabolic and cardiovascular diseases associated with cardiolipin remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165744. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Cardiolipin and mitochondrial function in health and disease. Antioxid. Redox Signal. 2014, 20, 1925–1953. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Kim, Y.J.; Balla, T.; Epand, R.M. Distinct properties of the two isoforms of CDP-diacylglycerol synthase. Biochemistry 2014, 53, 7358–7367. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Gomez-Espinosa, E.; Ashlin, T.G.; Cockcroft, S. Mitochondrial CDP-diacylglycerol synthase activity is due to the peripheral protein, TAMM41 and not due to the integral membrane protein, CDP-diacylglycerol synthase 1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 284–298. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kuge, O.; Chang, S.C.; Heacock, P.N.; Rho, M.; Suzuki, K.; Nishijima, M.; Dowhan, W. Isolation of a chinese hamster ovary (CHO) cDNA encoding phosphatidylglycerophosphate (PGP) synthase, expression of which corrects the mitochondrial abnormalities of a PGP synthase-defective mutant of CHO-K1 cells. J. Biol. Chem. 1999, 274, 1828–1834. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, Z.; Murphy, A.N.; Wiley, S.E.; Perkins, G.A.; Worby, C.A.; Engel, J.L.; Heacock, P.; Nguyen, O.K.; Wang, J.H.; et al. Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab. 2011, 13, 690–700. [Google Scholar] [CrossRef]
- Schlame, M.; Ren, M.; Xu, Y.; Greenberg, M.L.; Haller, I. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids 2005, 138, 38–49. [Google Scholar] [CrossRef]
- Beranek, A.; Rechberger, G.; Knauer, H.; Wolinski, H.; Kohlwein, S.D.; Leber, R. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J. Biol. Chem. 2009, 284, 11572–11578. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.J.; Kotzbauer, P.; Wozniak, D.F.; Sims, H.F.; Jenkins, C.M.; Guan, S.; Han, X.; Yang, K.; Sun, G.; Malik, I.; et al. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction. J. Biol. Chem. 2009, 284, 35632–35644. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J.; et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010, 12, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sutachan, J.J.; Plesken, H.; Kelley, R.I.; Schlame, M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Investig. 2005, 85, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; McCaffery, J.M.; Koehler, C.M. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 2006, 174, 379–390. [Google Scholar] [CrossRef]
- Taylor, W.A.; Hatch, G.M. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 2003, 278, 12716–12721. [Google Scholar] [CrossRef]
- Taylor, W.A.; Hatch, G.M. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1). J. Biol. Chem. 2009, 284, 30360–30371. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Lockwood, J.; Burn, P.; Shi, Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 2004, 279, 31727–31734. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Nie, J.; Andersen, J.P.; Rendon, S.; Zheng, Y.; Liu, X.; Tian, Z.; Shi, Y. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 3498–3511. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Qi, S.; Liu, Z.; Zhang, X.; Zheng, Y.; Andersen, J.P.; Zhang, W.; Strong, R.; Martinez, P.A.; et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell 2019, 18, e12941. [Google Scholar] [CrossRef]
- Liu, X.; Ye, B.; Miller, S.; Yuan, H.; Zhang, H.; Tian, L.; Nie, J.; Imae, R.; Arai, H.; Li, Y.; et al. Ablation of ALCAT1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy. Mol. Cell. Biol. 2012, 32, 4493–4504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y. In Search of the Holy Grail: Toward a Unified Hypothesis on Mitochondrial Dysfunction in Age-Related Diseases. Cells 2022, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Mohammadyani, D.; Angeli, J.P.; Baranov, S.V.; Klein-Seetharaman, J.; Friedlander, R.M.; Mallampalli, R.K.; Conrad, M.; et al. Cardiolipin signaling mechanisms: Collapse of asymmetry and oxidation. Antioxid. Redox Signal. 2015, 22, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Vaz, F.M. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. CMLS 2008, 65, 2493–2506. [Google Scholar] [CrossRef] [PubMed]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef]
- Schlattner, U.; Tokarska-Schlattner, M.; Rousseau, D.; Boissan, M.; Mannella, C.; Epand, R.; Lacombe, M.L. Mitochondrial cardiolipin/phospholipid trafficking: The role of membrane contact site complexes and lipid transfer proteins. Chem. Phys. Lipids 2014, 179, 32–41. [Google Scholar] [CrossRef]
