Evolutionary Comparison of the Complete Chloroplast Genomes in Convallaria Species and Phylogenetic Study of Asparagaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples, DNA Extraction, and Sequencing
2.2. Chloroplast Genome Assembly and Annotation
2.3. Comparative Analysis of Convallaria cp Genomes and Hotspot Regions Identification
2.4. Long Repeats and Simple Sequence Repeats
2.5. Phylogenetic Analysis
3. Results
3.1. Genome Organization and Features
3.2. Variation at IR/SC Boundaries
3.3. Comparative Analysis of Convallaria cp Genomes and Hotspot Regions Identification
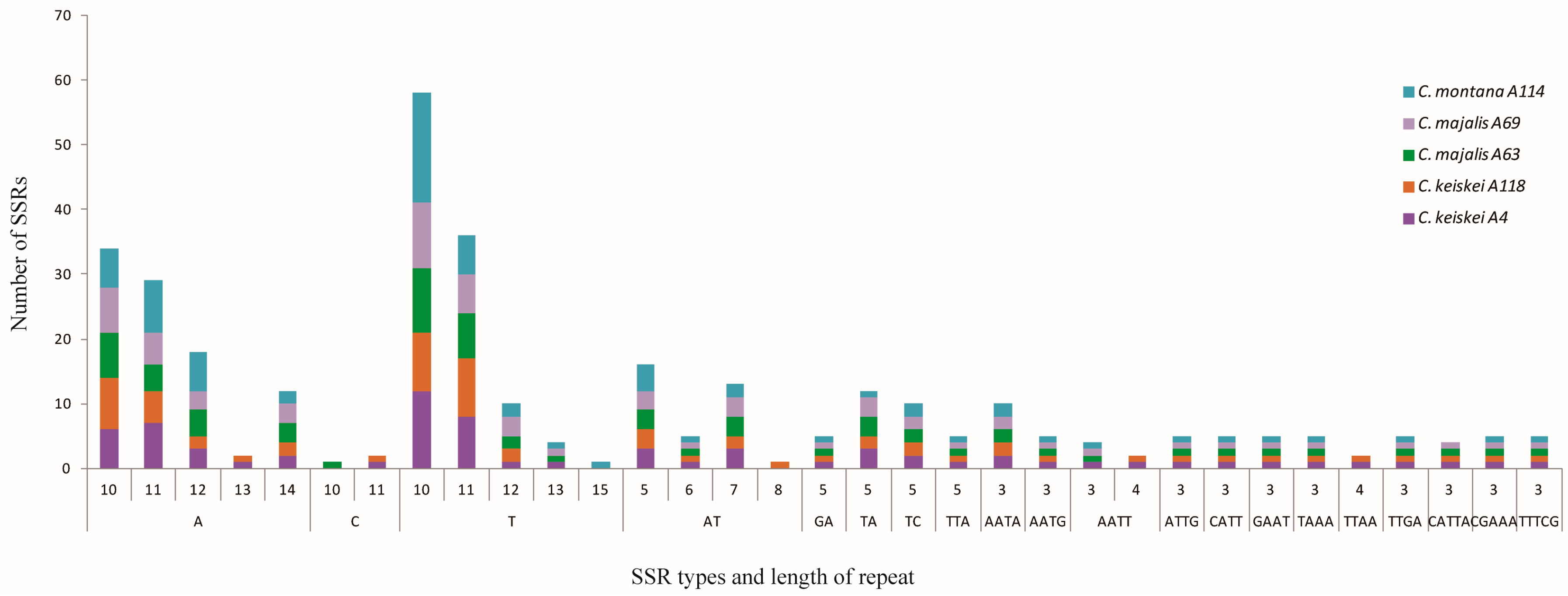
3.4. Long Repeats and Simple Sequence Repeats
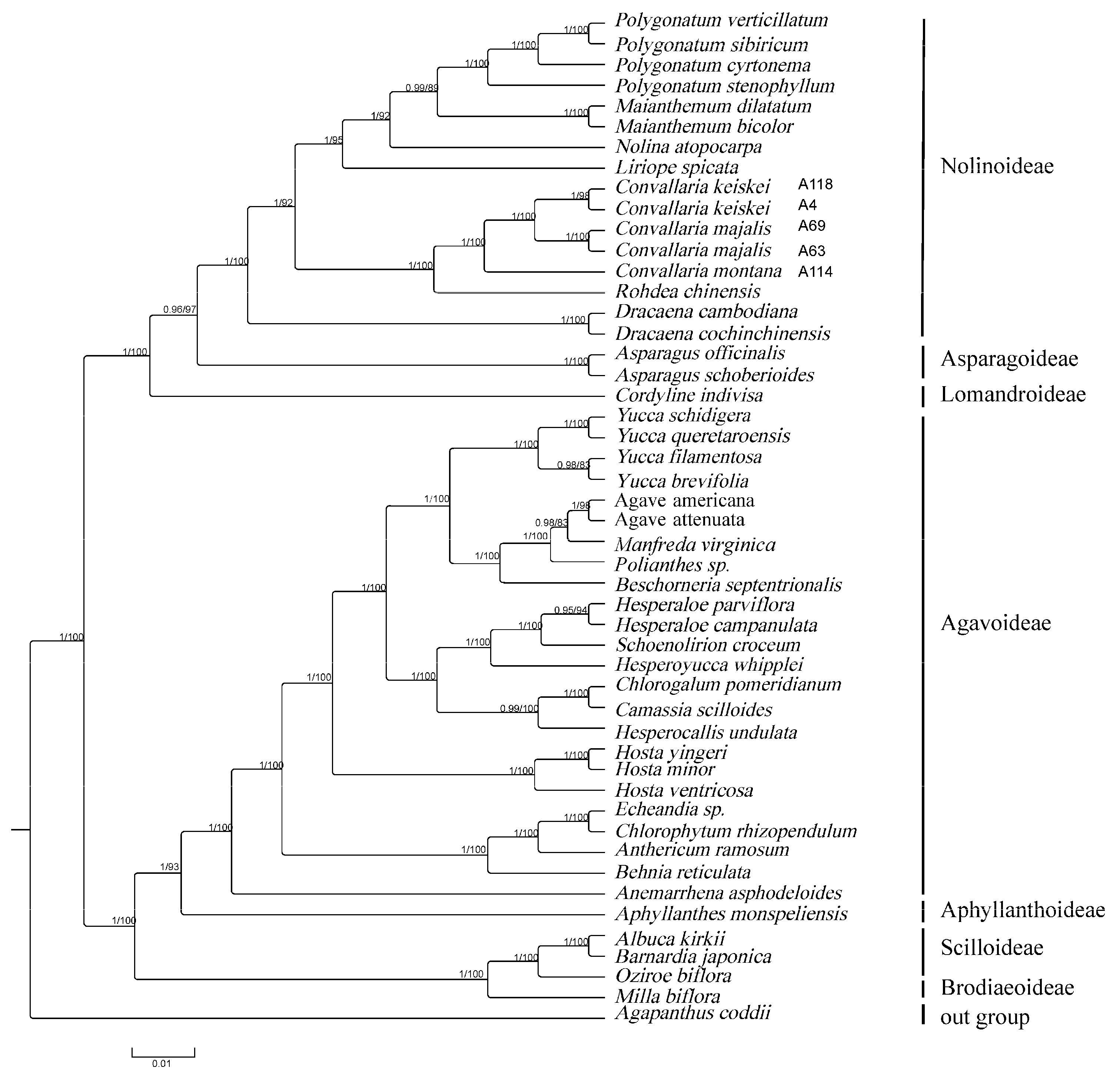
3.5. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, W.J.; Ni, D.P.; Wang, Y.J.; Shao, J.J.; Wang, X.C.; Yang, D.; Wang, J.S.; Chen, H.M.; Liu, C. Intraspecific and heteroplasmic variations, gene losses and inversions in the chloroplast genome of Astragalus membranaceus. Sci. Rep. 2016, 6, 21669. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Ohara, M. Reproductive demography of ramets and genets in a rhizomatous clonal plant Convallaria keiskei. J. Plant Res. 2008, 121, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.X.; Gao, J.; Wu, J.J.; Zhou, X.; Wu, X.; Li, M.D.; Wei, Y.K.; Wang, R.H.; Qi, Z.C.; Li, P. Development of 19 novel microsatellite markers of lily-of-the-valley (Convallaria, asparagaceae) from transcriptome sequencing. Mol. Biol. Rep. 2020, 47, 3041–3047. [Google Scholar] [CrossRef]
- Streveler, B. A taxonomic Study of the Genus Convallaria (Liliaceae). Masters Thesis, University of Wisconsin, Madison, WI, USA, 1966. [Google Scholar]
- Araki, K.; Shimatani, K.; Ohara, M. Floral distribution, clonal structure, and their effects on pollination success in a self-incompatible Convallaria keiskei population in northern Japan. Plant Ecol. 2007, 189, 175–186. [Google Scholar] [CrossRef][Green Version]
