Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management
Abstract
1. Introduction
2. Sclerosing Disorders
2.1. Osteopetrosis
2.1.1. Overview and Pathogenesis
2.1.2. Clinical, Biochemical and Radiographic Presentation
2.1.3. Treatment
2.2. Progressive Diaphyseal Dysplasia
2.2.1. Overview and Pathogenesis
2.2.2. Clinical, Biochemical and Radiographic Presentation
2.2.3. Treatment
2.3. Melorheostosis
2.3.1. Overview and Pathogenesis
2.3.2. Clinical, Biochemical and Radiographic Presentation
2.3.3. Treatment
2.4. Pyknodysostosis
2.4.1. Overview and Pathogenesis
2.4.2. Clinical, Biochemical and Radiographic Presentation
2.4.3. Treatment
2.5. High Bone Mass Associated with LRP5 and LRP6 Mutations
2.5.1. Overview and Pathogenesis
2.5.2. Clinical Presentation
2.5.3. Treatment
2.6. Pyle Disease
2.6.1. Overview and Pathogenesis
2.6.2. Clinical and Radiographic Presentation
2.6.3. Treatment
2.7. Hyperostosis Corticalis Generalisata
2.7.1. Overview and Pathogenesis
2.7.2. Clinical and Biochemical Presentation
2.7.3. Treatment
2.8. Sclerosteosis
2.8.1. Overview and Pathogenesis
2.8.2. Clinical Presentation
2.8.3. Treatment
2.9. Juvenile Paget’s Disease
2.9.1. Overview and Pathogenesis
2.9.2. Clinical, Biochemical and Radiographic Presentation
2.9.3. Treatment
2.10. Paget’s Disease
2.10.1. Overview and Pathogenesis
2.10.2. Clinical, Biochemical and Radiographic Presentation
2.10.3. Treatment
2.11. Osteopathia Striata
2.11.1. Overview and Pathogenesis
2.11.2. Clinical and Radiographic Presentation
2.11.3. Treatment
2.12. Osteopoikilosis
2.12.1. Overview and Pathogenesis
2.12.2. Clinical and Radiographic Presentation
2.12.3. Treatment
3. Disorders of Defective Bone Mineralization
3.1. Hypophosphatasia
3.1.1. Overview and Pathogenesis
3.1.2. Clinical and Biochemical Presentation
3.1.3. Treatment
3.2. Hypophosphatemic Rickets
3.2.1. Overview and Pathogenesis
3.2.2. Clinical and Biochemical Presentation
3.2.3. Treatment
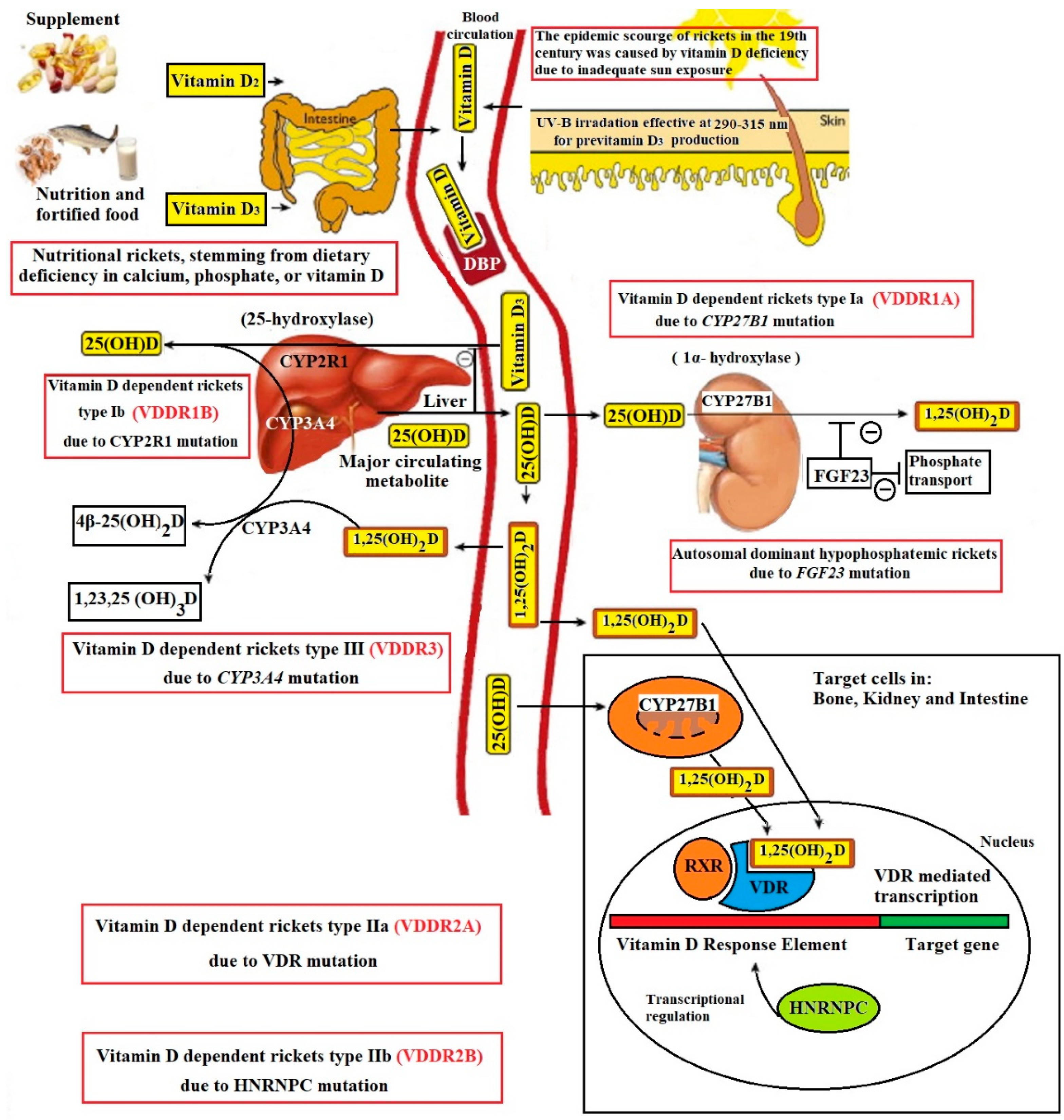
3.3. Vitamin D-Dependent Rickets
3.3.1. Overview and Pathogenesis
3.3.2. Clinical and Biochemical Presentation
3.3.3. Treatment
3.4. Axial Osteomalacia
3.4.1. Overview and Pathogenesis
3.4.2. Clinical and Biochemical Presentation
3.4.3. Treatment
4. Disorders of Bone Matrix and Cartilage Formation
4.1. Achondroplasia
4.1.1. Overview and Pathogenesis
4.1.2. Clinical Presentation
4.1.3. Treatment
4.2. Multiple Exostoses
4.2.1. Overview and Pathogenesis
4.2.2. Clinical and Radiographic Presentation
4.2.3. Treatment
4.3. Patchydermoperiostosis
4.3.1. Overview and Pathogenesis
4.3.2. Clinical Presentation
4.3.3. Treatment
4.4. Osteoporosis-Pseudoglioma Syndrome
4.4.1. Overview and Pathogenesis
4.4.2. Clinical Presentation
4.4.3. Treatment
4.5. Osteogenesis Imperfecta
4.5.1. Overview and Pathogenesis
4.5.2. Clinical Presentation
4.5.3. Treatment
4.6. Other Hereditary Connective Tissue Disorders including Ehlers-Danlos Syndrome, Marfan Syndrome and Loeys-Dietz Syndrome
4.6.1. Overview and Pathogenesis
4.6.2. Clinical Presentation and Diagnosis
4.6.3. Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Sayed, S.A.; Nezwek, T.A.; Varacallo, M. Physiology, Bone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Shahi, M.; Peymani, A.; Sahmani, M. Regulation of Bone Metabolism. Rep. Biochem. Mol. Biol. 2017, 5, 73–82. [Google Scholar]
- Guntur, A.R.; Rosen, C.J. Bone as an endocrine organ. Endocr. Pract. 2012, 18, 758–762. [Google Scholar] [CrossRef]
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J.; Miller, W.P.; Petryk, A.; Rush, E.T.; Shoback, D.M.; et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123. [Google Scholar] [CrossRef]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef]
- Palagano, E.; Menale, C.; Sobacchi, C.; Villa, A. Genetics of Osteopetrosis. Curr. Osteoporos. Rep. 2018, 16, 13–25. [Google Scholar] [CrossRef]
- Penna, S.; Capo, V.; Palagano, E.; Sobacchi, C.; Villa, A. One Disease, Many Genes: Implications for the Treatment of Osteopetroses. Front. Endocrinol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Nagai, R.; Kooh, S.W.; Balfe, J.W.; Fenton, T.; Halperin, M.L. Renal tubular acidosis and osteopetrosis with carbonic anhydrase II deficiency: Pathogenesis of impaired acidification. Pediatr. Nephrol. 1997, 11, 633–636. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Corradini, N.g.; Hadj-Rabia, S.; Fournet, J.-C.; Faivre, L.; Le Deist, F.o.; Durand, P.; Doffinger, R.; Smahi, A.; Israel, A.; et al. Osteopetrosis, Lymphedema, Anhidrotic Ectodermal Dysplasia, and Immunodeficiency in a Boy and Incontinentia Pigmenti in His Mother. Pediatrics 2002, 109, e97. [Google Scholar] [CrossRef]
- Helfrich, M.H.; Aronson, D.C.; Everts, V.; Mieremet, R.H.P.; Gerritsen, E.J.A.; Eckhardt, P.G.; Groot, C.G.; Scherft, J.P. Morphologic features of bone in human osteopetrosis. Bone 1991, 12, 411–419. [Google Scholar] [CrossRef]
- Charoenngam, N.; Cevik, M.B.; Holick, M.F. Diagnosis and management of pediatric metabolic bone diseases associated with skeletal fragility. Curr. Opin Pediatr. 2020, 32, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-C.; OuYang, X.-L.; Liu, G.-C.; Zhang, W.-J.; Zhang, X.-M. 99Tcm-MDP Imaging of Osteopetrosis: Case Report. Medicine 2015, 94, e929. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.-L.H.; Nabulsi, M.M.; Farhat, F.T.; Haidar, R.K.; Fuleihan, N.S. When bone becomes marble: Head and neck manifestations of osteopetrosis. Paediatr. Child Health 2006, 11, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Steward, C.G. Neurological aspects of osteopetrosis. Neuropathol. Appl. Neurobiol. 2003, 29, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Maurizi, A.; Ventura, L.; Rucci, N.; Teti, A. Effective Small Interfering RNA Therapy to Treat CLCN7-dependent Autosomal Dominant Osteopetrosis Type 2. Mol. Ther. Nucleic Acids 2015, 4, e248. [Google Scholar] [CrossRef]
- Kinoshita, A. Camurati-Engelmann disease. Nihon Rinsho 2015, 73, 2149–2159. [Google Scholar]
- Kinoshita, A.; Fukumaki, Y.; Shirahama, S.; Miyahara, A.; Nishimura, G.; Haga, N.; Namba, A.; Ueda, H.; Hayashi, H.; Ikegawa, S.; et al. TGFB1 mutations in four new families with Camurati-Engelmann disease: Confirmation of independently arising LAP-domain-specific mutations. Am. J. Med. Genet. A 2004, 127, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, W.; Boudin, E.; Vanhoenacker, F.M.; Mortier, G. Camurati–Engelmann Disease. Calcif. Tissue Int. 2019, 104, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, R.S.; Graham, C.B. Camurati-Engelmann disease. Genetics and clinical manifestations with a review of the literature. J. Med. Genet. 1972, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; Vanhoenacker, F.; Bonduelle, M.; Verbruggen, L.; Van Maldergem, L.; Ralston, S.; Guañabens, N.; Migone, N.; Wientroub, S.; Divizia, M.T.; et al. Camurati-Engelmann disease: Review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J. Med. Genet. 2006, 43, 1. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Y.; Alon, U.; Kaftori, J.K.; Berant, M. Progressive diaphyseal dysplasia: Evaluation of corticosteroid therapy. Pediatrics 1985, 75, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Rajan, R.; Paul, J.; Cherian, K.E.; Kapoor, N.; Paul, T.V. Losartan as a Steroid-Sparing Adjunct in a Patient With Features of Refractory Camurati-Engelmann Disease. AACE Clin. Case Rep. 2022, 8, 54–57. [Google Scholar] [CrossRef]
- Abdulla, M. Camurati-Engelmann disease with good treatment response to Losartan. Indian J. Nucl. Med. 2019, 34, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J. Targeting TGF-β Signaling for Therapeutic Gain. Cold Spring Harb. Perspect Biol. 2017, 9, a022301. [Google Scholar] [CrossRef]
