Alpelisib Efficacy in Hormone Receptor-Positive HER2-Negative PIK3CA-Mutant Advanced Breast Cancer Post-Everolimus Treatment
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Response to Everolimus and Alpelisib
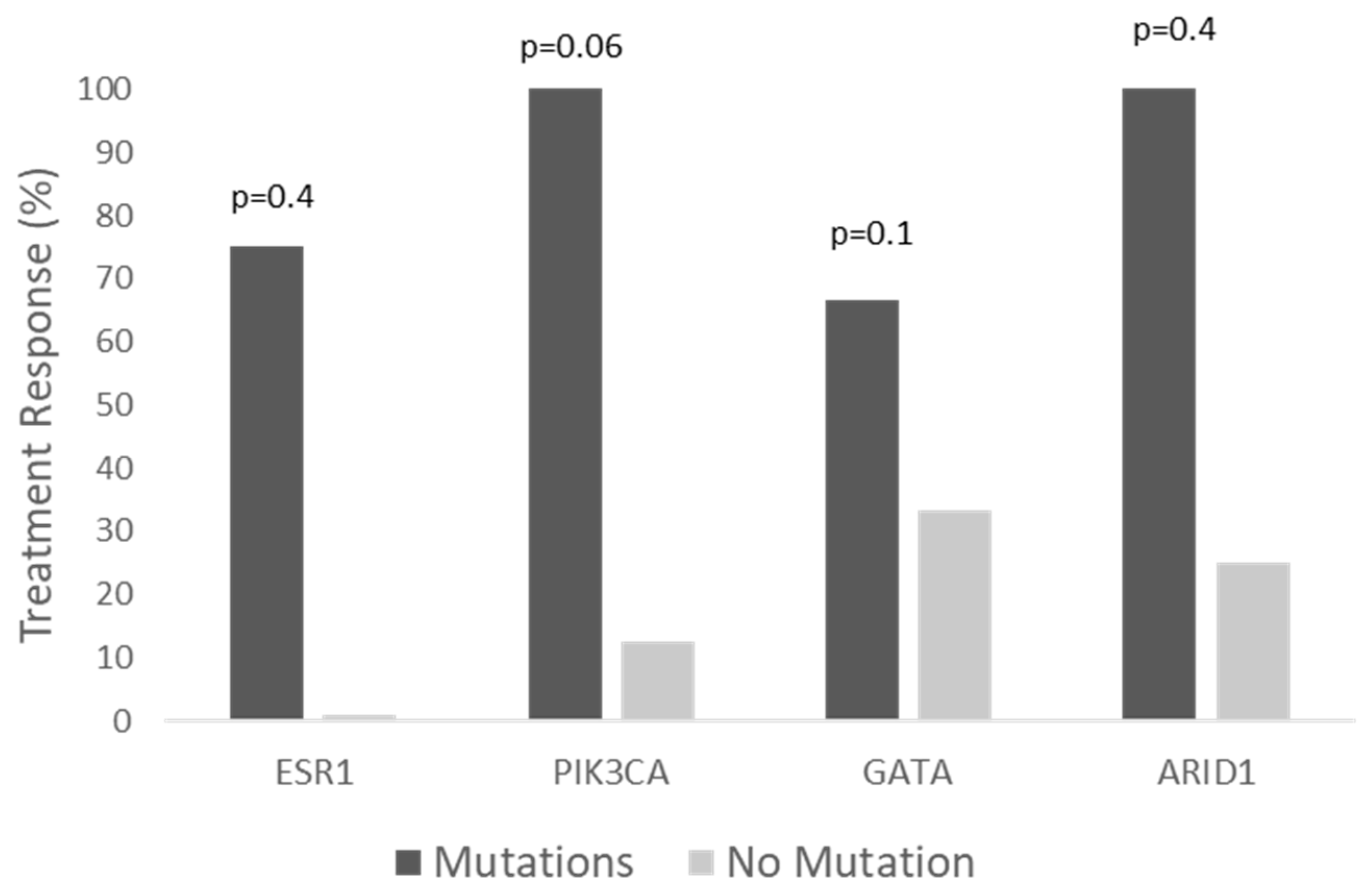
3.2. Clinical Benefit of Alpelisib by Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Miyoshi, Y. Mechanism of resistance to endocrine therapy in breast cancer: The important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 2018, 25, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, A.; Antony Thomas, R.; Levine, E.G.; Brufsky, A.; Takabe, K.; Hanna, M.G.; Attwood, K.; Miller, A.; Khoury, T.; Early, A.P.; et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib. Breast Cancer Basic Clin. Res. 2020, 14, 1178223420944864. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor—Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Matikas, A.; Kotsakis, A.; Perraki, M.; Hatzidaki, D.; Kalbakis, K.; Kontopodis, E.; Nikolaou, M.; Georgoulias, V. Objective Response to First-Line Treatment as a Predictor of Overall Survival in Metastatic Breast Cancer: A Retrospective Analysis from Two Centers over a 25-Year Period. Breast Care 2022, 17, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- Razavi, P.; Dickler, M.N.; Shah, P.D.; Toy, W.; Brown, D.N.; Won, H.H.; Li, B.T.; Shen, R.; Vasan, N.; Modi, S.; et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat. Cancer 2020, 1, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Neven, P.; Saffie, I.; Park, H.P.; Laurentiis, M.D.; Lerebours, F.; Ciruelos, E.M.; Turner, N.; Juric, D.; Gu, E.; et al. Alpelisib + fulvestrant in patients with PIK3CA-mutated, HR+, HER2—advanced breast cancer (ABC) who received chemotherapy or endocrine therapy (ET) as immediate prior treatment: BYLieve Cohort C primary results and exploratory biomarker analyses. In Proceedings of the 2021 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 7–10 December 2021; AACR: San Antonio, TX, USA, 2022. Abstract nr PD13-05. [Google Scholar]