- Utech, F.H.; Kawano, S. Floral vascular anatomy of Convallaria majalis L. and C. keiskei Miq. (Liliaceae-Convallariinae). Bot. Mag. 1976, 89, 173–182. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; De Visser, R. Provenancing flower bulbs by analytical fingerprinting: Convallaria majalis. Agriculture 2015, 5, 17–29. [Google Scholar] [CrossRef]
- Kanchi, G.N.; James, R.L.; James, Z.L. Nomenclatural and taxonomic analysis of Convallaria majalis, C. majuscula, and C. montana (Ruscaceae/Liliaceae). Phytoneuron 2012, 17, 1–4. [Google Scholar]
- Katrien, V.; Tim, D.M.; Hans, J.; Isabel, R.R.; Olivier, H. The impact of extensive clonal growth on fine-scale mating patterns: A full paternity analysis of a lily-of-the-valley population (Convallaria majalis). Ann. Bot. 2013, 111, 623–628. [Google Scholar]
- Vandepitte, K.; Roldan-Ruiz, I.; Jacquemyn, H.; Honnay, O. Extremely low genotypic diversity and sexual reproduction in isolated populations of the self-incompatible lily-of-the-valley (Convallaria majalis) and the role of the local forest environment. Ann. Bot. 2010, 105, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Chwedorzewska, K.J.; Galera, H.; Kosiński, I. Plantations of Convallaria majalis L. as a threat to the natural stands of the species: Genetic variability of the cultivated plants and natural populations. Biol. Conserv. 2008, 141, 2619–2624. [Google Scholar] [CrossRef]
- Peng, J.; Cui, J.M.; Kang, L.P.; Ma, B.P. Chemical constituents and pharmacological activities of Convallariaceae plants: Research advances. J. Int. Pharm. Res. 2013, 40, 726–735. [Google Scholar]
