Is Mitochondria DNA Variation a Biomarker for AD?
Abstract
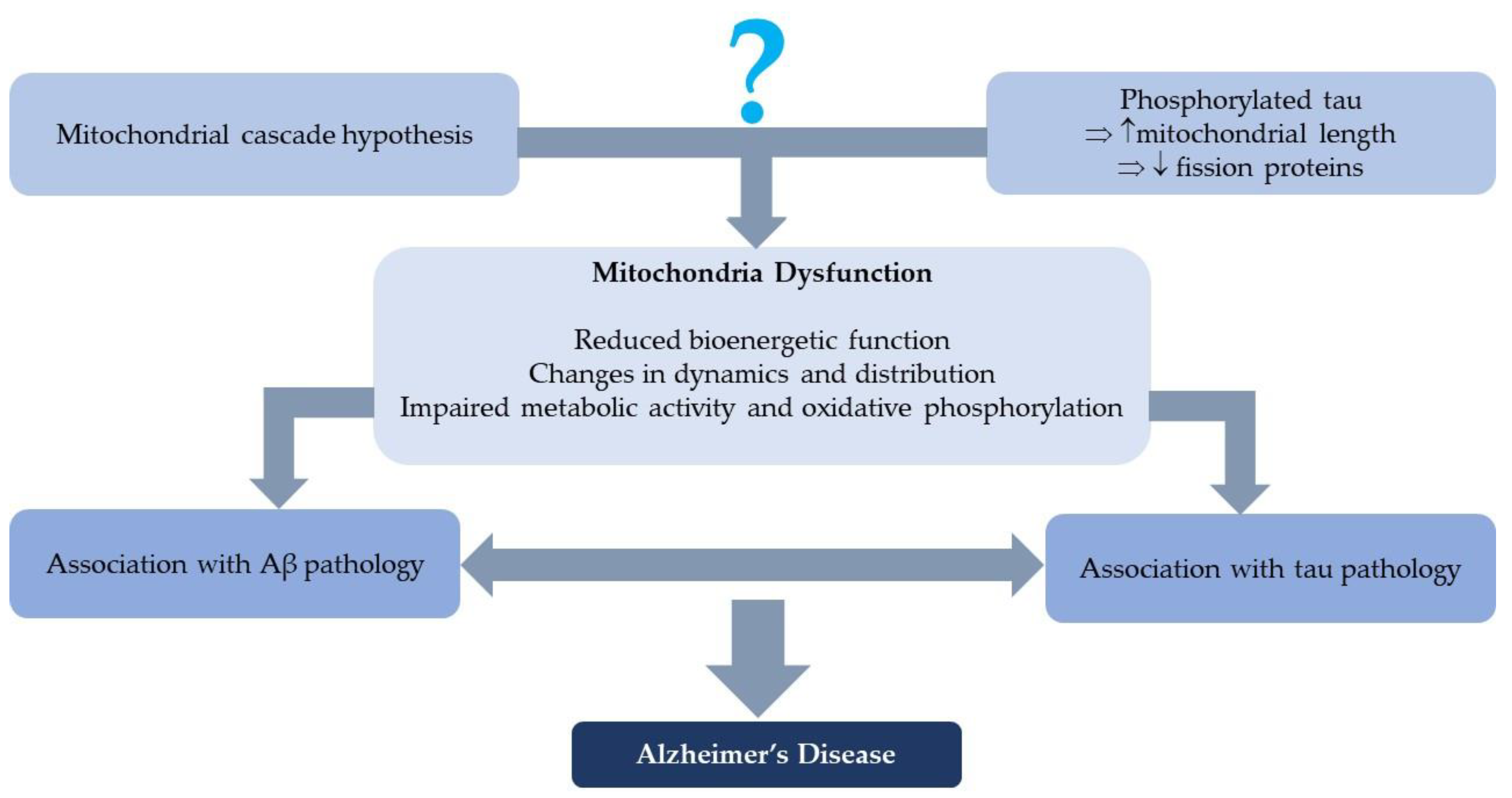
1. Introduction
2. MtDNA
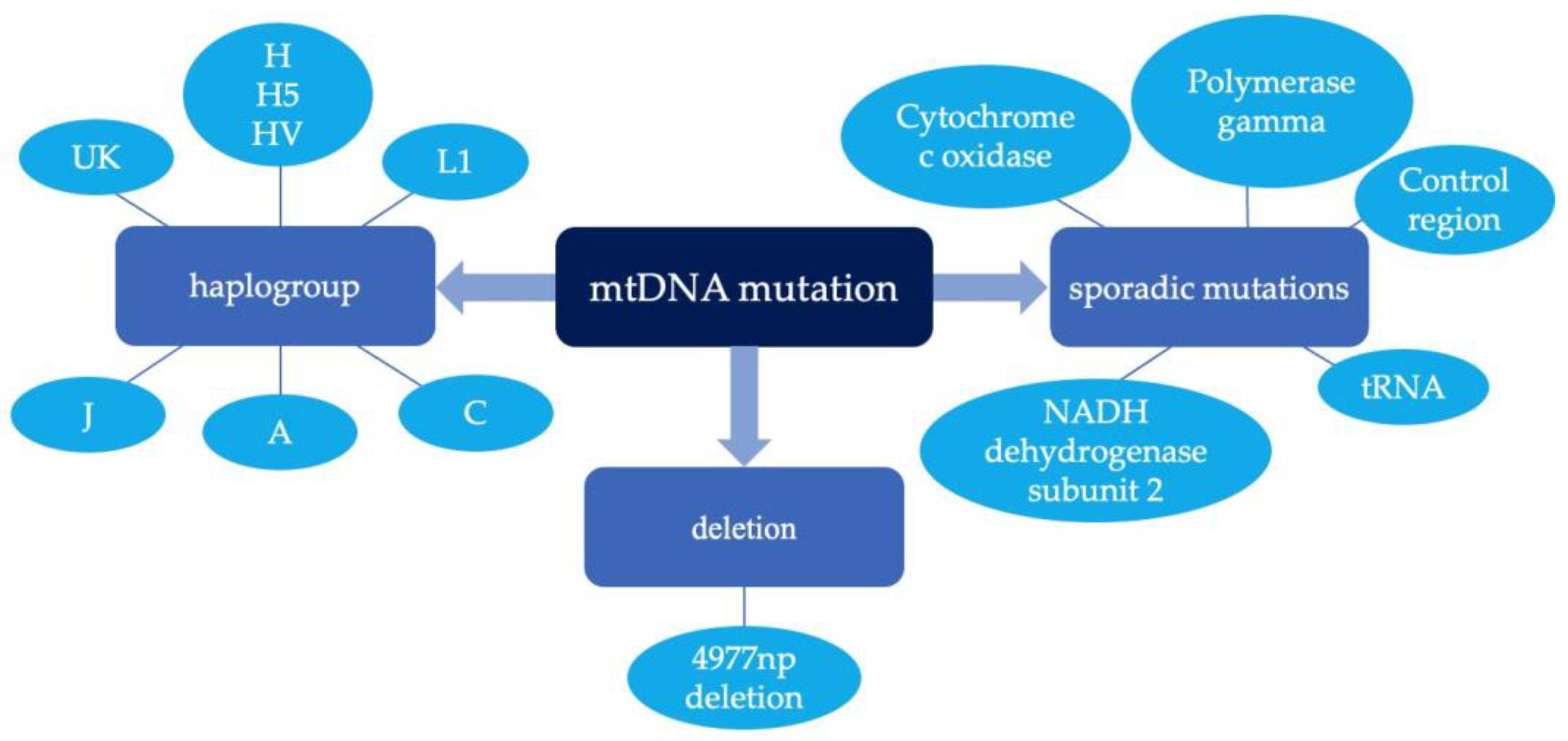
3. MtDNA Mutation
4. MtDNA Copy Number
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dumurgier, J.; Sabia, S. Epidemiology of Alzheimer’s disease: Latest trends. Rev. Prat. 2020, 70, 149–151. [Google Scholar] [PubMed]
- Guerreiro, R.; Bras, J. The age factor in Alzheimer’s disease. Genome Med. 2015, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Von Strauss, E.; Viitanen, M.; De Ronchi, D.; Winblad, B.; Fratiglioni, L. Aging and the Occurrence of Dementia: Findings From a Population-Based Cohort With a Large Sample of Nonagenarians. Arch. Neurol. 1999, 56, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Mega, S.M.; Cummings, L.J.; Fiorello, L.T.; Gornbein, L.J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996, 46, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Terzioğlu, G.; Örmeci, B.; Türksoy, Ö.; Sayman, C.; Çınar, N.; Öztürk, G.A.; Demirel, G.Y. Mitochondrial depletion in CD4+ and CD19+ peripheral lymphocytes in early stage Alzheimer’s disease. Mech. Ageing Dev. 2017, 167, 24–29. [Google Scholar] [CrossRef]
- DeMarshall, C.A.; Nagele, E.P.; Sarkar, A.; Acharya, N.K.; Godsey, G.; Goldwaser, E.L.; Kosciuk, M.; Thayasivam, U.; Han, M.; Belinka, B.; et al. Detection of Alzheimer’s disease at mild cognitive impairment and disease progression using autoantibodies as blood-based biomarkers. Alzheimers Dement 2016, 3, 51–62. [Google Scholar] [CrossRef]
- Reiss, A.B.; Ahmed, S.; Dayaramani, C.; Glass, A.D.; Gomolin, I.H.; Pinkhasov, A.; Stecker, M.M.; Wisniewski, T.; De Leon, J. The role of mitochondrial dysfunction in Alzheimer’s disease: A potential pathway to treatment. Exp. Gerontol. 2022, 164, 111828. [Google Scholar] [CrossRef] [PubMed]
- Opazo, F.; Vergara-Pulgar, K.; Perez, M.; Jara, C.; Osorio-Fuentealba, C.; Quintanilla, R. Mitochondrial Dysfunction Contributes to the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 509654. [Google Scholar] [CrossRef]
- Coppedè, F.; Stoccoro, A. Mitoepigenetics and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid cascade hypothesis: Are we poised for success or failure? J. Neurochem. 2016, 139, 237–252. [Google Scholar] [CrossRef]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial dysfunction: The missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Area-Gomez, E.; de Groof, A.; Bonilla, E.; Montesinos, J.; Tanji, K.; Boldogh, I.; Pon, L.; Schon, E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018, 9, 335. [Google Scholar] [CrossRef]