- Rossi, M.; Battafarano, G.; De Martino, V.; Scillitani, A.; Minisola, S.; Del Fattore, A. Looking for new anabolic treatment from rare diseases of bone formation. J. Endocrinol. 2021, 248, R29–R40. [Google Scholar] [CrossRef]
- Wordsworth, P.; Chan, M. Melorheostosis and Osteopoikilosis: A Review of Clinical Features and Pathogenesis. Calcif. Tissue Int. 2019, 104, 530–543. [Google Scholar] [CrossRef]
- Kotwal, A.; Clarke, B.L. Melorheostosis: A Rare Sclerosing Bone Dysplasia. Curr. Osteoporos. Rep. 2017, 15, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Couto, A.R.; Bruges-Armas, J.; Peach, C.A.; Chapman, K.; Brown, M.A.; Wordsworth, B.P.; Zhang, Y. A Novel LEMD3 Mutation Common to Patients with Osteopoikilosis With and Without Melorheostosis. Calcif. Tissue Int. 2007, 81, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jha, S.; Deng, Z.; Fratzl-Zelman, N.; Cabral, W.A.; Ivovic, A.; Meylan, F.; Hanson, E.P.; Lange, E.; Katz, J.; et al. Somatic activating mutations in MAP2K1 cause melorheostosis. Nat. Commun. 2018, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- Gnoli, M.; Staals, E.L.; Campanacci, L.; Bedeschi, M.F.; Faletra, F.; Gallone, S.; Gaudio, A.; Mattina, T.; Gurrieri, F.; Percesepe, A.; et al. Melorheostosis and Osteopoikilosis Clinical and Molecular Description of an Italian Case Series. Calcif. Tissue Int. 2019, 105, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Fick, C.N.; Fratzl-Zelman, N.; Roschger, P.; Klaushofer, K.; Jha, S.; Marini, J.C.; Bhattacharyya, T. Melorheostosis: A Clinical, Pathologic, and Radiologic Case Series. Am. J. Surg. Pathol. 2019, 43, 1554. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G.G.; Mahan, K.T. Melorheostosis: A Literature Review and Case Report with Surgical Considerations. J. Foot Ankle Surg. 2010, 49, 80–85. [Google Scholar] [CrossRef]
- Suresh, S.; Muthukumar, T.; Saifuddin, A. Classical and unusual imaging appearances of melorheostosis. Clin. Radiol. 2010, 65, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P.; Murphy, W.A.; Siegel, B.A. 99mTc-Pyrophosphate Bone Imaging in Osteopoikilosis, Osteopathia Striata, and Melorheostosis. Radiology 1978, 127, 439–443. [Google Scholar] [CrossRef]
- Schmidt, G.S.; Schacht, J.P.; Knee, T.S.; Shakir, M.K.M.; Hoang, T.D. Pyknodysostosis (Osteopetrosis Acro-Osteolytica). AACE Clin. Case Rep. 2020, 6, e257–e261. [Google Scholar] [CrossRef]
- LeBlanc, S.; Savarirayan, R. Pycnodysostosis. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Fratzl-Zelman, N.; Valenta, A.; Roschger, P.; Nader, A.; Gelb, B.D.; Fratzl, P.; Klaushofer, K. Decreased Bone Turnover and Deterioration of Bone Structure in Two Cases of Pycnodysostosis. J. Clin. Endocrinol. Metab. 2004, 89, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, K.K.K.; George, G.B.; Padiyath, S.; Sethuraman, R.; Cherian, B. Pyknodysostosis: Report of a rare case with review of literature. Imaging Sci. Dent. 2011, 41, 177–181. [Google Scholar] [CrossRef]
- Omer Sulaiman, H.; Thalange, N.K.S. Pycnodysostosis: A Growth Hormone Responsive Skeletal Dysplasia. AACE Clin. Case Rep. 2021, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Ramadan, M.A.F.; Sherif, A.; Aziz Bedair, E.-S.M.; Rizk, M.M. Pycnodysostosis: Clinical, radiologic, and endocrine evaluation and linear growth after growth hormone therapy. Metab.—Clin. Exp. 2001, 50, 905–911. [Google Scholar] [CrossRef]
- Testani, E.; Scarano, E.; Leoni, C.; Dittoni, S.; Losurdo, A.; Colicchio, S.; Gnoni, V.; Vollono, C.; Zampino, G.; Paludetti, G.; et al. Upper airway surgery of obstructive sleep apnea in pycnodysostosis: Case report and literature review. Am. J. Med. Genet. Part A 2014, 164, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Vieira Ortegosa, M.; Romeo Bertola, D.; Aguena, M.; Passos-Bueno, M.R.; Ae Kim, C.; Justamante De Faria, M.E. Challenges in the Orthodontic Treatment of a Patient with Pycnodysostosis. Cleft Palate-Craniofacial J. 2014, 51, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Niziolek, P.J.; MacDonald, B.T.; Zylstra, C.R.; Alenina, N.; Robinson, D.R.; Zhong, Z.; Matthes, S.; Jacobsen, C.M.; Conlon, R.A.; et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 2011, 17, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Boyden, L.M.; Mao, J.; Belsky, J.; Mitzner, L.; Farhi, A.; Mitnick, M.A.; Wu, D.; Insogna, K.; Lifton, R.P. High Bone Density Due to a Mutation in LDL-Receptor–Related Protein 5. N. Engl. J. Med. 2002, 346, 1513–1521. [Google Scholar] [CrossRef]
- Gregson, C.L.; Wheeler, L.; Hardcastle, S.A.; Appleton, L.H.; Addison, K.A.; Brugmans, M.; Clark, G.R.; Ward, K.A.; Paggiosi, M.; Stone, M.; et al. Mutations in Known Monogenic High Bone Mass Loci Only Explain a Small Proportion of High Bone Mass Cases. J. Bone Miner. Res. 2016, 31, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Kwee, M.L.; Balemans, W.; Cleiren, E.; Gille, J.J.P.; Van Der Blij, F.; Sepers, J.M.; Van Hul, W. An Autosomal Dominant High Bone Mass Phenotype in Association With Craniosynostosis in an Extended Family Is Caused by an LRP5 Missense Mutation. J. Bone Miner. Res. 2005, 20, 1254–1260. [Google Scholar] [CrossRef]
- Whyte, M.P.; McAlister, W.H.; Zhang, F.; Bijanki, V.N.; Nenninger, A.; Gottesman, G.S.; Lin, E.L.; Huskey, M.; Duan, S.; Dahir, K.; et al. New explanation for autosomal dominant high bone mass: Mutation of low-density lipoprotein receptor-related protein 6. Bone 2019, 127, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Brance, M.L.; Brun, L.R.; Cóccaro, N.M.; Aravena, A.; Duan, S.; Mumm, S.; Whyte, M.P. High bone mass from mutation of low-density lipoprotein receptor-related protein 6 (LRP6). Bone 2020, 141, 115550. [Google Scholar] [CrossRef] [PubMed]
- Pyle, E. A Case of Unusual Bone Development. JBJS 1931, 13, 874–876. [Google Scholar]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef]
- Beighton, P. Pyle disease (metaphyseal dysplasia). J. Med. Genet. 1987, 24, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Faden, M.A.; Krakow, D.; Ezgu, F.; Rimoin, D.L.; Lachman, R.S. The Erlenmeyer flask bone deformity in the skeletal dysplasias. Am. J. Med. Genet. A 2009, 149, 1334–1345. [Google Scholar] [CrossRef]
- Soares, D.X.; Almeida, A.M.; Barreto, A.R.F.; Alencar, E.; Silva, I.J.; de Castro, J.D.V.; Magalhães Pinto, F.J.; Dias, D.A.; Aguiar, L.B. Pyle disease (metaphyseal dysplasia) presenting in two adult sisters. Radiol. Case Rep. 2016, 11, 405–410. [Google Scholar] [CrossRef]
- van Lierop, A.H.; Hamdy, N.A.T.; van Egmond, M.E.; Bakker, E.; Dikkers, F.G.; Papapoulos, S.E. Van Buchem disease: Clinical, biochemical, and densitometric features of patients and disease carriers. J. Bone Miner. Res. 2013, 28, 848–854. [Google Scholar] [CrossRef]
- Nassar, K.; Rachidi, W.; Janani, S.; Mkinsi, O. Van Buchem’s Disease. Jt. Bone Spine 2016, 83, 737–738. [Google Scholar] [CrossRef]
- Van Hul, W.; Balemans, W.; Van Hul, E.; Dikkers, F.G.; Obee, H.; Stokroos, R.J.; Hildering, P.; Vanhoenacker, F.; Van Camp, G.; Willems, P.J. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am. J. Hum. Genet. 1998, 62, 391–399. [Google Scholar] [CrossRef]
- Sebastian, A.; Loots, G.G. Genetics of Sost/SOST in sclerosteosis and van Buchem disease animal models. Metabolism 2018, 80, 38–47. [Google Scholar] [CrossRef]
- van Lierop, A.H.; Hamdy, N.A.T.; Hamersma, H.; van Bezooijen, R.L.; Power, J.; Loveridge, N.; Papapoulos, S.E. Patients with sclerosteosis and disease carriers: Human models of the effect of sclerostin on bone turnover. J. Bone Miner. Res. 2011, 26, 2804–2811. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–544. [Google Scholar] [CrossRef]