- Epand, R.F.; Tokarska-Schlattner, M.; Schlattner, U.; Wallimann, T.; Epand, R.M. Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J. Mol. Biol. 2007, 365, 968–980. [Google Scholar] [CrossRef]
- Kondadi, A.K.; Anand, R.; Reichert, A.S. Cristae Membrane Dynamics-A Paradigm Change. Trends Cell Biol. 2020, 30, 923–936. [Google Scholar] [CrossRef]
- Boyd, K.J.; Alder, N.N.; May, E.R. Buckling Under Pressure: Curvature-Based Lipid Segregation and Stability Modulation in Cardiolipin-Containing Bilayers. Langmuir ACS J. Surf. Colloids 2017, 33, 6937–6946. [Google Scholar] [CrossRef]
- Kasahara, T.; Kubota-Sakashita, M.; Nagatsuka, Y.; Hirabayashi, Y.; Hanasaka, T.; Tohyama, K.; Kato, T. Cardiolipin is essential for early embryonic viability and mitochondrial integrity of neurons in mammals. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 1465–1480. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Guo, X.; Li, Y.; Ogurlu, R.; Lu, F.; Prondzynski, M.; de la Serna Buzon, S.; Ma, Q.; Zhang, D.; et al. Increased Reactive Oxygen Species-Mediated Ca2+/Calmodulin-Dependent Protein Kinase II Activation Contributes to Calcium Handling Abnormalities and Impaired Contraction in Barth Syndrome. Circulation 2021, 143, 1894–1911. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Nickel, A.; Kohlhaas, M.; Hohl, M.; Sequeira, V.; Brune, C.; Schwemmlein, J.; Abeßer, M.; Schuh, K.; Kutschka, I.; et al. Loss of Mitochondrial Ca2+ Uniporter Limits Inotropic Reserve and Provides Trigger and Substrate for Arrhythmias in Barth Syndrome Cardiomyopathy. Circulation 2021, 144, 1694–1713. [Google Scholar] [CrossRef] [PubMed]
- Bowron, A.; Honeychurch, J.; Williams, M.; Tsai-Goodman, B.; Clayton, N.; Jones, L.; Shortland, G.J.; Qureshi, S.A.; Heales, S.J.; Steward, C.G. Barth syndrome without tetralinoleoyl cardiolipin deficiency: A possible ameliorated phenotype. J. Inherit. Metab. Dis. 2015, 38, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Rodenburg, R.J.; Thiels, C.; van Lenthe, H.; Stet, F.; Poll-The, B.T.; Stone, J.E.; Steward, C.G.; Wanders, R.J.; Smeitink, J.; et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal. Biochem. 2009, 387, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sustarsic, E.G.; Ma, T.; Lynes, M.D.; Larsen, M.; Karavaeva, I.; Havelund, J.F.; Nielsen, C.H.; Jedrychowski, M.P.; Moreno-Torres, M.; Lundh, M.; et al. Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab. 2018, 28, 159–174.e11. [Google Scholar] [CrossRef] [PubMed]
- Phoon, C.K.; Acehan, D.; Schlame, M.; Stokes, D.L.; Edelman-Novemsky, I.; Yu, D.; Xu, Y.; Viswanathan, N.; Ren, M. Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J. Am. Heart Assoc. 2012, 1, e000455. [Google Scholar] [CrossRef]
- Acehan, D.; Xu, Y.; Stokes, D.L.; Schlame, M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab. Investig. 2007, 87, 40–48. [Google Scholar] [CrossRef]
- Acehan, D.; Vaz, F.; Houtkooper, R.H.; James, J.; Moore, V.; Tokunaga, C.; Kulik, W.; Wansapura, J.; Toth, M.J.; Strauss, A.; et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 2011, 286, 899–908. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M.E. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101. [Google Scholar] [CrossRef]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef]
- Laage, S.; Tao, Y.; McDermott, A.E. Cardiolipin interaction with subunit c of ATP synthase: Solid-state NMR characterization. Biochim. Biophys. Acta 2015, 1848, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Kim, A.A.; Yaguzhinsky, L.S.; Dagda, R.K. Non-bilayer structures in mitochondrial membranes regulate ATP synthase activity. Biochim. Biophys. Acta Biomembr. 2018, 1860, 586–599. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, M.; Horvath, S.E.; Pfanner, N. Mitochondrial contact site and cristae organizing system. Curr. Opin. Cell Biol. 2016, 41, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Stephan, T.; Brüser, C.; Deckers, M.; Steyer, A.M.; Balzarotti, F.; Barbot, M.; Behr, T.S.; Heim, G.; Hübner, W.; Ilgen, P.; et al. MICOS assembly controls mitochondrial inner membrane remodeling and crista junction redistribution to mediate cristae formation. EMBO J. 2020, 39, e104105. [Google Scholar] [CrossRef]
- Anand, R.; Kondadi, A.K.; Meisterknecht, J.; Golombek, M.; Nortmann, O.; Riedel, J.; Peifer-Weiß, L.; Brocke-Ahmadinejad, N.; Schlütermann, D.; Stork, B.; et al. MIC26 and MIC27 cooperate to regulate cardiolipin levels and the landscape of OXPHOS complexes. Life Sci. Alliance 2020, 3, e202000711. [Google Scholar] [CrossRef]