- Adlimoghaddam, A.; Snow, W.M.; Stortz, G.; Perez, C.; Djordjevic, J.; Goertzen, A.L.; Ko, J.H.; Albensi, B.C. Regional hypometabolism in the 3xTg mouse model of Alzheimer’s disease. Neurobiol. Dis. 2019, 127, 264–277. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2022. [Google Scholar] [CrossRef]
- Pérez, M.J.; Jara, C.; Quintanilla, R.A. Contribution of Tau Pathology to Mitochondrial Impairment in Neurodegeneration. Front. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial Respiratory-Chain Diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Silva, P.; Enriquez, J.A.; Montoya, J. Replication and Transcription of Mammalian Mitochondrial DNA. Exp. Physiol. 2003, 88, 41–56. [Google Scholar] [CrossRef]
- Cruz, A.C.P.; Ferrasa, A.; Muotri, A.R.; Herai, R.H. Frequency and association of mitochondrial genetic variants with neurological disorders. Mitochondrion 2019, 46, 345–360. [Google Scholar] [CrossRef]
- Phillips, N.R.; Simpkins, J.W.; Roby, R.K. Mitochondrial DNA deletions in Alzheimer’s brains: A review. Alzheimers Dement. 2014, 10, 393–400. [Google Scholar] [CrossRef]
- Zambrano, K.; Barba, D.; Castillo, K.; Robayo, P.; Argueta-Zamora, D.; Sanon, S.; Arizaga, E.; Caicedo, A.; Gavilanes, A.W.D. The war against Alzheimer, the mitochondrion strikes back! Mitochondrion 2022, 64, 125–135. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Carl, S.M.; Weber, S.G.; Ramanujan, S.A.; Festoff, B.W.; Linseman, D.A.; Swerdlow, R.H. Mitochondrial Lysates Induce Inflammation and Alzheimer’s Disease-Relevant Changes in Microglial and Neuronal Cells. J. Alzheimer’s Dis. 2015, 45, 305–318. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J. Neurosci. Res. 2007, 85, 3416–3428. [Google Scholar] [CrossRef]
- Gambardella, S.; Limanaqi, F.; Ferese, R.; Biagioni, F.; Campopiano, R.; Centonze, D.; Fornai, F. ccf-mtDNA as a Potential Link Between the Brain and Immune System in Neuro-Immunological Disorders. Front. Immunol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Reeve, A.K.; Krishnan, K.J.; Turnbull, D. Mitochondrial DNA Mutations in Disease, Aging, and Neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; Jacq, C.; Rouvière-Yaniv, J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc. Natl. Acad. Sci. USA 1979, 76, 4265–4269. [Google Scholar] [CrossRef]
- Kazak, L.; Reyes, A.; Holt, I.J. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 2012, 13, 659–671. [Google Scholar] [CrossRef]