- Bhadada, S.K.; Rastogi, A.; Steenackers, E.; Boudin, E.; Arya, A.; Dhiman, V.; Bhansali, A.; Van Hul, W. Novel SOST gene mutation in a sclerosteosis patient and her parents. Bone 2013, 52, 707–710. [Google Scholar] [CrossRef]
- Fijalkowski, I.; Geets, E.; Steenackers, E.; Van Hoof, V.; Ramos, F.J.; Mortier, G.; Fortuna, A.M.; Van Hul, W.; Boudin, E. A Novel Domain-Specific Mutation in a Sclerosteosis Patient Suggests a Role of LRP4 as an Anchor for Sclerostin in Human Bone. J. Bone Miner. Res. 2016, 31, 874–881. [Google Scholar] [CrossRef]
- Bukowska-Olech, E.; Sowińska-Seidler, A.; Szczałuba, K.; Jamsheer, A. A novel biallelic splice-site variant in the LRP4 gene causes sclerosteosis 2. Birth Defects Res. 2020, 112, 652–659. [Google Scholar] [CrossRef]
- Beighton, P.; Durr, L.; Hamersma, H. The Clinical Features of Sclerosteosis. Ann. Intern. Med. 1976, 84, 393–397. [Google Scholar] [CrossRef]
- Beighton, P.; Barnard, A.; Hamersma, H.; Wouden, A.v.d. The syndromic status of sclerosteosis and van Buchem disease. Clin. Genet. 1984, 25, 175–181. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Cundy, T.; Mantzoros, C.S. Juvenile Paget disease. Metab.—Clin. Exp. 2018, 80, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Michou, L.; Collet, C.; Laplanche, J.-L.; Orcel, P.; Cornélis, F. Genetics of Paget’s disease of bone. Jt. Bone Spine 2006, 73, 243–248. [Google Scholar] [CrossRef]
- Osteoprotegerin Deficiency and Juvenile Paget’s Disease. N. Engl. J. Med. 2002, 347, 1622–1623. [CrossRef] [PubMed]
- Kerr, N.M.; Cassinelli, H.R.; DiMeglio, L.A.; Tau, C.; Tüysüz, B.; Cundy, T.; Vincent, A.L. Ocular Manifestations of Juvenile Paget Disease. Arch. Ophthalmol. 2010, 128, 698–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naot, D.; Choi, A.; Musson, D.S.; Simsek Kiper, P.Ö.; Utine, G.E.; Boduroglu, K.; Peacock, M.; DiMeglio, L.A.; Cundy, T. Novel homozygous mutations in the osteoprotegerin gene TNFRSF11B in two unrelated patients with juvenile Paget’s disease. Bone 2014, 68, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Makras, P.; Anastasilakis, A.D.; Mintziori, G.; Kita, M.; Papatheodorou, A.; Kokkoris, P.; Terpos, E. Periostin and sclerostin levels in juvenile Paget’s disease. Clin. Cases Miner. Bone Metab. 2017, 14, 269–271. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Litsas, I.; Efstathiadou, Z.; Kita, M.; Arsos, G.; Moralidis, E.; Papatheodorou, A.; Terpos, E. Profound hypocalcemia following effective response to zoledronic acid treatment in a patient with juvenile Paget’s disease. J. Bone Miner. Metab. 2010, 28, 706–712. [Google Scholar] [CrossRef]
- Tüysüz, B.; Mercimek, S.; Üngür, S.; Deniz, M. Calcitonin treatment in osteoectasia with hyperphosphatasia (juvenile Paget’s disease): Radiographic changes after treatment. Pediatr. Radiol. 1999, 29, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Singhellakis, P.N.; Naot, D.; Adamidou, F.; Malandrinou, F.C.; Anastasilakis, A.D.; Polymerou, V.; Kita, M. Denosumab Treatment for Juvenile Paget’s Disease: Results From Two Adult Patients With Osteoprotegerin Deficiency (“Balkan” Mutation in the TNFRSF11B Gene). J. Clin. Endocrinol. Metab. 2014, 99, 703–707. [Google Scholar] [CrossRef][Green Version]
- Cundy, T.; Davidson, J.; Rutland, M.D.; Stewart, C.; DePaoli, A.M. Recombinant Osteoprotegerin for Juvenile Paget’s Disease. N. Engl. J. Med. 2005, 353, 918–923. [Google Scholar] [CrossRef]
- Tan, A.; Ralston, S.H. Paget’s disease of bone. QJM Int. J. Med. 2014, 107, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Wall, C. Paget’s disease of bone: A clinical update. Aust. J. Gen. Pract. 2021, 50, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mirra, J.M. Pathogenesis of Paget’s disease based on viral etiology. Clin. Orthop. Relat. Res. 1987, 217, 162–170. [Google Scholar] [CrossRef]
- Hocking, L.; Slee, F.; Haslam, S.I.; Cundy, T.; Nicholson, G.; van Hul, W.; Ralston, S.H. Familial Paget’s disease of bone: Patterns of inheritance and frequency of linkage to chromosome 18q. Bone 2000, 26, 577–580. [Google Scholar] [CrossRef]
- Alonso, N.; Calero-Paniagua, I.; Del Pino-Montes, J. Clinical and Genetic Advances in Paget’s Disease of Bone: A Review. Clin. Rev. Bone Miner. Metab. 2017, 15, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, F.; Rendina, D.; Di Stefano, M.; Mingione, A.; Esposito, T.; Merlotti, D.; Gallone, S.; Magliocca, S.; Goode, A.; Formicola, D.; et al. A nonsynonymous TNFRSF11A variation increases NFκB activity and the severity of Paget’s disease. J. Bone Miner. Res. 2012, 27, 443–452. [Google Scholar] [CrossRef]
- Makaram, N.S.; Ralston, S.H. Genetic Determinants of Paget’s Disease of Bone. Curr. Osteoporos. Rep. 2021, 19, 327–337. [Google Scholar] [CrossRef]
- Beyens, G.; Daroszewska, A.; de Freitas, F.; Fransen, E.; Vanhoenacker, F.; Verbruggen, L.; Zmierczak, H.-G.; Westhovens, R.; Van Offel, J.; Ralston, S.H.; et al. Identification of Sex-Specific Associations Between Polymorphisms of the Osteoprotegerin Gene, TNFRSF11B, and Paget’s Disease of Bone. J. Bone Miner. Res. 2007, 22, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Rabjohns, E.M.; Hurst, K.; Ghosh, A.; Cuellar, M.C.; Rampersad, R.R.; Tarrant, T.K. Paget’s Disease of Bone: Osteoimmunology and Osteoclast Pathology. Curr. Allergy Asthma Rep. 2021, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.; Lalam, R.; Cassar-Pullicino, V. Imaging of Paget’s disease of bone. Wien. Med. Wochenschr. 2017, 167, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.; Lyles, K.; Brown, J.P.; Fraser, W.D.; Miller, P.; Curiel, M.D.; Devogelaer, J.-P.; Hooper, M.; Su, G.; Zelenakas, K.; et al. Long-Term Control of Bone Turnover in Paget’s Disease With Zoledronic Acid and Risedronate. J. Bone Miner. Res. 2007, 22, 142–148. [Google Scholar] [CrossRef]
- Reid, I.R.; Sharma, S.; Kalluru, R.; Eagleton, C. Treatment of Paget’s Disease of Bone with Denosumab: Case Report and Literature Review. Calcif. Tissue Int. 2016, 99, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Perdu, B.; Freitas, F.d.; Frints, S.G.M.; Schouten, M.; Schrander-Stumpel, C.; Barbosa, M.; Pinto-Basto, J.; Reis-Lima, M.; Vernejoul, M.-C.d.; Becker, K.; et al. Osteopathia striata with cranial sclerosis owing to WTX gene defect. J. Bone Miner. Res. 2010, 25, 82–90. [Google Scholar] [CrossRef]
- Holman, S.K.; Morgan, T.; Baujat, G.; Cormier-Daire, V.; Cho, T.J.; Lees, M.; Samanich, J.; Tapon, D.; Hove, H.D.; Hing, A.; et al. Osteopathia striata congenita with cranial sclerosis and intellectual disability due to contiguous gene deletions involving the WTX locus. Clin. Genet. 2013, 83, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Rauch, F.; Travers, R.; Roy, M.; Montes, J.; Chabot, G.; Glorieux, F.H. Osteopathia striata with cranial sclerosis: Clinical, radiological, and bone histological findings in an adolescent girl. Am. J. Med. Genet. Part A 2004, 129, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gear, R.; Savarirayan, R. Osteopathia Striata with Cranial Sclerosis. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Sultana, A.; Sjöström, M. Orthognathic surgery in a patient with osteopathia striata combined with cranial sclerosis: A case report. Oral Maxillofac. Surg. Cases 2020, 6, 100194. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, Z.H.; Dong, C.S.; Yang, F.; Xie, Y.H.; Jin, P. Identification of a novel LEMD3 Y871X mutation in a three-generation family with osteopoikilosis and review of the literature. J. Endocrinol. Investig. 2016, 39, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowsky, T.G.; Monticielo, M.R.; Keiserman, B.; Monticielo, O.A. Osteopoikilosis: What does the rheumatologist must know about it? Clin. Rheumatol. 2012, 31, 745–748. [Google Scholar] [CrossRef]