- Matsuo, Y.; Shinoda, D.; Nakamaru, A.; Kamohara, K.; Sakagami, H.; Mimaki, Y. Steroidal glycosides from Convallaria majalis whole plants and their cytotoxic activity. Int. J. Mol. Sci. 2017, 18, 2358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Nam, J.S.; Shin, J.A.; Hong, I.S.; Yang, I.H.; You, M.J.; Cho, S.D. Convallaria keiskei as a novel therapeutic alternative for salivary gland cancer treatment by targeting myeloid cell leukemia-1. Head Neck 2016, 38, E761–E770. [Google Scholar] [CrossRef] [PubMed]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Chase, M.W.; Reveal, J.L.; Fay, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2010, 161, 132–136. [Google Scholar] [CrossRef]
- Seberg, O.; Petersen, G.; Davis, J.I.; Pires, J.C.; Stevenson, D.W.; Chase, M.W.; Fay, M.F.; Devey, D.S.; Jorgensen, T.; Sytsma, K.J.; et al. Phylogeny of the asparagales based on three plastid and two mitochondrial genes. Am. J. Bot. 2012, 99, 875–889. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.K.; Forest, F.; Fay, M.F. Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences. Ann. Bot. 2010, 106, 775. [Google Scholar] [CrossRef]
- Ruhsam, M.; Rai, H.S.; Mathews, S.; Ross, T.G.; Hollingsworth, P.M. Does complete plastid genome sequencing improve species discrimination and phylogenetic resolution in Araucaria? Mol. Ecol. Resour. 2015, 15, 1067–1078. [Google Scholar] [CrossRef]
- Jansen, R.K.; Ruhlman, T.A. Plastid genomes of seed plants. In Genomics of Chloroplasts and Mitochondria; Springer: Berlin/Heidelberg, Germany, 2012; pp. 103–126. [Google Scholar]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef]
- Zhai, W.; Duan, X.S.; Zhang, R.; Guo, C.C.; Li, L. Chloroplast genomic data provide new and robust insights into the phylogeny and evolution of the Ranunculaceae. Mol. Phylogenetics Evol. 2019, 135, 12–21. [Google Scholar] [CrossRef]