- Linnane, A.; Ozawa, T.; Marzuki, S.; Tanaka, M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1989, 333, 642–645. [Google Scholar] [CrossRef]
- Antonyová, V.; Kejík, Z.; Brogyányi, T.; Kaplánek, R.; Pajková, M.; Talianová, V.; Hromádka, R.; Masařík, M.; Sýkora, D.; Mikšátková, L.; et al. Role of mtDNA disturbances in the pathogenesis of Alzheimer’s and Parkinson’s disease. DNA Repair 2020, 91–92, 102871. [Google Scholar] [CrossRef]
- Kukreja, L.; Kujoth, G.C.; Prolla, T.A.; Van Leuven, F.; Vassar, R. Increased mtDNA mutations with aging promotes amyloid accumulation and brain atrophy in the APP/Ld transgenic mouse model of Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Corral-Debrinski, M.; Horton, T.; Lott, M.T.; Shoffner, J.M.; McKee, A.C.; Beal, M.F.; Graham, B.H.; Wallace, D.C. Marked Changes in Mitochondrial DNA Deletion Levels in Alzheimer Brains. Genomics 1994, 23, 471–476. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Davis, R.E.; Miller, S.; Herrnstadt, C.; Ghosh, S.S.; Fahy, E.; Shinobu, L.A.; Galasko, D.; Thal, L.J.; Beal, M.F.; Howell, N.; et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 4526–4531. [Google Scholar] [CrossRef]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. The Whole Structure of the 13-Subunit Oxidized Cytochrome c Oxidase at 2.8 Å. Science 1996, 272, 1136–1144. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, Y.; Zhou, M. Two point mutations in mitochondrial DNA of cytochrome c oxidase coexist with normal mtDNA in a patient with Alzheimer’s disease. Brain Res. 2001, 893, 261–263. [Google Scholar] [CrossRef]
- Shoffner, J.M.; Brown, M.D.; Torroni, A.; Lott, M.T.; Cabell, M.F.; Mirra, S.S.; Beal, M.F.; Yang, C.-C.; Gearing, M.; Salvo, R.; et al. Mitochondrial DNA Variants Observed in Alzheimer Disease and Parkinson Disease Patients. Genomics 1993, 17, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Hutchin, T.; Cortopassi, G. A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc. Natl. Acad. Sci. USA 1995, 92, 6892–6895. [Google Scholar] [CrossRef]
- Egensperger, R.; Kösel, S.; Schnopp, N.M.; Mehraein, P.; Graeber, M.B. Association of the mitochondrial tRNAA4336G mutation with Alzheimer’s and Parkinson’s diseases. Neuropathol. Appl. Neurobiol. 1997, 23, 315–321. [Google Scholar] [CrossRef]
- Edland, S.D.; Tobe, V.O.; Rieder, M.J.; Bowen, J.D.; McCormick, W.; Teri, L.; Schellenberg, G.D.; Larson, E.B.; Nickerson, D.A.; Kukull, W.A. Mitochondrial Genetic Variants and Alzheimer Disease: A Case-Control Study of the T4336C and G5460A Variants. Alzheimer Dis. Assoc. Disord 2002, 16, 1–7. [Google Scholar] [CrossRef]