- Rampelt, H.; Wollweber, F.; Gerke, C.; de Boer, R.; van der Klei, I.J.; Bohnert, M.; Pfanner, N.; van der Laan, M. Assembly of the Mitochondrial Cristae Organizer Mic10 Is Regulated by Mic26-Mic27 Antagonism and Cardiolipin. J. Mol. Biol. 2018, 430, 1883–1890. [Google Scholar] [CrossRef]
- Friedman, J.R.; Mourier, A.; Yamada, J.; McCaffery, J.M.; Nunnari, J. MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. eLife 2015, 4, e07739. [Google Scholar] [CrossRef]
- Koob, S.; Barrera, M.; Anand, R.; Reichert, A.S. The non-glycosylated isoform of MIC26 is a constituent of the mammalian MICOS complex and promotes formation of crista junctions. Biochim. Biophys. Acta 2015, 1853, 1551–1563. [Google Scholar] [CrossRef]
- Musatov, A.; Sedlák, E. Role of cardiolipin in stability of integral membrane proteins. Biochimie 2017, 142, 102–111. [Google Scholar] [CrossRef]
- Musatov, A.; Robinson, N.C. Bound cardiolipin is essential for cytochrome c oxidase proton translocation. Biochimie 2014, 105, 159–164. [Google Scholar] [CrossRef]
- Planas-Iglesias, J.; Dwarakanath, H.; Mohammadyani, D.; Yanamala, N.; Kagan, V.E.; Klein-Seetharaman, J. Cardiolipin Interactions with Proteins. Biophys. J. 2015, 109, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, J.A. Supramolecular Organization of Respiratory Complexes. Annu. Rev. Physiol. 2016, 78, 533–561. [Google Scholar] [CrossRef]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Arnarez, C.; Marrink, S.J.; Periole, X. Molecular mechanism of cardiolipin-mediated assembly of respiratory chain supercomplexes. Chem. Sci. 2016, 7, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef]
- Böttinger, L.; Horvath, S.E.; Kleinschroth, T.; Hunte, C.; Daum, G.; Pfanner, N.; Becker, T. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J. Mol. Biol. 2012, 423, 677–686. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Lazarou, M.; Thorburn, D.R.; Ryan, M.T. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 2006, 361, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids 2014, 179, 42–48. [Google Scholar] [CrossRef]
- Schlame, M.; Ren, M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim. Biophys. Acta 2009, 1788, 2080–2083. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nagao, S.; Hirota, S. Characterization of the Cytochrome c Membrane-Binding Site Using Cardiolipin-Containing Bicelles with NMR. Angew. Chem. Int. Ed. Engl. 2016, 55, 14019–14022. [Google Scholar] [CrossRef]
- Lee, R.G.; Gao, J.; Siira, S.J.; Shearwood, A.M.; Ermer, J.A.; Hofferek, V.; Mathews, J.C.; Zheng, M.; Reid, G.E.; Rackham, O.; et al. Cardiolipin is required for membrane docking of mitochondrial ribosomes and protein synthesis. J. Cell Sci. 2020, 133, jcs240374. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Klingenberg, M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry 1985, 24, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L.I.; Drees, M.; Beyer, K.; Klingenberg, M.; Marsh, D. Lipid-protein interactions in ADP-ATP carrier/egg phosphatidylcholine recombinants studied by spin-label ESR spectroscopy. Biochemistry 1990, 29, 10664–10669. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, F.; Palmieri, F. Specific elution from hydroxylapatite of the mitochondrial phosphate carrier by cardiolipin. Biochim. Biophys. Acta 1984, 766, 386–394. [Google Scholar] [CrossRef]
- Nałecz, K.A.; Bolli, R.; Wojtczak, L.; Azzi, A. The monocarboxylate carrier from bovine heart mitochondria: Partial purification and its substrate-transporting properties in a reconstituted system. Biochim. Biophys. Acta 1986, 851, 29–37. [Google Scholar] [CrossRef]
- Giudetti, A.M.; Siculella, L.; Gnoni, G.V. Citrate carrier activity and cardiolipin level in eel (Anguilla anguilla) liver mitochondria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 227–234. [Google Scholar] [CrossRef]
- Raja, V.; Joshi, A.S.; Li, G.; Maddipati, K.R.; Greenberg, M.L. Loss of Cardiolipin Leads to Perturbation of Acetyl-CoA Synthesis. J. Biol. Chem. 2017, 292, 1092–1102. [Google Scholar] [CrossRef]
- Li, Y.; Lou, W.; Raja, V.; Denis, S.; Yu, W.; Schmidtke, M.W.; Reynolds, C.A.; Schlame, M.; Houtkooper, R.H.; Greenberg, M.L. Cardiolipin-induced activation of pyruvate dehydrogenase links mitochondrial lipid biosynthesis to TCA cycle function. J. Biol. Chem. 2019, 294, 11568–11578. [Google Scholar] [CrossRef]
- Claypool, S.M.; Oktay, Y.; Boontheung, P.; Loo, J.A.; Koehler, C.M. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008, 182, 937–950. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion 2001, 1, 151–159. [Google Scholar] [CrossRef]