- Firetti, F.; Zuntini, A.R.; Gaiarsa, J.W.; Oliveira, R.S.; Lohmann, L.G.; Sluys, M.A.V. Complete chloroplast genome sequences contribute to plant species delimitation: A case study of the Anemopaegma species complex. Am. J. Bot. 2017, 104, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Attigala, L.; Wysocki, W.P.; Duvall, M.R.; Clark, L.G. Phylogenetic estimation and morphological evolution of Arundinarieae (Bambusoideae: Poaceae) based on plastome phylogenomic analysis. Mol. Phylogenetics Evol. 2016, 101, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jose, C.C.; Roberto, A.; Victoria, I.; Javier, T.; Manuel, T.; Joaquin, D. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035. [Google Scholar]
- Li, R.; Ma, P.F.; Wen, J.; Yi, T.S. Complete sequencing of five Araliaceae chloroplast genomes and the phylogenetic implications. PLoS ONE 2013, 8, e78568. [Google Scholar] [CrossRef]
- Leonie, D.; Barbara, G.; Youri, L.; Yavuz, A.; Thomas, C.; Klaas, V. The complete chloroplast genome of 17 individuals of pest species Jacobaea vulgaris: SNPs, microsatellites and barcoding markers for population and phylogenetic studies. DNA Res. 2011, 18, 93–105. [Google Scholar]
- Shepherd, L.D.; McLay, T.G.B. Two micro-scale protocols for the isolation of DNA from polysaccharide-rich plant tissue. J. Plant Res. 2011, 124, 311–314. [Google Scholar] [CrossRef]
- Cronn, R.; Liston, A.; Parks, M.; Gernandt, D.S.; Shen, R.; Mockler, T. Multiplex sequencing of plant chloroplast genomes using Solexa sequencing-by-synthesis technology. Nucleic Acids Res. 2008, 36, e122. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Wang, Y.C.; Shi, X.W.; Mao, S.L. The complete chloroplast genome sequence of Campylandra chinensis (Liliaceae). Mitochondrial DNA B Resour. 2018, 3, 780–781. [Google Scholar] [CrossRef]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Schattner, P.; Brooks, A.N.; Lowe, T.M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005, 33, W686–W689. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef]