- Tanno, Y.; Okuizumi, K.; Tsuji, S. mtDNA Polymorphisms in Japanese Sporadic Alzheimer’s Disease. Neurobiol. Aging 1998, 19, S47–S51. [Google Scholar] [CrossRef]
- Chagnon, P.; Gee, M.; Filion, M.; Robitaille, Y.; Belouchi, M.; Gauvreau, D. Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. Am. J. Med. Genet. 1999, 85, 20–30. [Google Scholar] [CrossRef]
- Lin, F.-H.; Lin, R.; Wisniewski, H.M.; Hwang, Y.-W.; Grundke-Iqbal, I.; Healy-Louie, G.; Iqbal, K. Detection of point mutations in codon 331 of mitochondrial NADH dehydrogenase subunit 2 in alzheimer’s brains. Biochem. Biophys. Res. Commun. 1992, 182, 238–246. [Google Scholar] [CrossRef]
- Janetzky, B.; Schmid, C.; Bischof, F.; Frölich, L.; Gsell, W.; Kalaria, R.N.; Riederer, P.; Reichmann, H. Investigations on the Point Mutations at nt 5460 of the mtDNA in Different Neurodegenerative and Neuromuscular Diseases. Eur. Neurol. 1996, 36, 149–153. [Google Scholar] [CrossRef]
- Kosel, S.; Egensperger, R.; Mehraein, P.; Graeber, M.B. No Association of Mutations at Nucleotide 5460 of Mitochondrial NADH Dehydrogenase with Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 1994, 203, 745–749. [Google Scholar] [CrossRef]
- Grazina, M.; Silva, F.; Santana, I.; Pratas, J.; Santiago, B.; Oliveira, M.; Carreira, I.; Cunha, L.; Oliveira, C. Mitochondrial DNA Variants in a Portuguese Population of Patients with Alzheimer’s Disease. Eur. Neurol. 2005, 53, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.E.; Beal, M.F.; Wallace, D.C. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc. Natl. Acad. Sci. USA 2004, 101, 10726–10731. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.E.; Wyrembak, J.; Derbereva, O.; Melkonian, G.; Doran, E.; Lott, I.T.; Head, E.; Cotman, C.W.; Wallace, D.C. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S293–S310. [Google Scholar] [CrossRef] [PubMed]
- Coto, E.; Gómez, J.; Alonso, B.; Corao, A.I.; Díaz, M.; Menéndez, M.; Martínez, C.; Calatayud, M.T.; Morís, G.; Álvarez, V. Late-onset Alzheimer’s disease is associated with mitochondrial DNA 7028C/haplogroup H and D310 poly-C tract heteroplasmy. Neurogenetics 2011, 12, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Yokoyama, J.S.; Katzman, S.M.; Nalls, M.A.; Newman, A.B.; Harris, T.B.; Cesari, M.; Manini, T.M.; Schork, N.J.; Cummings, S.R.; et al. Mitochondrial DNA sequence associations with dementia and amyloid-β in elderly African Americans. Neurobiol. Aging 2014, 35, 442.e1–442.e8. [Google Scholar] [CrossRef]