- Chen, Y.C.; Taylor, E.B.; Dephoure, N.; Heo, J.M.; Tonhato, A.; Papandreou, I.; Nath, N.; Denko, N.C.; Gygi, S.P.; Rutter, J. Identification of a protein mediating respiratory supercomplex stability. Cell Metab. 2012, 15, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, G.K.; Vikulina, A.S.; Volodkin, D.; Vladimirov, Y.A. Structure of the complex of cytochrome c with cardiolipin in non-polar environment. Chem. Phys. Lipids 2018, 214, 35–45. [Google Scholar] [CrossRef]
- Ott, M.; Robertson, J.D.; Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 2002, 99, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Huang, C.H.; Lin, Y.H.; Wang, C.C.; Jan, T.R. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic. Biol. Med. 2018, 124, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Casanova, G.; Matera, M.; Ruggiero, F.M.; Paradies, G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: Induction of permeability transition and cytochrome c release. FEBS Lett. 2006, 580, 6311–6316. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.A.; Greenberg, M.L. Cardiolipin-mediated cellular signaling. Adv. Exp. Med. Biol. 2013, 991, 195–213. [Google Scholar] [CrossRef]
- Nomura, K.; Imai, H.; Koumura, T.; Kobayashi, T.; Nakagawa, Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000, 351, 183–193. [Google Scholar] [CrossRef]
- Lutter, M.; Fang, M.; Luo, X.; Nishijima, M.; Xie, X.; Wang, X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000, 2, 754–761. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Schug, Z.T.; Houtkooper, R.H.; MacKenzie, E.D.; Brooks, D.G.; Wanders, R.J.; Petit, P.X.; Vaz, F.M.; Gottlieb, E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 2008, 183, 681–696. [Google Scholar] [CrossRef]
- Heit, B.; Yeung, T.; Grinstein, S. Changes in mitochondrial surface charge mediate recruitment of signaling molecules during apoptosis. Am. J. Physiol. Cell Physiol. 2011, 300, C33–C41. [Google Scholar] [CrossRef]
- Sani, M.A.; Dufourc, E.J.; Gröbner, G. How does the Bax-alpha1 targeting sequence interact with mitochondrial membranes? The role of cardiolipin. Biochim. Biophys. Acta 2009, 1788, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Gasanoff, E.S.; Yaguzhinsky, L.S.; Garab, G. Cardiolipin, Non-Bilayer Structures and Mitochondrial Bioenergetics: Relevance to Cardiovascular Disease. Cells 2021, 10, 1721. [Google Scholar] [CrossRef] [PubMed]
- Corrado, M.; Edwards-Hicks, J.; Villa, M.; Flachsmann, L.J.; Sanin, D.E.; Jacobs, M.; Baixauli, F.; Stanczak, M.; Anderson, E.; Azuma, M.; et al. Dynamic Cardiolipin Synthesis Is Required for CD8+ T Cell Immunity. Cell Metab. 2020, 32, 981–995.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.M.; Liu, X.; Shen, J.; Jovanovic, O.; Pohl, E.E.; Gerson, S.L.; Finkel, T.; Broxmeyer, H.E.; Qu, C.K. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 2013, 12, 62–74. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, W.M.; Shen, J.; Kang, S.; Hambardzumyan, D.; Li, J.Y.; Shen, Y.; Kenney, A.M.; Chen, J.; Qu, C.K. Mitochondrial oxidation of the carbohydrate fuel is required for neural precursor/stem cell function and postnatal cerebellar development. Sci. Adv. 2018, 4, eaat2681. [Google Scholar] [CrossRef]
- Tu, C.; Xiong, H.; Hu, Y.; Wang, W.; Mei, G.; Wang, H.; Li, Y.; Zhou, Z.; Meng, F.; Zhang, P.; et al. Cardiolipin Synthase 1 Ameliorates NASH Through Activating Transcription Factor 3 Transcriptional Inactivation. Hepatology 2020, 72, 1949–1967. [Google Scholar] [CrossRef]
- Soustek, M.S.; Falk, D.J.; Mah, C.S.; Toth, M.J.; Schlame, M.; Lewin, A.S.; Byrne, B.J. Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum. Gene Ther. 2011, 22, 865–871. [Google Scholar] [CrossRef]
- Cole, L.K.; Mejia, E.M.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Han, X.; Dedousis, N.; Kaufman, B.A.; Dolinsky, V.W.; Hatch, G.M. Cardiolipin deficiency elevates susceptibility to a lipotoxic hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2020, 144, 24–34. [Google Scholar] [CrossRef]
- Cole, L.K.; Kim, J.H.; Amoscato, A.A.; Tyurina, Y.Y.; Bay, R.H.; Karimi, B.; Siddiqui, T.J.; Kagan, V.E.; Hatch, G.M.; Kauppinen, T.M. Aberrant cardiolipin metabolism is associated with cognitive deficiency and hippocampal alteration in tafazzin knockdown mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3353–3367. [Google Scholar] [CrossRef]
- Cole, L.K.; Mejia, E.M.; Vandel, M.; Sparagna, G.C.; Claypool, S.M.; Dyck-Chan, L.; Klein, J.; Hatch, G.M. Impaired Cardiolipin Biosynthesis Prevents Hepatic Steatosis and Diet-Induced Obesity. Diabetes 2016, 65, 3289–3300. [Google Scholar] [CrossRef]