- Frazer, K.A.; Lior, P.; Alexander, P.; Rubin, E.M.; Inna, D. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273. [Google Scholar] [CrossRef]
- Darling, A.; Mau, B.; Blattner, F.R.; Perna, A. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Szczecińska, M.; Sawicki, J. Genomic resources of three Pulsatilla species reveal evolutionary hotspots, species-specific sites and variable plastid structure in the family Ranunculaceae. Int. J. Mol. Sci. 2015, 16, 22258–22279. [Google Scholar] [CrossRef]
- Ma, J.; Yang, B.X.; Zhu, W.; Sun, L.L.; Tian, J.K.; Wang, X.M. The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms. Gene 2013, 528, 120–131. [Google Scholar] [CrossRef]
- Vanin, E.F. Processed pseudogenes: Characteristics and evolution. Annu. Rev. Genet. 1985, 19, 253–272. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Ayala, F.J. Pseudogenes: Are they “Junk” or functional DNA? Annu. Rev. Genet. 2003, 37, 123–151. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, T.; Yang, J.; Sun, J.J.; Ju, M.M.; Zhao, Y.M.; Zhao, G.F. Comparative analyses of chloroplast genomes of Cucurbitaceae species: Lights into selective pressures and phylogenetic relationships. Molecules 2018, 23, 2165. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Zhou, T.; Han, K.; Yang, Y.; Zhao, J.H.; Liu, Z.L. The complete chloroplast genome sequences of six Rehmannia species. Genes 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, A.; Brisson, N. Recombination and the maintenance of plant organelle genome stability. New Phytol. 2010, 186, 299–317. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, H.L. Complete chloroplast genome sequences from korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004, 11, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Cheng, C.L.; Chang, C.C.; Wu, C.L.; Su, T.M.; Chaw, S.M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008, 8, 36. [Google Scholar] [CrossRef]