- Santoro, A.; Balbi, V.; Balducci, E.; Pirazzini, C.; Rosini, F.; Tavano, F.; Achilli, A.; Siviero, P.; Minicuci, N.; Bellavista, E.; et al. Evidence for Sub-Haplogroup H5 of Mitochondrial DNA as a Risk Factor for Late Onset Alzheimer’s Disease. PLoS ONE 2010, 5, e12037. [Google Scholar] [CrossRef]
- Maruszak, A.; Canter, J.A.; Styczyńska, M.; Żekanowski, C.; Barcikowska, M. Mitochondrial haplogroup H and Alzheimer’s disease—Is there a connection? Neurobiol. Aging 2009, 30, 1749–1755. [Google Scholar] [CrossRef]
- Lakatos, A.; Derbeneva, O.; Younes, D.; Keator, D.; Bakken, T.; Lvova, M.; Brandon, M.; Guffanti, G.; Reglodi, D.; Saykin, A.; et al. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol. Aging 2010, 31, 1355–1363. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhao, H.; Du, Y.; Liu, X. The heterogeneity among subgroups of haplogroup J. influencing Alzheimer’s disease risk. J. Adv. Res. 2021, 33, 117–126. [Google Scholar] [CrossRef]
- Watts, A.; Chalise, P.; Hu, J.; Hui, D.; Pa, J.; Andrews, S.J.; Michaelis, E.K.; Swerdlow, R.H. A Mitochondrial DNA Haplogroup Defines Patterns of Five-Year Cognitive Change. J. Alzheimer’s Dis. 2022, 89, 913–922. [Google Scholar] [CrossRef]
- Reid, D.M.; Barber, R.C.; Thorpe, R.J.; Sun, J.; Zhou, Z.; Phillips, N.R. Mitochondrial DNA oxidative mutations are elevated in Mexican American women potentially implicating Alzheimer’s disease. Npj Aging 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Simon, D.K.; Ahn, C.H.; Kim, L.M.; Beal, M.F. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum. Mol. Genet. 2002, 11, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.T.; Pereira, C.P.M.; Rowies, F.T.; Farfel, J.M.; Grinberg, L.T.; Suemoto, C.K.; Leite, R.E.P.; Rodriguez, R.D.; Ericson, N.G.; Bielas, J.H.; et al. Lower mitochondrial DNA content but not increased mutagenesis associates with decreased base excision repair activity in brains of AD subjects. Neurobiol. Aging 2019, 73, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, T.; Bhattacharjee, P.; Bhattacharjee, S.; Bhattacharjee, P. Hypomethylation of mitochondrial D-loop and ND6 with increased mitochondrial DNA copy number in the arsenic-exposed population. Toxicology 2018, 408, 54–61. [Google Scholar] [CrossRef]
- St. John, J.C. Mitochondrial DNA copy number and replication in reprogramming and differentiation. Semin. Cell Dev. Biol. 2016, 52, 93–101. [Google Scholar] [CrossRef]
- Wei, W.; Keogh, M.J.; Wilson, I.; Coxhead, J.; Ryan, S.; Rollinson, S.; Griffin, H.; Kurzawa-Akanbi, M.; Santibanez-Koref, M.; Talbot, K.; et al. Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human brains. Acta Neuropathol. Commun. 2017, 5, 13. [Google Scholar] [CrossRef]