- Zegallai, H.M.; Abu-El-Rub, E.; Cole, L.K.; Field, J.; Mejia, E.M.; Gordon, J.W.; Marshall, A.J.; Hatch, G.M. Tafazzin deficiency impairs mitochondrial metabolism and function of lipopolysaccharide activated B lymphocytes in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e22023. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Agarwal, P.; Doucette, C.A.; Fonseca, M.; Xiang, B.; Sparagna, G.C.; Seshadri, N.; Vandel, M.; Dolinsky, V.W.; Hatch, G.M. Tafazzin Deficiency Reduces Basal Insulin Secretion and Mitochondrial Function in Pancreatic Islets From Male Mice. Endocrinology 2021, 162, bqab102. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xu, Y.; Ma, Q.; Lin, Z.; Schlame, M.; Bezzerides, V.J.; Strathdee, D.; Pu, W.T. AAV Gene Therapy Prevents and Reverses Heart Failure in a Murine Knockout Model of Barth Syndrome. Circ. Res. 2020, 126, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Konjar, Š.; Frising, U.C.; Ferreira, C.; Hinterleitner, R.; Mayassi, T.; Zhang, Q.; Blankenhaus, B.; Haberman, N.; Loo, Y.; Guedes, J.; et al. Mitochondria maintain controlled activation state of epithelial-resident T lymphocytes. Sci. Immunol. 2018, 3, eaan2543. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.J.; Sims, H.F.; Han, X.; Jenkins, C.M.; Guan, S.P.; Yang, K.; Moon, S.H.; Pietka, T.; Abumrad, N.A.; Schlesinger, P.H.; et al. Genetic ablation of calcium-independent phospholipase A2gamma leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J. Biol. Chem. 2007, 282, 34611–34622. [Google Scholar] [CrossRef]
- Elimam, H.; Papillon, J.; Kaufman, D.R.; Guillemette, J.; Aoudjit, L.; Gross, R.W.; Takano, T.; Cybulsky, A.V. Genetic Ablation of Calcium-independent Phospholipase A2γ Induces Glomerular Injury in Mice. J. Biol. Chem. 2016, 291, 14468–14482. [Google Scholar] [CrossRef]
- Yoda, E.; Hachisu, K.; Taketomi, Y.; Yoshida, K.; Nakamura, M.; Ikeda, K.; Taguchi, R.; Nakatani, Y.; Kuwata, H.; Murakami, M.; et al. Mitochondrial dysfunction and reduced prostaglandin synthesis in skeletal muscle of Group VIB Ca2+-independent phospholipase A2gamma-deficient mice. J. Lipid Res. 2010, 51, 3003–3015. [Google Scholar] [CrossRef]
- Moon, S.H.; Mancuso, D.J.; Sims, H.F.; Liu, X.; Nguyen, A.L.; Yang, K.; Guan, S.; Dilthey, B.G.; Jenkins, C.M.; Weinheimer, C.J.; et al. Cardiac Myocyte-specific Knock-out of Calcium-independent Phospholipase A2γ (iPLA2γ) Decreases Oxidized Fatty Acids during Ischemia/Reperfusion and Reduces Infarct Size. J. Biol. Chem. 2016, 291, 19687–19700. [Google Scholar] [CrossRef]
- Moon, S.H.; Dilthey, B.G.; Liu, X.; Guan, S.; Sims, H.F.; Gross, R.W. High-fat diet activates liver iPLA(2)γ generating eicosanoids that mediate metabolic stress. J. Lipid Res. 2021, 62, 100052. [Google Scholar] [CrossRef]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef]
- Bo, W.; Ma, Y.; Xi, Y.; Liang, Q.; Cai, M.; Tian, Z. The Roles of FGF21 and ALCAT1 in Aerobic Exercise-Induced Cardioprotection of Postmyocardial Infarction Mice. Oxidative Med. Cell. Longev. 2021, 2021, 8996482. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Li, J.; Song, C.; Shi, Y. Pharmacological inhibition of ALCAT1 mitigates amyotrophic lateral sclerosis by attenuating SOD1 protein aggregation. Mol. Metab. 2022, 63, 101536. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Nie, J.; Zhang, J.; Kimball, S.R.; Zhang, H.; Zhang, W.J.; Jefferson, L.S.; Cheng, Z.; Ji, Q.; et al. ALCAT1 controls mitochondrial etiology of fatty liver diseases, linking defective mitophagy to steatosis. Hepatology 2015, 61, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.L.; Bogomolovas, J.; Chen, J. Understanding the molecular basis of cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H181–H233. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat Shock Protein 60 in Cardiovascular Physiology and Diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef]
- Lee, H.J.; Mayette, J.; Rapoport, S.I.; Bazinet, R.P. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006, 5, 2. [Google Scholar] [CrossRef]
- Pepe, S.; Tsuchiya, N.; Lakatta, E.G.; Hansford, R.G. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am. J. Physiol. 1999, 276, H149–H158. [Google Scholar] [CrossRef]
- Saini-Chohan, H.K.; Holmes, M.G.; Chicco, A.J.; Taylor, W.A.; Moore, R.L.; McCune, S.A.; Hickson-Bick, D.L.; Hatch, G.M.; Sparagna, G.C. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid Res. 2009, 50, 1600–1608. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Slabe, T.J.; Stoll, M.S.; Minkler, P.E.; Hoppel, C.L. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2770–H2778. [Google Scholar] [CrossRef]
- Heerdt, P.M.; Schlame, M.; Jehle, R.; Barbone, A.; Burkhoff, D.; Blanck, T.J. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann. Thorac. Surg. 2002, 73, 1216–1221. [Google Scholar] [CrossRef]