- Yao, X.H.; Tang, P.; Li, Z.Z.; Li, D.W.; Liu, Y.F.; Huang, H.W. The first complete chloroplast genome sequences in Actinidiaceae: Genome structure and comparative analysis. PLoS ONE 2015, 10, e0129347. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, A.G.; Carlsen, M.; Lohmann, L.G. Complete chloroplast genome of Tanaecium tetragonolobum: The first Bignoniaceae plastome. PLoS ONE 2015, 10, e0129930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Du, L.W.; Liu, A.; Chen, J.J.; Wu, L.; Hu, W.M.; Zhang, W.; Kim, K.; Lee, S.C.; Yang, T.J. The complete chloroplast genome sequences of five Epimedium species: Lights into phylogenetic and taxonomic analyses. Front. Plant Sci. 2016, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, B.G. Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: An example from the compositae. Mol. Phylogenetics Evol. 1992, 1, 3–16. [Google Scholar] [CrossRef]
- Taberlet, P. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Yin, J.L.; Guo, H.Y.; Zhang, Y.Y.; Xiao, W.; Sun, C.; Wu, J.Y.; Qu, X.B.; Yu, J.; Wang, X.M. The complete chloroplast genome provides insight into the evolution and polymorphism of Panax ginseng. Front. Plant Sci. 2015, 5, 696. [Google Scholar] [CrossRef]
- Bodin, S.S.; Kim, J.S.; Kim, J.H. Complete chloroplast genome of Chionographis japonica (Willd.) Maxim. (Melanthiaceae): Comparative genomics and evaluation of universal primers for Liliales. Plant Mol. Biol. Report. 2013, 31, 1407–1421. [Google Scholar] [CrossRef]
- Perdereau, A.; Klaas, M.; Barth, S.; Hodkinson, T.R. Plastid genome sequencing reveals biogeographical structure and extensive population genetic variation in wild populations of Phalaris arundinacea L. in north-western Europe. Gcb Bioenergy 2017, 9, 46–56. [Google Scholar] [CrossRef]