- Rice, A.C.; Keeney, P.M.; Algarzae, N.K.; Ladd, A.C.; Thomas, R.R.; Bennett, J.P., Jr. Mitochondrial DNA Copy Numbers in Pyramidal Neurons are Decreased and Mitochondrial Biogenesis Transcriptome Signaling is Disrupted in Alzheimer’s Disease Hippocampi. J. Alzheimer’s Dis. 2014, 40, 319–330. [Google Scholar] [CrossRef]
- Rodríguez-Santiago, B.; Casademont, J.; Nunes, V. Is mitochondrial DNA depletion involved in Alzheimer’s disease? Eur. J. Hum. Genet. 2001, 9, 279–285. [Google Scholar] [CrossRef]
- Klein, H.-U.; Trumpff, C.; Yang, H.-S.; Lee, A.J.; Picard, M.; Bennett, D.A.; De Jager, P.L. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Mol. Neurodegener. 2021, 16, 75. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Podlesniy, P.; Figueiro-Silva, J.; Llado, A.; Antonell, A.; Sanchez-Valle, R.; Alcolea, D.; Lleo, A.; Molinuevo, J.L.; Serra, N.; Trullas, R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann. Neurol. 2013, 74, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Podlesniy, P.; Llorens, F.; Puigròs, M.; Serra, N.; Sepúlveda-Falla, D.; Schmidt, C.; Hermann, P.; Zerr, I.; Trullas, R. Cerebrospinal Fluid Mitochondrial DNA in Rapid and Slow Progressive Forms of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6298. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Carles, L.; Alcolea, D.; Estanga, A.; Ecay-Torres, M.; Izagirre, A.; Clerigué, M.; García-Sebastián, M.; Villanúa, J.; Escalas, C.; Blesa, R.; et al. Cerebrospinal fluid mitochondrial DNA in the Alzheimer’s disease continuum. Neurobiol. Aging 2017, 53, e1–e192. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [CrossRef]
- Delbarba, A.; Abate, G.; Prandelli, C.; Marziano, M.; Buizza, L.; Arce Varas, N.; Novelli, A.; Cuetos, F.; Martinez, C.; Lanni, C.; et al. Mitochondrial Alterations in Peripheral Mononuclear Blood Cells from Alzheimer’s Disease and Mild Cognitive Impairment Patients. Oxid. Med. Cell. Longev. 2016, 2016, 5923938. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, D.; Ge, B.; Chen, H.; Du, Y.; Liu, S.; Ji, Y.; Sun, C.; Wang, G.; Gao, Y.; et al. Association of Folate Metabolites and Mitochondrial Function in Peripheral Blood Cells in Alzheimer’s Disease: A Matched Case-Control Study. J. Alzheimer’s Dis. 2019, 70, 1133–1142. [Google Scholar] [CrossRef]
- Liou, C.-W.; Chen, S.-H.; Lin, T.-K.; Tsai, M.-H.; Chang, C.-C. Oxidative Stress Biomarkers and Mitochondrial DNA Copy Number Associated with APOE4 Allele and Cholinesterase Inhibitor Therapy in Patients with Alzheimer’s Disease. Antioxidants 2021, 10, 1971. [Google Scholar] [CrossRef]