- Vasan, N.; Razavi, P.; Johnson, J.L.; Shao, H.; Shah, H.; Antoine, A.; Ladewig, E.; Gorelick, A.; Lin, T.Y.; Toska, E.; et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science 2019, 366, 714–723. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Ringeisen, F.; Hortobagyi, G.N.; et al. Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR(+), HER2(−) advanced breast cancer: Results from BOLERO-2. Br. J. Cancer 2017, 116, 726–730. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Chen, D.; Piccart, M.; Rugo, H.S.; Burris, H.A., 3rd; Pritchard, K.I.; Campone, M.; Noguchi, S.; Perez, A.T.; Deleu, I.; et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2. J. Clin. Oncol. 2016, 34, 419–426. [Google Scholar] [CrossRef]



| Characteristic | |
|---|---|
| Age, years, median (range) | 68 (49–81) |
| Previous systemic treatment lines, n | |
| 3 | 3 (23%) |
| 4 | 5 (31%) |
| ≥5 | 5 (38.5%) |
| Received chemotherapy before alpelisib | 4 (31%) |
| Median time from metastasis to alpelisib treatment, years (range) | 3.5 (1.4–11.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raphael, A.; Salmon-Divon, M.; Epstein, J.; Zahavi, T.; Sonnenblick, A.; Shachar, S.S. Alpelisib Efficacy in Hormone Receptor-Positive HER2-Negative PIK3CA-Mutant Advanced Breast Cancer Post-Everolimus Treatment. Genes 2022, 13, 1763. https://doi.org/10.3390/genes13101763
Raphael A, Salmon-Divon M, Epstein J, Zahavi T, Sonnenblick A, Shachar SS. Alpelisib Efficacy in Hormone Receptor-Positive HER2-Negative PIK3CA-Mutant Advanced Breast Cancer Post-Everolimus Treatment. Genes. 2022; 13(10):1763. https://doi.org/10.3390/genes13101763
Chicago/Turabian StyleRaphael, Ari, Mali Salmon-Divon, Jessica Epstein, Tamar Zahavi, Amir Sonnenblick, and Shlomit S. Shachar. 2022. "Alpelisib Efficacy in Hormone Receptor-Positive HER2-Negative PIK3CA-Mutant Advanced Breast Cancer Post-Everolimus Treatment" Genes 13, no. 10: 1763. https://doi.org/10.3390/genes13101763
APA StyleRaphael, A., Salmon-Divon, M., Epstein, J., Zahavi, T., Sonnenblick, A., & Shachar, S. S. (2022). Alpelisib Efficacy in Hormone Receptor-Positive HER2-Negative PIK3CA-Mutant Advanced Breast Cancer Post-Everolimus Treatment. Genes, 13(10), 1763. https://doi.org/10.3390/genes13101763