- Krishnappa, R.; Kumar, N.; Alva, K.; Shetty, P.; Dang, M.; Mohamed, S. Plasma cell myeloma with unusual presentations. J. Orthop. Traumatol. Rehabil. 2013, 6, 84–86. [Google Scholar]
- Ozdemirel, A.E.; Cakit, B.D.; Erdem, H.R.; Koc, B. A rare benign disorder mimicking metastasis on radiographic examination: A case report of osteopoikilosis. Rheumatol. Int. 2011, 31, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, M.; Caner, B. Osteopoikilosis: A major diagnostic problem solved by bone scintigraphy. Rev. Española Med. Nucl. Imagen Mol. 2012, 31, 93–96. [Google Scholar] [CrossRef]
- Ellanti, P.; Clarke, B.; Gray, J. Osteopoikilosis. Ir. J. Med. Sci. 2010, 179, 615–616. [Google Scholar] [CrossRef]
- Khan, A.A.; Josse, R.; Kannu, P.; Villeneuve, J.; Paul, T.; Van Uum, S.; Greenberg, C.R. Hypophosphatasia: Canadian update on diagnosis and management. Osteoporos. Int. 2019, 30, 1713–1722. [Google Scholar] [CrossRef]
- Mornet, E. Hypophosphatasia. Orphanet J. Rare Dis. 2007, 2, 40. [Google Scholar] [CrossRef]
- Whyte, M.P. Hypophosphatasia: Enzyme Replacement Therapy Brings New Opportunities and New Challenges. J. Bone Miner. Res. 2017, 32, 667–675. [Google Scholar] [CrossRef]
- Whyte, M.P. Hypophosphatasia—Aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2016, 12, 233–246. [Google Scholar] [CrossRef]
- Mornet, E. Hypophosphatasia. Metab. —Clin. Exp. 2018, 82, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Morbach, H.; Richl, P.; Stenzel, M.; Girschick, H.J. How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol. Int. 2009, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lahorgue Nunes, M.; Mugnol, F.; Bica, I.; Machado Fiori, R. Pyridoxine-Dependent Seizures Associated With Hypophosphatasia in a Newborn. J. Child Neurol. 2002, 17, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, Z.; Yi, S.; Wei, H.; Zhou, X.z.; Shen, F. Case Report: Variations in the ALPL Gene in Chinese Patients With Hypophosphatasia. Front. Genet. 2021, 12, 732621. [Google Scholar] [CrossRef]
- Millán, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Int. 2016, 98, 398–416. [Google Scholar] [CrossRef]
- Chodirker, B.N.; Coburn, S.P.; Seargeant, L.E.; Whyte, M.P.; Greenberg, C.R. Increased plasma pyridoxal-5′-phosphate levels before and after pyridoxine loading in carriers of perinatal/infantile hypophosphatasia. J. Inherit. Metab. Dis. 1990, 13, 891–896. [Google Scholar] [CrossRef]
- Conti, F.; Ciullini, L.; Pugliese, G. Hypophosphatasia: Clinical manifestation and burden of disease in adult patients. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2017, 14, 230–234. [Google Scholar] [CrossRef]
- Fraser, D. Hypophosphatasia. Am. J. Med. 1957, 22, 730–746. [Google Scholar] [CrossRef]
- Whyte, M.P. Hypophosphatasia: An overview For 2017. Bone 2017, 102, 15–25. [Google Scholar] [CrossRef]
- Whyte, M.P.; Wenkert, D.; Zhang, F. Hypophosphatasia: Natural history study of 101 affected children investigated at one research center. Bone 2016, 93, 125–138. [Google Scholar] [CrossRef]
- Bowden, S.A.; Foster, B.L. Profile of asfotase alfa in the treatment of hypophosphatasia: Design, development, and place in therapy. Drug Des. Dev. Ther. 2018, 12, 3147–3161. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P.; Greenberg, C.R.; Salman, N.J.; Bober, M.B.; McAlister, W.H.; Wenkert, D.; Van Sickle, B.J.; Simmons, J.H.; Edgar, T.S.; Bauer, M.L.; et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. New Engl. J. Med. 2012, 366, 904–913. [Google Scholar] [CrossRef]
- Simon, S.; Resch, H.; Klaushofer, K.; Roschger, P.; Zwerina, J.; Kocijan, R. Hypophosphatasia: From Diagnosis to Treatment. Curr. Rheumatol. Rep. 2018, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Sigl, S.; Haberlandt, E.; Mumm, S.; Scholl-Bürgi, S.; Sergi, C.; Ryan, L.; Ericson, K.L.; Whyte, M.P.; Högler, W. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677T>C, p.M226T; c.1112C>T, p.T371I) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007, 40, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, S.S.; Mughal, Z.; Haffner, D.; Nilsson, O.; Levtchenko, E.; Ariceta, G.; de Lucas Collantes, C.; Schnabel, D.; Jandhyala, R.; Mäkitie, O. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J. Rare Dis. 2019, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Prié, D.; Friedlander, G. Reciprocal Control of 1,25-Dihydroxyvitamin D and FGF23 Formation Involving the FGF23/Klotho System. Clin. J. Am. Soc. Nephrol. 2010, 5, 1717. [Google Scholar] [CrossRef]
- Endo, I.; Fukumoto, S.; Ozono, K.; Namba, N.; Inoue, D.; Okazaki, R.; Yamauchi, M.; Sugimoto, T.; Minagawa, M.; Michigami, T.; et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: Prevalence, biochemical data and treatment. Endocr. J. 2015, 62, 811–816. [Google Scholar] [CrossRef]
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef]
- Rafaelsen, S.; Johansson, S.; Ræder, H.; Bjerknes, R. Hereditary hypophosphatemia in Norway: A retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur. J. Endocrinol. 2016, 174, 125–136. [Google Scholar] [CrossRef]
- Dixon, P.H.; Christie, P.T.; Wooding, C.; Trump, D.; Grieff, M.; Holm, I.; Gertner, J.M.; Schmidtke, J.; Shah, B.; Shaw, N.; et al. Mutational Analysis of PHEX Gene in X-Linked Hypophosphatemia1. J. Clin. Endocrinol. Metab. 1998, 83, 3615–3623. [Google Scholar] [CrossRef]
- White, K.E.; Evans, W.E.; O’Riordan, J.L.H.; Speer, M.C.; Econs, M.J.; Lorenz-Depiereux, B.; Grabowski, M.; Meitinger, T.; Strom, T.M. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Levy-Litan, V.; Hershkovitz, E.; Avizov, L.; Leventhal, N.; Bercovich, D.; Chalifa-Caspi, V.; Manor, E.; Buriakovsky, S.; Hadad, Y.; Goding, J.; et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am. J. Hum. Genet. 2010, 86, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Farrow, E.G.; Davis, S.I.; Ward, L.M.; Summers, L.J.; Bubbear, J.S.; Keen, R.; Stamp, T.C.B.; Baker, L.R.I.; Bonewald, L.F.; White, K.E. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone 2009, 44, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Petje, G.; Meizer, R.; Radler, C.; Aigner, N.; Grill, F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin. Orthop. Relat. Res. 2008, 466, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Linglart, A.; Biosse-Duplan, M.; Briot, K.; Chaussain, C.; Esterle, L.; Guillaume-Czitrom, S.; Kamenicky, P.; Nevoux, J.; Prié, D.; Rothenbuhler, A.; et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 2014, 3, R13–R30. [Google Scholar] [CrossRef]
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef]
- Baroncelli, G.I.; Toschi, B.; Bertelloni, S. Hypophosphatemic rickets. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 460–467. [Google Scholar] [CrossRef]
- Choy, M. Pharmaceutical Approval Update. P T A Peer-Rev. J. Formul. Manag. 2018, 43, 326–327. [Google Scholar]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Högler, W.; Linglart, A.; Padidela, R.; van’t Hoff, W.; Mao, M.; Chen, C.-Y.; et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. New Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef]