- Lunnon, K.; Keohane, A.; Pidsley, R.; Newhouse, S.; Riddoch-Contreras, J.; Thubron, E.B.; Devall, M.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; et al. Mitochondrial genes are altered in blood early in Alzheimer’s disease. Neurobiol. Aging 2017, 53, 36–47. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, L.; Han, M.; Liu, X.; Li, F.; Zhou, X.; Wang, Y.; Bi, J. Altered mitochondrial DNA methylation and mitochondrial DNA copy number in an APP/PS1 transgenic mouse model of Alzheimer disease. Biochem. Biophys. Res. Commun. 2019, 520, 41–46. [Google Scholar] [CrossRef]
- Andrews, S.J.; Goate, A.M. Mitochondrial DNA copy number is associated with cognitive impairment. Alzheimers. Dement. 2020, 16, e047543. [Google Scholar] [CrossRef]
- Silzer, T.; Barber, R.; Sun, J.; Pathak, G.; Johnson, L.; O’Bryant, S.; Phillips, N. Circulating mitochondrial DNA: New indices of type 2 diabetes-related cognitive impairment in Mexican Americans. PLoS ONE 2019, 14, e0213527. [Google Scholar] [CrossRef] [PubMed]
- Thubron, E.B.; Rosa, H.S.; Hodges, A.; Sivaprasad, S.; Francis, P.T.; Pienaar, I.S.; Malik, A.N. Regional mitochondrial DNA and cell-type changes in post-mortem brains of non-diabetic Alzheimer’s disease are not present in diabetic Alzheimer’s disease. Sci. Rep. 2019, 9, 11386. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Park, K.D.; Im, J.-A.; Kim, M.Y.; Lee, D.-C. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin. Chim. Acta 2010, 411, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.H.; Lee, D.C. Combined Impact of Telomere Length and Mitochondrial DNA Copy Number on Cognitive Function in Community-Dwelling Very Old Adults. Dement. Geriatr. Cogn. Disord. 2017, 44, 232–243. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.-G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
| Source | Tissue/Cell Type | Technique | Ratio | Trend | Disease Stage | Reference |
|---|---|---|---|---|---|---|
| Brain | mean mtDNA read depth/mean exome read depth | decrease | AD | [66] | ||
| Brain | Hippocampus | Multiplex qPCR | comparing to a standard curve of circular human mtDNA run on the same plate | decrease | AD | [67] |
| Brain | Frontal cortex | RT-PCR | mtND2/r18S | decrease | AD | [68] |
| Brain | Hippocampus and CE | RT-PCR | mtND2/r18S | No change | AD | [68] |
| Blood | RT-PCR | mtND2/r18S | No change | AD | [68] | |
| Brain | Frontal cortex | qRT-PCR | mtND2/r18S | decrease | [52] | |