- Reibel, D.K.; O’Rourke, B.; Foster, K.A.; Hutchinson, H.; Uboh, C.E.; Kent, R.L. Altered phospholipid metabolism in pressure-overload hypertrophied hearts. Am. J. Physiol. 1986, 250, H1–H6. [Google Scholar] [CrossRef] [PubMed]
- Sparagna, G.C.; Johnson, C.A.; McCune, S.A.; Moore, R.L.; Murphy, R.C. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 2005, 46, 1196–1204. [Google Scholar] [CrossRef]
- Momoi, N.; Chang, B.; Takeda, I.; Aoyagi, Y.; Endo, K.; Ichida, F. Differing clinical courses and outcomes in two siblings with Barth syndrome and left ventricular noncompaction. Eur. J. Pediatr. 2012, 171, 515–520. [Google Scholar] [CrossRef]
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Van der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.; Van‘t Veer-Korthof, E.T.; Van der Harten, J.J.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef]
- Richter-Dennerlein, R.; Korwitz, A.; Haag, M.; Tatsuta, T.; Dargazanli, S.; Baker, M.; Decker, T.; Lamkemeyer, T.; Rugarli, E.I.; Langer, T. DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate cardiolipin remodeling. Cell Metab. 2014, 20, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, M.; Nolte, H.; König, T.; Saita, S.; Ananjew, M.; Krüger, M.; Tatsuta, T.; Langer, T. Acylglycerol Kinase Mutated in Sengers Syndrome Is a Subunit of the TIM22 Protein Translocase in Mitochondria. Mol. Cell 2017, 67, 471–483.e7. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Di Venosa, N.; Serena, D.; Ruggiero, F.M. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic. Biol. Med. 1999, 27, 42–50. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Tandler, B.; Hoppel, C.L. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 535–565. [Google Scholar] [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Kloner, R.A.; Hale, S.L.; Dai, W.; Gorman, R.C.; Shuto, T.; Koomalsingh, K.J.; Gorman, J.H., 3rd; Sloan, R.C.; Frasier, C.R.; Watson, C.A.; et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J. Am. Heart Assoc. 2012, 1, e001644. [Google Scholar] [CrossRef]
- Oemer, G.; Koch, J.; Wohlfarter, Y.; Alam, M.T.; Lackner, K.; Sailer, S.; Neumann, L.; Lindner, H.H.; Watschinger, K.; Haltmeier, M.; et al. Phospholipid Acyl Chain Diversity Controls the Tissue-Specific Assembly of Mitochondrial Cardiolipins. Cell Rep. 2020, 30, 4281–4291.e4. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, M.M.; Henry, A.E.; Kelly, R.I. Barth syndrome is associated with a cognitive phenotype. J. Dev. Behav. Pediatr. 2007, 28, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, M.M.; Kelley, R.I. Preliminary evidence for a cognitive phenotype in Barth syndrome. Am. J. Med. Genet. 2001, 102, 372–378. [Google Scholar] [CrossRef]
- Jefferies, J.L. Barth syndrome. Am. J. Med. Genet. C Semin Med. Genet. 2013, 163C, 198–205. [Google Scholar] [CrossRef]
- Raches, D.; Mazzocco, M.M. Emergence and nature of mathematical difficulties in young children with Barth syndrome. J. Dev. Behav. Pediatr. 2012, 33, 328–335. [Google Scholar] [CrossRef]
- Sen, T.; Sen, N.; Jana, S.; Khan, F.H.; Chatterjee, U.; Chakrabarti, S. Depolarization and cardiolipin depletion in aged rat brain mitochondria: Relationship with oxidative stress and electron transport chain activity. Neurochem. Int. 2007, 50, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lores-Arnaiz, S.; Lombardi, P.; Karadayian, A.G.; Cutrera, R.; Bustamante, J. Changes in motor function and brain cortex mitochondrial active oxygen species production in aged mice. Exp. Gerontol. 2019, 118, 88–98. [Google Scholar] [CrossRef]
- Petrosillo, G.; Matera, M.; Casanova, G.; Ruggiero, F.M.; Paradies, G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008, 53, 126–131. [Google Scholar] [CrossRef]
- Sen, T.; Sen, N.; Tripathi, G.; Chatterjee, U.; Chakrabarti, S. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem. Int. 2006, 49, 20–27. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Oliveira, M.M.; Melo, T.; Domingues, M.R.; Moreira, P.I.; Ferreiro, E.; Peixoto, F.; Videira, R.A. Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimer Dis. JAD 2015, 43, 1375–1392. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Amoscato, A.A.; Fink, A.B.; Anthonymuthu, T.; New, L.A.; Kochanek, P.M.; Watkins, S.; Kagan, V.E.; Bayir, H. Imaging mass spectrometry reveals loss of polyunsaturated cardiolipins in the cortical contusion, hippocampus, and thalamus after traumatic brain injury. J. Neurochem. 2016, 139, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Anthonymuthu, T.S.; Kenny, E.M.; Amoscato, A.A.; Lewis, J.; Kochanek, P.M.; Kagan, V.E.; Bayir, H. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Anthonymuthu, T.S.; Kenny, E.M.; Amoscato, A.A.; Cole, L.K.; Hatch, G.M.; Ji, J.; Kagan, V.E.; Bayir, H. Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury. JCI Insight 2018, 3, e97677. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Geens, A.; Bohm, C.; Deaulmerie, L.; Swerts, J.; Rossi, M.; Craessaerts, K.; Leites, E.P.; Seibler, P.; Rakovic, A.; et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J. Cell Biol. 2017, 216, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Tyurina, Y.Y.; Polimova, A.M.; Maciel, E.; Tyurin, V.A.; Kapralova, V.I.; Winnica, D.E.; Vikulina, A.S.; Domingues, M.R.; McCoy, J.; Sanders, L.H.; et al. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: Implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic. Res. 2015, 49, 681–691. [Google Scholar] [CrossRef]
- Phan, K.; He, Y.; Pickford, R.; Bhatia, S.; Katzeff, J.S.; Hodges, J.R.; Piguet, O.; Halliday, G.M.; Kim, W.S. Uncovering pathophysiological changes in frontotemporal dementia using serum lipids. Sci. Rep. 2020, 10, 3640. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Kenny, E.M.; Hier, Z.E.; Clark, R.S.B.; Kochanek, P.M.; Kagan, V.E.; Bayir, H. Detection of brain specific cardiolipins in plasma after experimental pediatric head injury. Exp. Neurol. 2019, 316, 63–73. [Google Scholar] [CrossRef]
- Breda, C.N.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Camara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Kudlaty, E.; Agnihotri, N.; Khojah, A. Hypogammaglobulinaemia and B cell lymphopaenia in Barth syndrome. BMJ Case Rep. 2022, 15, e249254. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Frank, M. Haematological features in Barth syndrome. Curr. Opin. Hematol. 2013, 20, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.G.; Valianpour, F.; Bowen, V.M.; Lam, J.; Duran, M.; Vaz, F.M.; Wanders, R.J. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): An update. Am. J. Med. Genet. A 2004, 126A, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Homburg, C.H.; de Haas, M.; von dem Borne, A.E.; Verhoeven, A.J.; Reutelingsperger, C.P.; Roos, D. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood 1995, 85, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Fadok, V. Corpse clearance defines the meaning of cell death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.W.; Maianski, N.A.; Tool, A.T.; Becker, K.; Plecko, B.; Valianpour, F.; Wanders, R.J.; Pereira, R.; Van Hove, J.; Verhoeven, A.J.; et al. Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood 2004, 103, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Milosevic, J.; Brouse, T.; Aziz, N.; Elkhoury, J.; Wang, S.; Hauschild, A.; van Gastel, N.; Cetinbas, M.; Tufa, S.F.; et al. A new murine model of Barth syndrome neutropenia links TAFAZZIN deficiency to increased ER stress-induced apoptosis. Blood Adv. 2022, 6, 2557–2577. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Yang, K.; Zhao, Z.; Abendschein, D.R.; Gross, R.W. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry 2007, 46, 6417–6428. [Google Scholar] [CrossRef]
| Genes | Mouse Models | Major Phenotypes | Ref. |
|---|---|---|---|
| Ptpmt1 | Global KO 1 | Embryonic lethality before E8.5 | [29] |
| Cardiac-specific KO by Tnt-Cre | Abnormal heart development, embryonic lethality at around E16.5, mitochondrial cristae malformation, and mitochondrial dysfunction | [13] | |
| Induced cardiac-specific KO in adult mice by αMHC-MerCreMer | No obvious baseline phenotype | ||
| T-cell-specific KO by CD4-Cre | Reduced CD8+ cell numbers and abnormal memory CD8+ T cell function | [103] | |
| HSC-specific KO by Vav1-Cre | Failure of HSC differentiation, pancytopenia, anemia, and premature lethality | [104] | |
| Granulocyte-macrophage progenitors-specific KO by LysM-Cre | No obvious baseline phenotype | ||
| T-cell-specific KO by LCK-Cre | No obvious baseline phenotype | ||
| B-cell-specific KO by CD19-Cre | No obvious baseline phenotype | ||
| Neural cell-specific KO by Nestin-Cre | Cell cycle arrest of neuronal progenitors, growth retardation, ataxia, and premature lethality | [105] | |
| Purkinje cell-specific KO by PCP2-Cre | Minimal structural changes in cerebellum and altered walking gaits | ||
| Granule cell-specific KO by Atoh1-Cre | Abnormal anterior lobules and altered walking gaits | ||
| Dual neural-cell-specific KO by PCP2-Cre and Atoh1-Cre | Abnormal anterior lobules and altered walking gaits | ||
| Induced KO in adult mice by CAG-CreER | Transient defects, including ataxia, tremor, and impaired motor coordination, but soon recovered | ||
| Crls1 | Global KO | Early embryonic lethality at the peri-implantation stage | [50] |