- Mäkitie, O.; Doria, A.; Kooh, S.W.; Cole, W.G.; Daneman, A.; Sochett, E. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 2003, 88, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.; Guegan, K.; Offiah, A.; Neill, R.O.; Hiorns, M.P.; Ellard, S.; Bockenhauer, D.; Hoff, W.V.t.; Waters, A.M. Growth in PHEX-associated X-linked hypophosphatemic rickets: The importance of early treatment. Pediatr. Nephrol. 2012, 27, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Levine, M.A. Diagnosis and Management of Vitamin D Dependent Rickets. Front. Pediatr. 2020, 8, 315. [Google Scholar] [CrossRef]
- Chen, H.; Hewison, M.; Hu, B.; Adams, J.S. Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: A cause of vitamin D resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 6109–6114. [Google Scholar] [CrossRef]
- Roizen, J.D.; Li, D.; O’Lear, L.; Javaid, M.K.; Shaw, N.J.; Ebeling, P.R.; Nguyen, H.H.; Rodda, C.P.; Thummel, K.E.; Thacher, T.D.; et al. CYP3A4 mutation causes vitamin D-dependent rickets type 3. J. Clin. Investig. 2018, 128, 1913–1918. [Google Scholar] [CrossRef]
- Balsan, S.; Garabédian, M.; Larchet, M.; Gorski, A.M.; Cournot, G.; Tau, C.; Bourdeau, A.; Silve, C.; Ricour, C. Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J. Clin. Investig. 1986, 77, 1661–1667. [Google Scholar] [CrossRef]
- Whyte, M.P.; Fallon, M.D.; Murphy, W.A.; Teitelbaum, S.L. Axial osteomalacia: Clinical, laboratory and genetic investigation of an affected mother and son. Am. J. Med. 1981, 71, 1041–1049. [Google Scholar] [CrossRef]
- Nelson, A.M.; Riggs, B.L.; Jowsey, J.O. Atypical axial osteomalacia. report of four cases with two having features of ankylosing spondylitis. Arthritis Rheum. 1978, 21, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Vajo, Z.; Francomano, C.A.; Wilkin, D.J. The Molecular and Genetic Basis of Fibroblast Growth Factor Receptor 3 Disorders: The Achondroplasia Family of Skeletal Dysplasias, Muenke Craniosynostosis, and Crouzon Syndrome with Acanthosis Nigricans. Endocr. Rev. 2000, 21, 23–39. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Legeai-Mallet, L. Achondroplasia: Development, pathogenesis, and therapy. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2017, 246, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Pauli, R.M. Achondroplasia: A comprehensive clinical review. Orphanet J. Rare Dis. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, P.L.; Shiang, R.; Thompson, L.M.; Zhu, Y.-Z.; Wilkin, D.J.; Lachman, R.S.; Wilcox, W.R.; Rimoin, D.L.; Cohn, D.H.; Wasmuth, J.J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat. Genet. 1995, 9, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Wang, Y.; Liu, A.M.; Tung, J.C. Thanatophoric dysplasia type I. Acta Paediatr. Taiwan 2001, 42, 39–41. [Google Scholar] [PubMed]
- Yolanda, N.; Yulianto, F.; Arina, S.; Edwin, J. A full-term infant with type II thanatophoric dysplasia. Case Rep. Perinat. Med. 2019, 8. [Google Scholar] [CrossRef]
- Bober, M.B.; Bellus, G.A.; Nikkel, S.M.; Tiller, G.E. Hypochondroplasia. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Yamashita, A.; Morioka, M.; Kishi, H.; Kimura, T.; Yahara, Y.; Okada, M.; Fujita, K.; Sawai, H.; Ikegawa, S.; Tsumaki, N. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature 2014, 513, 507–511. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.; Duncan, G.; Goutsos, K.T.; Tufaro, F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. USA 2000, 97, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M. The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 71–72, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, G.; Ristori, G.; Scoccianti, G.; Tamburini, A.; Capanna, R. Hereditary Multiple Exostoses: A review of clinical appearance and metabolic pattern. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2016, 13, 110–118. [Google Scholar] [CrossRef]
- Kose, O.; Ertas, A.; Celiktas, M.; Kisin, B. Fracture of an osteochondroma treated successfully with total excision: Two case reports. Cases J. 2009, 2, 8062. [Google Scholar] [CrossRef]
- Errani, C.; Vanel, D.; Donati, D.; Picci, P.; Faldini, C. Spontaneous healing of an osteochondroma fracture. Diagn. Interv. Imaging 2015, 96, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.M.; Kuo, S.; Epstein, R. Non-Traumatic Fracture of an Osteochondroma Mimicking Malignant Degeneration in an Adult with Hereditary Multiple Exostoses. Radiol. Case Rep. 2015, 3, 99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, P.; Che, M.; Liu, J.; Biswas, S.; Ma, G.; He, L.; Wei, Z.; Zhang, Z.; Yang, Y.; et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. eLife 2019, 8, e50208. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M. Hereditary multiple exostoses: Are there new plausible treatment strategies? Expert Opin. Orphan Drugs 2018, 6, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.R.A.; Jason, W.L.C.; Nasruddin, A.B. Pachydermoperiostosis: A rare mimicker of acromegaly. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 17-0029. [Google Scholar] [CrossRef]
- Mittal, A.; Gupta, N.; Soneja, M. Touraine-Solente-Gole syndrome. BMJ Case Rep. 2019, 12, e232238. [Google Scholar] [CrossRef] [PubMed]
- Akaranuchat, N.; Limsuvan, P. Touraine–Solente–Gole syndrome: Clinical manifestation with bilateral true eyelid ptosis. JPRAS Open 2019, 21, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Nepal, G.; Shing, Y.K.; Panthi, H.P.; Baral, S. Pachydermoperiostosis (Touraine-Solente-Gole syndrome): A case report. J. Med. Case Rep. 2019, 13, 39. [Google Scholar] [CrossRef]
- Zhang, Z.; He, J.-W.; Fu, W.-Z.; Zhang, C.-Q.; Zhang, Z.-L. Mutations in the SLCO2A1 Gene and Primary Hypertrophic Osteoarthropathy: A Clinical and Biochemical Characterization. J. Clin. Endocrinol. Metab. 2013, 98, E923–E933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, R.; Wang, G.; Zhang, H.; Yang, X.; Li, Z.; Guan, J.; Gai, Z.; Liu, Y. Establishment of a novel human iPSC line (SDQLCHi032-A) derived from a patient with primary hypertrophic osteoarthropathy caused by HPGD homozygous mutation. Stem Cell Res. 2021, 52, 102217. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Diggle, C.P.; Carr, I.M.; Fishwick, C.W.G.; Ahmed, M.; Ibrahim, G.H.; Helliwell, P.S.; Latos-Bieleńska, A.; Phillips, S.E.V.; Markham, A.F.; et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat. Genet. 2008, 40, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: Two case reports. Medicine 2017, 96, e8865. [Google Scholar] [CrossRef]
- Flayell, G. Reversal of pulmonary hypertrophic osteoarthropathy by vagotomy. Lancet 1956, 267, 260–262. [Google Scholar] [CrossRef]
- Bingol, U.A.; Cinar, C. Pachydermoperiostosis: Aesthetic Treatment of Prematurely Aging Face With Facelift and Botulinum Toxin A. J. Craniofacial Surg. 2014, 25, e563–e564. [Google Scholar] [CrossRef]
- Ai, M.; Heeger, S.; Bartels, C.F.; Schelling, D.K.; Osteoporosis-Pseudoglioma Collaborative, G. Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am. J. Hum. Genet. 2005, 77, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H. Complex genetics of familial exudative vitreoretinopathy and related pediatric retinal detachments. Taiwan J. Ophthalmol. 2015, 5, 56–62. [Google Scholar] [CrossRef]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Joyal, J.-S.; Juan, A.M.; Hatton, C.J.; Aderman, C.M.; Dennison, R.J.; Willett, K.L.; et al. Retinal Expression of Wnt-Pathway Mediated Genes in Low-Density Lipoprotein Receptor-Related Protein 5 (Lrp5) Knockout Mice. PLoS ONE 2012, 7, e30203. [Google Scholar] [CrossRef] [PubMed]
- Toomes, C.; Bottomley, H.M.; Jackson, R.M.; Towns, K.V.; Scott, S.; Mackey, D.A.; Craig, J.E.; Jiang, L.; Yang, Z.; Trembath, R.; et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 2004, 74, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.; Moore, A.; Allgrove, J. Bilateral retinal detachments at birth: The osteoporosis pseudoglioma syndrome. Br. J. Ophthalmol. 2001, 85, 1139. [Google Scholar] [CrossRef][Green Version]