| Brain | DLPFC, PCC | WGS | median sequence coverages of the autosomal chromosomes covnuc and of the mitochondrial genome covmt (covmt/covnuc) × 2 | decrease | AD | [69] |
| Brain | CE | WGS | median sequence coverages of the autosomal chromosomes covnuc and of the mitochondrial genome covmt (covmt/covnuc) × 2 | No change | AD | [69] |
| Brain | TC | ddPCR | mitochondrial (Walker)/nuclear (RPP30) loci | decrease | AD | [63] |
| Brain | CE | ddPCR | mitochondrial (Walker)/nuclear (RPP30) loci | No change | AD | [63] |
| Brain | CSF | qPCR; ddPCR | 1 copy of mtDNA corresponds to 18.16 attogram | decrease | symptomatic AD | [71] |
| Brain | CSF | ddPCR | copies/µL of CSF | decrease | AD patients progressed faster | [72] |
| Brain | CSF | ddPCR | copies/µL of CSF | increase | AD | [73] |
| Brain | Pyramidal neurons | In situ hybridization | increase | AD | [74] | |
| Blood | PBMC | qRT-PCR | mtDNA/a reference single copy gene | decrease | MCI and AD | [76] |
| Blood | Leukocyte | qRT-PCR | mtDNA/a reference single copy gene | decrease | AD | [76] |
| Blood | CD4+, CD19+ and CD56+ peripheral lymphocytes | RT-PCR | mtDNA/β globin | decrease | early- and late-stage AD | [9] |
| Blood | CD8+ peripheral lymphocytes | RT-PCR | mtDNA/β globin | decrease | late-stage AD | [9] |
| Blood | CD56+ peripheral lymphocytes | RT-PCR | mtDNA/β globin | decrease | early- and late-stage AD | [9] |
| APP/PS1 transgenic mice model | qRT-PCR | 12 S rRNA/18 S rRNA | decrease | [79] | ||
| Brain | WGS | mitochondrial genomes/nuclear genome | decrease | cognitive impaired | [80] | |
| Blood | Buffy coat | RT-PCR | nuclear DNA/mtDNA | decrease | MCI and AD | [81] |
| Brain | Parietal cortex | qRT-PCR | mtDNA/β-2-microglobulin | decrease | MCI and AD | [82] |
| Blood | RT-PCR | mtND1/β globin | decrease | cognitive impaired | [83] | |
| Blood | RT-PCR | mtDNA/β globin | decrease | cognitive dysfunction | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Ma, S.L. Is Mitochondria DNA Variation a Biomarker for AD? Genes 2022, 13, 1789. https://doi.org/10.3390/genes13101789
Gao R, Ma SL. Is Mitochondria DNA Variation a Biomarker for AD? Genes. 2022; 13(10):1789. https://doi.org/10.3390/genes13101789
Chicago/Turabian StyleGao, Ruonan, and Suk Ling Ma. 2022. "Is Mitochondria DNA Variation a Biomarker for AD?" Genes 13, no. 10: 1789. https://doi.org/10.3390/genes13101789
APA StyleGao, R., & Ma, S. L. (2022). Is Mitochondria DNA Variation a Biomarker for AD? Genes, 13(10), 1789. https://doi.org/10.3390/genes13101789