| Neuron-specific KO by Camk2α-Cre | Neuronal loss and gliosis in the forebrain, and lethality at age 12–14 months | ||
| Adipocyte-specific KO by Adipoq-Cre | Less adipose tissue and reduced cold tolerance and insulin sensitivity | [55] | |
| Induced brown and beige adipose-specific KO by UCP1-CreER | Paler brown fat, insulin resistance | ||
| Hepatocyte-specific KO by Alb-Cre | Exacerbated insulin resistance and hepatic steatosis induced by high-fat diet, and aggravated inflammatory response and fibrosis induced by a high-fat and high-cholesterol diet | [106] | |
| Taz | Doxycycline-induced global shRNA KD 2 | Prenatal and perinatal lethality, embryonic diastolic dysfunction, and myocardial noncompaction | [56] |
| Doxycycline-induced global shRNA KD | Decreased body weight and left ventricular dilation and dysfunction at an age of 8 months | [58] | |
| Impaired skeletal muscle force generation at an age of 2 months and reduced ejection fraction at an age of 7–10 months | [107] | ||
| Increased cardiac lipotoxicity due to high-fat diet | [108] | ||
| Cognitive deficiency and hippocampal alteration | [109] | ||
| Resistant to high-fat-induced obesity, insulin resistance, and hepatic steatosis | [110] | ||
| Reduced cell proliferation of LPS-stimulated B cells | [111] | ||
| Reduced plasma insulin, impaired insulin secretion under low-glucose conditions, and increased fibrosis in pancreatic islets | [112] | ||
| Heart failure with a preserved ejection fraction and age-dependent progression of diastolic dysfunction, and prolonged QRS duration | [52] | ||
| Global KO | 20% die prenatally, survivors develop neutropenia, premature lethality, growth retardation, skeletal myopathy, and heart failure | [113] | |
| Reduced number and impaired function of CD8+ T cells | [104] | ||
| Bone marrow KO chimeras and reduced activities of intraepithelial lymphocytes in response to anti-CD3 and parasite infection | [114] | ||
| Cardiac-specific KO by αMHC-Cre | Progressive dilated cardiomyopathy and cardiac fibrosis without fetal and perinatal lethality | [113] | |
| Increased vulnerability to arrhythmia | [51] | ||
| Cardiac-specific KO by Xmlc2-Cre | No embryonic lethality, ~5% die before an age of 2 months, while survivors exhibit ventricular dilation and contractile dysfunction | [14] | |
| PNPLA8 | Global KO | Growth retardation, cold intolerance, reduced exercise endurance, and greatly increased mortality from cardiac stress after transverse aortic constriction | [115] |
| Deficiency in spatial learning and memory performance | [32] | ||
| Loss of podocytes in aging mice; higher albuminuria and more podocyte injury and loss in response to nephritis | [116] | ||
| Global KO | Growth retardation, kyphosis, and muscle weakness | [117] | |
| Induced cardiac-specific KO in adult mice by αMHC-MerCreMer | Protective from myocardial ischemia/reperfusion injury | [118] | |
| Hepatocyte-specific KO by MMAP-Cre | Enhances glucose clearance and reduces FA accumulation after high-fat diet | [119] | |
| ALCAT1 | Global KO | Impairs kidney injury after myocardial infarction | [120] |
| Improves cardiac performance after myocardial infarction | [121] | ||
| Global KO | Inhibits MPTP-induced neurotoxicity, apoptosis, and motor deficits | [40] | |
| Mitigates hyperthyroid cardiomyopathy and ventricular fibrosis | [41] | ||
| Reduced high-fat-diet-induced obesity and insulin resistance | [33] | ||
| Mitigates CHD and its related pathogenesis, including dilated cardiomyopathy, left ventricle dysfunction, inflammation, fibrosis, and apoptosis | [39] | ||
| Attenuates motor neuron dysfunction, neuronal inflammation, and skeletal muscle atrophy in SOD1G93A mice | [122] | ||
| Attenuates hepatic lipogenesis and fibrosis and prevents the onset of diet-induced nonalcoholic fatty liver diseases | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Shen, T.; Huynh, H.; Fang, X.; Han, Z.; Ouyang, K. Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells. Genes 2022, 13, 1889. https://doi.org/10.3390/genes13101889
Jiang Z, Shen T, Huynh H, Fang X, Han Z, Ouyang K. Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells. Genes. 2022; 13(10):1889. https://doi.org/10.3390/genes13101889
Chicago/Turabian StyleJiang, Zhitong, Tao Shen, Helen Huynh, Xi Fang, Zhen Han, and Kunfu Ouyang. 2022. "Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells" Genes 13, no. 10: 1889. https://doi.org/10.3390/genes13101889
APA StyleJiang, Z., Shen, T., Huynh, H., Fang, X., Han, Z., & Ouyang, K. (2022). Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells. Genes, 13(10), 1889. https://doi.org/10.3390/genes13101889