- Gowda, V.K.; Vegda, H.; Shivappa, S.K.; Benakappa, N. Osteoporosis Pseudoglioma Syndrome. J. Pediatr. Neurosci. 2020, 15, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Streeten, E.A.; McBride, D.; Puffenberger, E.; Hoffman, M.E.; Pollin, T.I.; Donnelly, P.; Sack, P.; Morton, H. Osteoporosis-pseudoglioma syndrome: Description of 9 new cases and beneficial response to bisphosphonates. Bone 2008, 43, 584–590. [Google Scholar] [CrossRef][Green Version]
- Miyazawa, S.; Nakamura, Y.; Kosho, T.; Kawame, H.; Narumi, S.; Hasegawa, T.; Suzuki, T.; Kato, H. Efficacy of denosumab therapy for osteoporosis-pseudoglioma syndrome with osteoporosis: A case report. Mod. Rheumatol. Case Rep. 2019, 3, 45–48. [Google Scholar] [CrossRef]
- Homaei, A.; Chegini, V.; Saffari, F. Clinical Response to Treatment with Teriparatide in an Adolescent with Osteoporosis-Pseudoglioma Syndrome (OPPG): A Case Report. Int. J. Endocrinol. Metab. 2022, 20, e121031. [Google Scholar] [CrossRef] [PubMed]
- Tosi, L.L.; Oetgen, M.E.; Floor, M.K.; Huber, M.B.; Kennelly, A.M.; McCarter, R.J.; Rak, M.F.; Simmonds, B.J.; Simpson, M.D.; Tucker, C.A.; et al. Initial report of the osteogenesis imperfecta adult natural history initiative. Orphanet J. Rare Dis. 2015, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Basel, D.; Steiner, R.D. Osteogenesis imperfecta: Recent findings shed new light on this once well-understood condition. Genet. Med. 2009, 11, 375–385. [Google Scholar] [CrossRef]
- Tournis, S.; Dede, A.D. Osteogenesis imperfecta—A clinical update. Metab.—Clin. Exp. 2018, 80, 27–37. [Google Scholar] [CrossRef]
- Dubail, J.; Brunelle, P.; Baujat, G.; Huber, C.; Doyard, M.; Michot, C.; Chavassieux, P.; Khairouni, A.; Topouchian, V.; Monnot, S.; et al. Homozygous Loss-of-Function Mutations in CCDC134 Are Responsible for a Severe Form of Osteogenesis Imperfecta. J. Bone Miner. Res. 2020, 35, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, K.; Langdahl, B.; Ljunggren, Ö.; Kindmark, A. THERAPY OF ENDOCRINE DISEASE: Treatment of osteogenesis imperfecta in adults. Eur. J. Endocrinol. 2014, 171, R79–R90. [Google Scholar] [CrossRef]
- Subramanian, S.; Viswanathan, V.K. Osteogenesis Imperfecta. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.D.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Prim. 2017, 3, 17052. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.H.; DiMeglio, L.A. Advances in the Classification and Treatment of Osteogenesis Imperfecta. Curr. Osteoporos. Rep. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palomo, T.; Fassier, F.; Ouellet, J.; Sato, A.; Montpetit, K.; Glorieux, F.H.; Rauch, F. Intravenous Bisphosphonate Therapy of Young Children With Osteogenesis Imperfecta: Skeletal Findings During Follow Up Throughout the Growing Years. J. Bone Miner. Res. 2015, 30, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Hennedige, A.A.; Jayasinghe, J.; Khajeh, J.; Macfarlane, T.V. Systematic review on the incidence of bisphosphonate related osteonecrosis of the jaw in children diagnosed with osteogenesis imperfecta. J. Oral Maxillofac. Res. 2014, 4, e1. [Google Scholar] [CrossRef]
- Hoyer-Kuhn, H.; Franklin, J.; Allo, G.; Kron, M.; Netzer, C.; Eysel, P.; Hero, B.; Schoenau, E.; Semler, O. Safety and efficacy of denosumab in children with osteogenesis imperfect--a first prospective trial. J. Musculoskelet. Neuronal Interact. 2016, 16, 24–32. [Google Scholar]
- Trifirò, G.; Mora, S.; Marelli, S.; Luzi, L.; Pini, A. Increased fracture rate in children and adolescents with Marfan syndrome. Bone 2020, 135, 115333. [Google Scholar] [CrossRef]
- Carter, N.; Duncan, E.; Wordsworth, P. Bone mineral density in adults with Marfan syndrome. Rheumatology 2000, 39, 307–309. [Google Scholar] [CrossRef]
- Rolfes, M.C.; Deyle, D.R.; King, K.S.; Hand, J.L.; Graff, A.H.; Derauf, C. Fracture incidence in Ehlers-Danlos syndrome—A population-based case-control study. Child Abus. Negl. 2019, 91, 95–101. [Google Scholar] [CrossRef]
- Banica, T.; Coussens, M.; Verroken, C.; Calders, P.; De Wandele, I.; Malfait, F.; Zmierczak, H.G.; Goemaere, S.; Lapauw, B.; Rombaut, L. Higher fracture prevalence and smaller bone size in patients with hEDS/HSD—A prospective cohort study. Osteoporos. Int. 2020, 31, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.W.; Offoha, R.U.; Oswald, G.L.; Skolasky, R.L.; Dewan, A.K.; Zhen, G.; Shapiro, J.R.; Dietz, H.C.; Cao, X.; Sponseller, P.D. Increased fracture risk and low bone mineral density in patients with loeys–dietz syndrome. Am. J. Med. Genet. Part A 2013, 161, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am. J. Med. Genet. Part C: Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Robinson, P.N.; Godfrey, M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J. Med. Genet. 2000, 37, 9. [Google Scholar] [CrossRef]
- Mizuguchi, T.; Collod-Beroud, G.; Akiyama, T.; Abifadel, M.; Harada, N.; Morisaki, T.; Allard, D.; Varret, M.; Claustres, M.; Morisaki, H.; et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 2004, 36, 855–860. [Google Scholar] [CrossRef] [PubMed]
- MacCarrick, G.; Black, J.H., 3rd; Bowdin, S.; El-Hamamsy, I.; Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Sponseller, P.D.; Loeys, B.; Dietz, H.C., 3rd. Loeys-Dietz syndrome: A primer for diagnosis and management. Genet. Med. Off. J. Am. Coll. Med. Genet. 2014, 16, 576–587. [Google Scholar] [CrossRef]
- Hussein, D.; Olsson, C.; Lagerstedt-Robinson, K.; Moreira, T. Novel Mutation of the TGF-β 3 Protein (Loeys-Dietz Type 5) Associated With Aortic and Carotid Dissections. Neurol. Genet. 2021, 7, e625. [Google Scholar] [CrossRef]
- Beighton, P.; Paepe, A.D.; Steinmann, B.; Tsipouras, P.; Wenstrup, R.J. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Am. J. Med. Genet. 1998, 77, 31–37. [Google Scholar] [CrossRef]
- von Kodolitsch, Y.; De Backer, J.; Schüler, H.; Bannas, P.; Behzadi, C.; Bernhardt, A.M.; Hillebrand, M.; Fuisting, B.; Sheikhzadeh, S.; Rybczynski, M.; et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl. Clin. Genet. 2015, 8, 137–155. [Google Scholar] [CrossRef]
- Carbone, L.; Tylavsky, F.A.; Bush, A.J.; Koo, W.; Orwoll, E.; Cheng, S. Bone Density in Ehlers-Danlos Syndrome. Osteoporos. Int. 2000, 11, 388–392. [Google Scholar] [CrossRef]
- Dolan, A.L.; Arden, N.K.; Grahame, R.; Spector, T.D. Assessment of bone in Ehlers Danlos syndrome by ultrasound and densitometry. Ann. Rheum. Dis. 1998, 57, 630. [Google Scholar] [CrossRef] [PubMed]
- Moura, B.; Tubach, F.; Sulpice, M.; Boileau, C.; Jondeau, G.; Muti, C.; Chevallier, B.; Ounnoughene, Y.; Le Parc, J.-M. Bone mineral density in Marfan syndrome. A large case-control study. Jt. Bone Spine 2006, 73, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Hossein-Nezhad, A.; Tabatabaei, F. Multiple fractures in infants who have Ehlers-Danlos/hypermobility syndrome and or vitamin D deficiency: A case series of 72 infants whose parents were accused of child abuse and neglect. Dermato-Endocrinology 2017, 9, e1279768. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Shirvani, A.; Charoenngam, N. Fetal Fractures in an Infant with Maternal Ehlers-Danlos Syndrome, CCDC134 Pathogenic Mutation and a Negative Genetic Test for Osteogenesis Imperfecta. Children 2021, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- OSTEOGENESIS IMPERFECTA, TYPE XXII.; OI22. Available online: https://www.omim.org/entry/619795 (accessed on 6 April 2022).
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef] [PubMed]
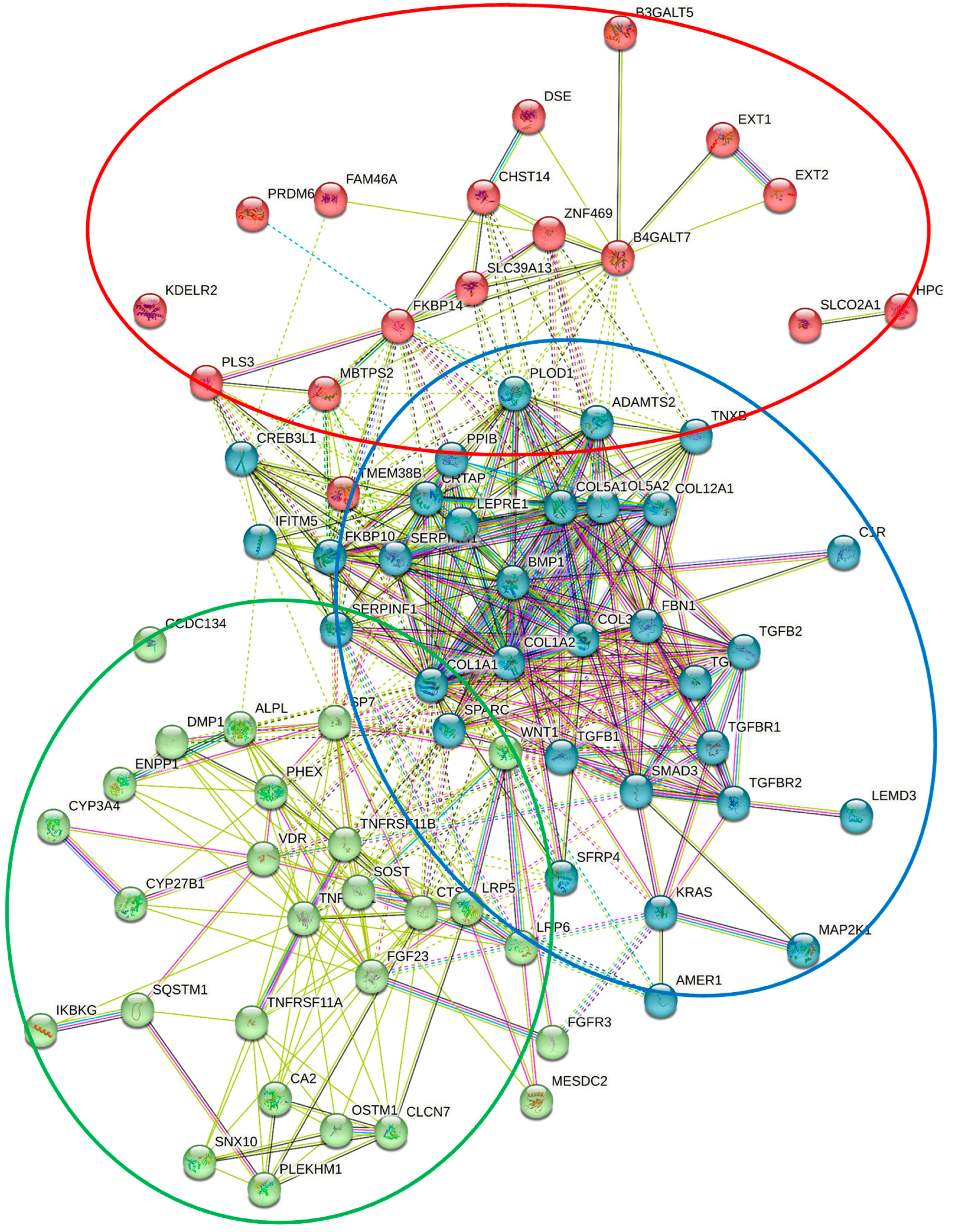
| Disorder | Clinical Manifestations | Causative Genetic Variations |
|---|---|---|
| Sclerosing disorders | ||
| Autosomal dominant osteopetrosis | Increased BMD, bony sclerosis, bone fragility, metaphyseal deformity, osteomyelitis, tooth eruption defects, dental caries, moderate bone marrow failure, cranial nerves impingement (II, VII, VIII) |
|
| Classic autosomal recessive osteopetrosis | Increased BMD, bony sclerosis, bone fragility, metaphyseal deformity, osteomyelitis, tooth eruption defects, dental caries, hydrocephalus, hypocalcemia, severe bone marrow failure, extramedullary hematopoiesis, hepatosplenomegaly, cranial nerves impingement (II, VII, VIII) |
|
| X-linked osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia and immunodeficiency | Increased BMD, bony sclerosis, bone fragility, metaphyseal deformity, osteomyelitis, tooth eruption defects, dental caries, anhidrotic ectodermal dysplasia, lymphedema, immunodeficiency |
|
| Autosomal recessive osteopetrosis with renal tubular acidosis | Increased BMD, bony sclerosis, bone fragility, metaphyseal deformity, osteomyelitis, tooth eruption defects, dental caries, renal tubular acidosis, developmental delay, intracranial calcification, cranial nerves impingement, bone marrow failure (rare) |
|
| Progressive Diaphyseal Dysplasia | Symmetric periosteal/endosteal thickening of long bone diaphysis (primarily femur and tibia), increased BMD, leg pain, muscle weakness, fatigue, slim limbs, tender bones, cranial enlargement, prominent forehead, cranial nerve palsy, hydrocephalus, hypocalciuria, hypocalcemia |
|
| Melorheostosis | Bone resembles dripping wax from a melting candle (affecting appendicular skeleton and adjacent soft tissue), dense hyperostosis of periosteal/endosteal surfaces, pain, bony swelling, joint and limb deformities, limited motion, numbness, weakness |
|
| Juvenile Paget’s Disease | Rapid bone turnover in children, bone deformities and fractures, short stature, elevated bone alkaline phosphatase, hearing loss, retinopathy, vascular calcification, internal carotid artery aneurysm |
|
| Paget’s disease | Bone pain, fractures and deformity, headache, hearing loss, nerve compression, spinal stenosis, high-output cardiac failure |
|
| High bone mass associated with LRP5 mutation | Extremely high BMD, increased calvarial thickness, craniosynostosis, striking square jaw, torus palatinus, thickened cortices of long bone |
|
| High bone mass associated with LRP6 mutation | Extremely high BMD, absence of adult maxillary incisors, broad jaw, torus palatinus, thickening of the skull, optic nerve dilatation, narrowing of optic and autidory canals |
|
| Pyle disease | Genu valgum, “Erlenmeyer flask” deformity, metaphyseal fracture, dental abnormalities, prognathism |
|
| Hyperostosis Corticalis Generalista | Endosteal hyperostosis of the mandible, skull, ribs, clavicles, and diaphysis of long bones, facial nerve palsy, hearing loss, optic atrophy |
|
| Sclerosteosis | Generalized bone overgrowth, jaw enlargement, facial abnormality, cranial nerve impingement, increased intracranial pressure, syndactyly, tall stature |
|
| Pyknodysostosis | short-limbed, short stature, dysmorphic facial features (small jaw, obtuse mandibular angle, and convex nasal ridge), osteosclerosis, bone fragility and fractures, dental and nail abnormalities, kyphoscoliosis, chest deformity, high arched palate, proptosis, blue sclera |
|
| Osteopathia Striata | Linear striations within the metaphyseal areas of long bones, macrocephaly, characteristic facial features (frontal bossing, hypertelorism, depressed nasal bridge, prominent mandible, and epicanthal folds), hearing loss, orofacial clefting, mild developmental delay In males: congenital, musculoskeletal defects (in mild cases). Multiple-malformation syndrome (in severe cases) |
|
| Osteopoikilosis | Numerous bone islands that typically affect the appendicular skeleton, usually free of any major symptoms |
|
| Demineralization disorders | ||
| Hypophosphatasia | ||
| Odonto hypophosphatasia | Premature loss of primary teeth |
|
| Adult hypophosphatasia | Bone pain, pseudogout, calcium pyrophosphate dihydrate crystal deposition in ligaments, and soft tissues. | |
| Childhood hypophosphatasia | Premature loss of primary teeth, bone pain, craniosynostosis, rachitic rosary, flaring of metaphysis, bowed legs, waddling gait | |
| Infantile hypophosphatasia | Skeletal rachitic deformities, brachycephaly, hypertelorism, increased intracranial pressure, tracheomalacia, chest wall deformity, recurrent pneumonia, hypercalcemia, hypercalciuria, pyridoxine-dependent seizure | |
| Perinatal hypophosphatasia | In utero limb deformities, cardiopulmonary failure, brain hemorrhage, myelophthisic anemia | |
| Hypophosphatemic rickets | Proximal muscle weakness, waddling gait, short stature, defective limb growth with preserved trunk growth, delayed tooth eruption, increased risk of dental abscesses, frontal bossing, parietal flattening, craniosynostosis, genu valgum/varum, intoeing or extoeing leg deformities, thickening of costochondral junctions |
|
| Vitamin D-dependent rickets | Proximal muscle weakness, waddling gait, short stature, sweating, delayed tooth eruption, sweating, craniotabes, frontal bossing, widened fontanelles, rachitic rosary, sternal protrusion, ribs deformities, flattened pelvic bones, bowing deformities of arms and legs, genu valgum/varum, flattened pelvic bones, hypocalcemic tetany, seizures, laryngospasm, cardiomyopathy, alopecia (for vitamin D-dependent rickets type II) |
|
| Axial osteomalacia | Bone pain along the axial skeleton, findings resembling ankylosing spondylosis |
|
| Disorders of bone matrix and cartilage formation | ||
| Achondroplasia | Short statue, prominent abdomen and buttocks, macrocephaly, frontal bossing, depressed nasal bridge, short extremities, limited range of motion at the elbows |
|
| Hypochondroplasia | Short stature without macrocephaly, short appendicular bone (less pronounced than achondroplasia) | |
| Thanatophoric dysplasia | Short extremities, redundant skin on the arms and legs, respiratory failure, flattened spine and curved femurs (type 1), straight femur and cloverleaf skull (type 2) | |
| Multiple exostosis | Multiple osteochondroma causing impingement of nerves and muscle tendons, nontraumatic fractures of osteochondroma |
|
| Patchydermoperiostosis | Clubbing of the fingers and toes, furrowing and thickening of the facial skin and the scalp, cylindrical enlargement of upper and lower extremities, hyperhydrosis, arthalgia |
|
| Osteoporosis-pseudoglioma syndrome | Juvenile-onset osteoporosis, fragility fractures, scoliosis, short stature, limb deformities, craniotabes, visual impairment, intellectual disability, hypotonia, joint hypermobility, seizures |
|
| Osteogenesis imperfecta | ||
| OI type I | Mild, normal or short stature, blue sclerae, late-onset hearing loss |
|
| OI type II | Perinatally lethal, minimal calvarial mineralization |
|
| OI type III | Severe, progressively deforming bones |
|
| OI type IV | More severe than type I, lesser severe than type II and type III, short stature, bone deformity |
|
| OI type V | Normal-to-severe skeletal deformity, hyperplastic callus formation, intraosseous membrane ossifications |
|
| OI type VI | Presence of osteoid, fish-scale appearance of the lamellar bone pattern |
|
| OI type VII | Severe to lethal, rhizomelia |
|
| OI type VIII | Severe to lethal, rhizomelia, coxa vara, popcorn metaphyses |
|
| OI type IX | Short bowed femurs with anterior, bowing of the tibiae, grey sclerae |
|
| OI type X | Severe skeletal deformity, blue sclerae, dentinogenesis imperfecta, skin abnormalities, inguinal hernia |
|
| OI type XI | Joint contractures (distorted lamellar, structure and a fish scale-like pattern), normal to grey sclerae |
|
| OI type XII | Fractures, mild bone deformations, generalized osteoporosis, delayed teeth eruption, progressive hearing loss, no dentinogenesis imperfecta, white sclerae |
|
| OI type XIII | Severe skeletal deformity, delayed tooth eruption, facial hypoplasia |
|
| OI type XIV | Severe bone deformity, normal-to-blue sclerae |
|
| OI type XV | Fractures, bone deformities, short stature, blue sclerae |
|
| OI type XVI | Prenatal onset of multiple fractures of ribs and long bones, blue sclerae, decreased ossification of the skull, and severe demineralization. |
|
| OI type XVII | Fractures, motor delay, muscle hypotonia, lower extremity weakness, decreased calf muscle mass, joint hyperlaxity, and soft skin |
|
| OI type XVIII | Congenital bowing of the long bones, wormian bones, blue sclerae, vertebral collapse, multiple fractures |
|
| OI type XIX | Moderate short stature, blue sclerae, pectus carinatum, bowing of lower extremity long bones, multiple fractures |
|
| OI type XX | Osteopenia, skeletal deformity, multiple fractures, respiratory failure |
|
| OI type XXI | Short stature, failure to thrive, wormian bones, bowed limbs, chest deformity, hypotonia, joint hypermobility, dysmorphic facies, blue sclerae, dentinogenesis imperfecta, scoliosis, fractures, platyspondyly. |
|
| OI type XXII | Intrauterine growth retardation, short stature, multiple fractures, decreased thoracic size, short limbs, blue sclerae |
|
| Ehlers-Danlos syndrome | ||
| Classical EDS, types I and II | Joint hypermobility, hyperextensible skin, easy bruisability, doughy-velvety skin, atrophic scars |
|
| Classical-like EDS | Hyperextensible skin, doughy-velvety skin texture, atropic scars, easy bruisability, joint hypermobility |
|
| Hypermobile EDS, type III | Joint hypermobility, joint dislocations-subluxations including temporal mandibular joint, hyperextensible skin, doughy-velvety skin, hernias, gastroparesis, high palate with dental crowding, bone fractures, vascular fragility, mast cell hyperactivity, postural orthostatic tachycardia syndrome, atrophic and hypertrophic scarring, poor wound healing, pieziogenic blisters on heels |
|
| Vascular EDS, type IV | Arterial rupture (aorta, mesenteric, cerebrovascular, splenic, renal arteries), organ rupture (colon, uterus), easy bruising, translucent skin |
|
| Cardiac-valvular type EDS | Progressive weakening of heart valves, hyperextensible skin, atrophic scars, easy bruisability, joint hypermobility |
|
| Kyphoscoliotic EDS, type VI | Joint hypermobility, kyphoscoliosis, osteopenia, hypotonia at birth, blue sclerae, Marfanoid habitus |
|
| Arthrochalasia EDS, types VIIA and VIIB | Congenital hip dislocations, recurrent subluxations, joint hypermobility, hyperextensible skin, muscle hypotonia, osteopenia |
|
| Dermatosparaxis EDS, type VIIC | Severe bruisability, blue sclerae, severe skin fragility, sagging skin, visceral fragility, growth retardation |
|
| Periodontal EDS, type VIII | Early onset severe periodontitis, unattached gingiva, pretibial plaques, hyperextensible skin, Marfanoid features, joint hypermobility |
|
| Spondylodysplastic EDS | Short stature, delayed eruption of teeth, hypodontia, limb bowing, joint laxity, osteopenia, hyperextensible, thin skin, delayed wound healing with atrophic scars |
|
| Musculocontractural EDS | Congenital contractures (thumb, finger, club feet), severe kyphoscoliosis, recurrent dislocations, easy bruisability, craniofacial features (broad forehead, small mouth, micrognathia, protruding jaw), hyperextensible fragile skin, atrophic scars |
|
| Brittle cornea syndrome | Thin cornea, retinal detachment, globe rupture, blue sclerae, keratoconus, high myopia |
|
| Myopathic EDS | Congenital hypotonia, proximal joint contractures, distal joint hypermobility, atrophic scars |
|
| Marfan’s syndrome | Aortic disease: aortic root disease, leading to aneurysmal dilatation, aortic regurgitation and dissection; Cardiac disease: mitral valve prolapse; Skeletal findings: arachnodactyly, pectus carinatum, pectus excavatum, abnormal upper/lower segments and arm span/height, scoliosis, kyphosis, malar hypoplasia, retrognathia; Ocular abnormalities: ectopia lentis, flat cornea, miosis, retinal detachment, glaucoma. Dural ectasia, emphysematous, pneumothorax, skin striae, arm span to height ratio >1.5 |
|
| Loeys-Dietz syndrome | Craniosynostosis, scoliosis, pectus excavatum/carinatum, clubfoot, pes planus, elongated limbs, joint instability/contracture, dural ectasia, bruising, abnormal scar, striae, skin translucency, spontaneous pneumothorax, hernias, hypertelorism, strabismus, bifid uvula, cleft palate, increased risk of immune disorders (i.e., allergies, asthma, eczema, inflammatory bowel disease) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenngam, N.; Nasr, A.; Shirvani, A.; Holick, M.F. Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management. Genes 2022, 13, 1880. https://doi.org/10.3390/genes13101880
Charoenngam N, Nasr A, Shirvani A, Holick MF. Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management. Genes. 2022; 13(10):1880. https://doi.org/10.3390/genes13101880
Chicago/Turabian StyleCharoenngam, Nipith, Aryan Nasr, Arash Shirvani, and Michael F. Holick. 2022. "Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management" Genes 13, no. 10: 1880. https://doi.org/10.3390/genes13101880
APA StyleCharoenngam, N., Nasr, A., Shirvani, A., & Holick, M. F. (2022). Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management. Genes, 13(10), 1880. https://doi.org/10.3390/genes13101880