- Tong, W.; Kim, T.S.; Park, Y.J. Rice chloroplast genome variation architecture and phylogenetic dissection in diverse Oryza species assessed by whole-genome resequencing. Rice 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Park, S.; Lee, E.M.; Park, S. Evidence of mitochondrial DNA in the chloroplast genome of Convallaria keiskei and its subsequent evolution in the Asparagales. Sci. Rep. 2019, 9, 5028. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.X.; Ye, W.Q.; Lu, R.S.; Xu, W.Q.; Qiu, Y.X. Phylogenomic and comparative analyses of complete plastomes of Croomia and Stemona (Stemonaceae). Int. J. Mol. Sci. 2018, 19, 2383. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cameron, K.M.; Kim, J. Molecular systematics and historical biogeography of Maianthemum s.s. Am. J. Bot. 2017, 104, 939–952. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, Y.P.; Hang, S.; Wen, J.; Deng, T.; Nie, Z.L.; Meng, Y.; Berthold, H. The biogeographic south-north divide of Polygonatum (Asparagaceae tribe Polygonateae) within Eastern Asia and its recent dispersals in the Northern Hemisphere. PLoS ONE 2016, 11, e0166134. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.I.; Abbott, R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar]
- Xiang, Q.J.; Soltis, D.E. Dispersal-vicariance analyses of intercontinental disjuncts: Historical biogeographical implications for Angiosperms in the Northern Hemisphere. Int. J. Plant Sci. 2001, 162, S29–S39. [Google Scholar] [CrossRef]
- Wen, J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annu. Rev. Ecol. Syst. 1999, 30, 421–455. [Google Scholar] [CrossRef]
- Raman, G.; Lee, E.M.; Park, S. Intracellular DNA transfer events restricted to the genus Convallaria within the Asparagaceae family: Possible mechanisms and potential as genetic markers for biographical studies. Genomics 2021, 113, 2906–2918. [Google Scholar] [CrossRef] [PubMed]
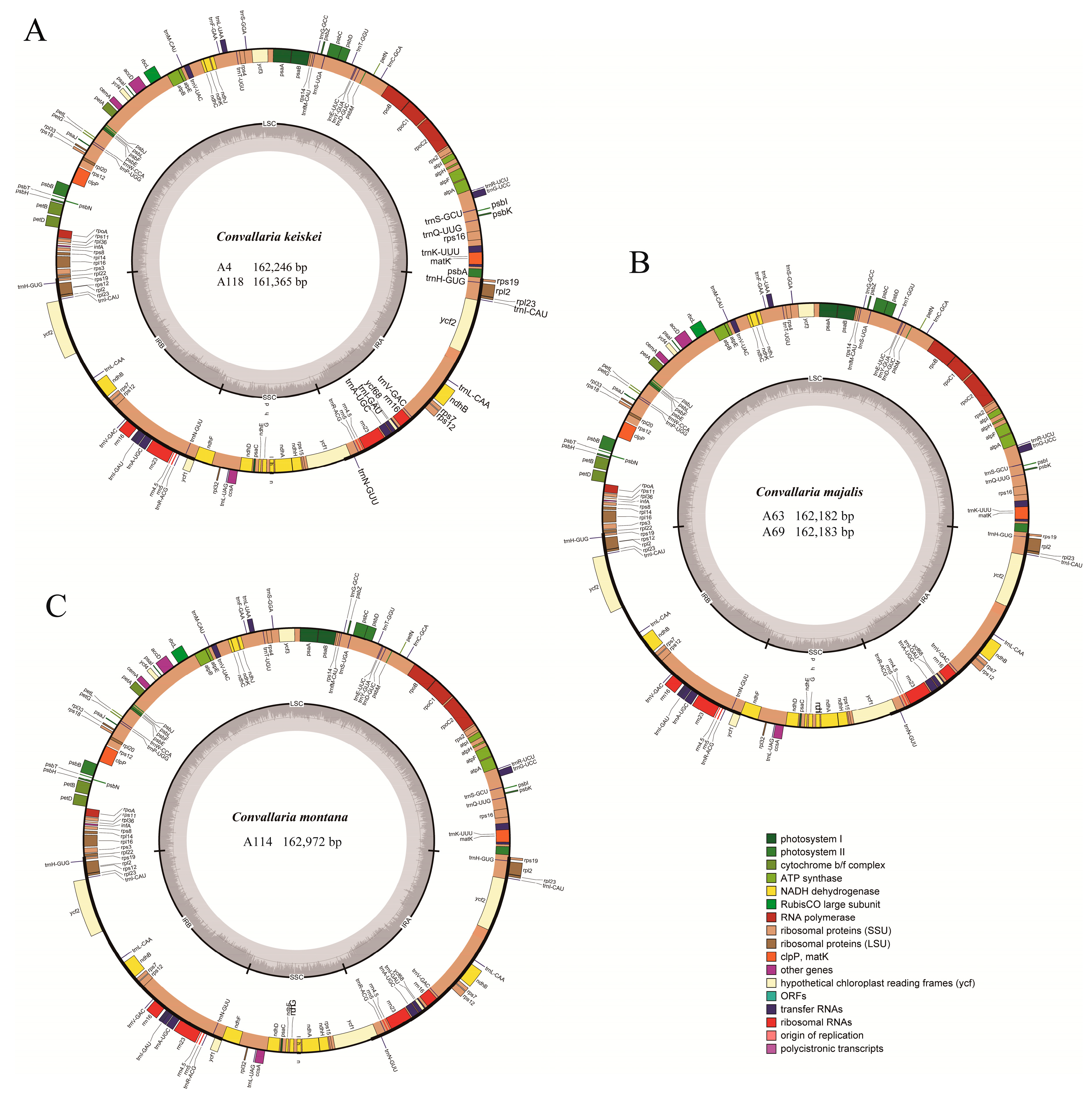
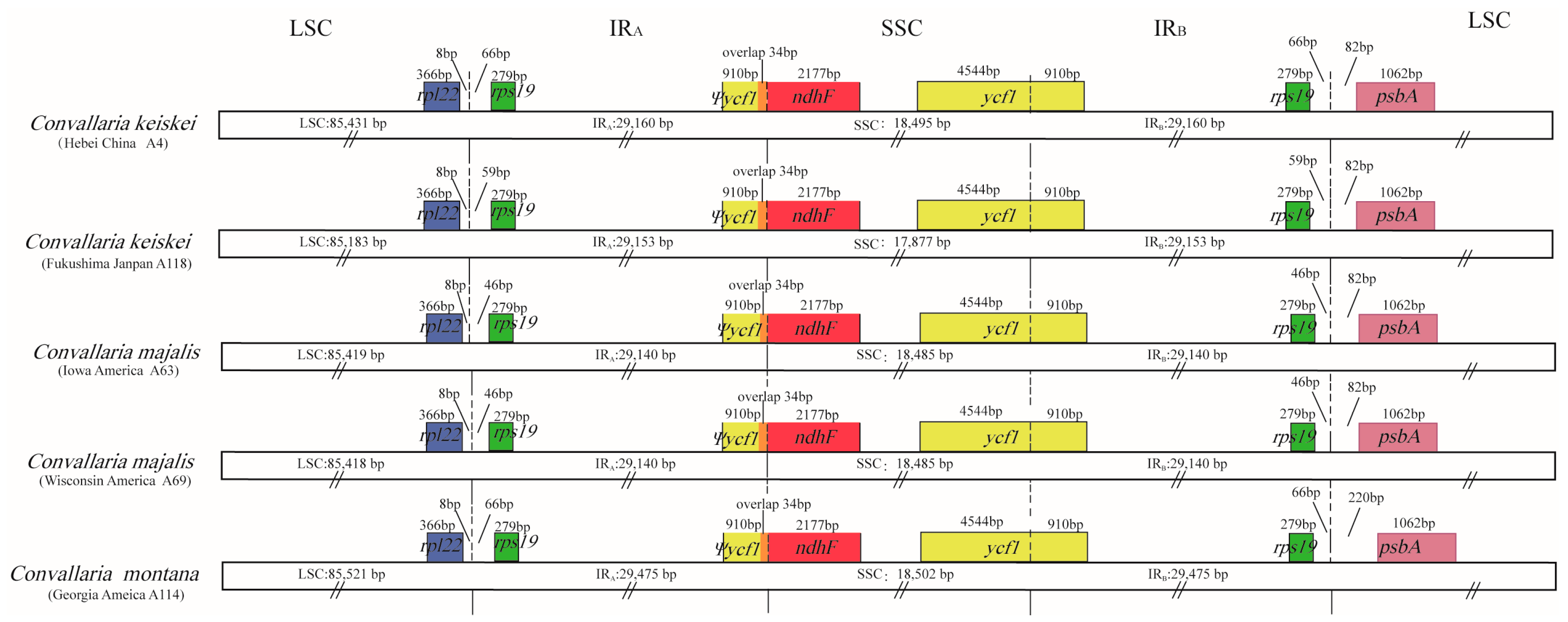

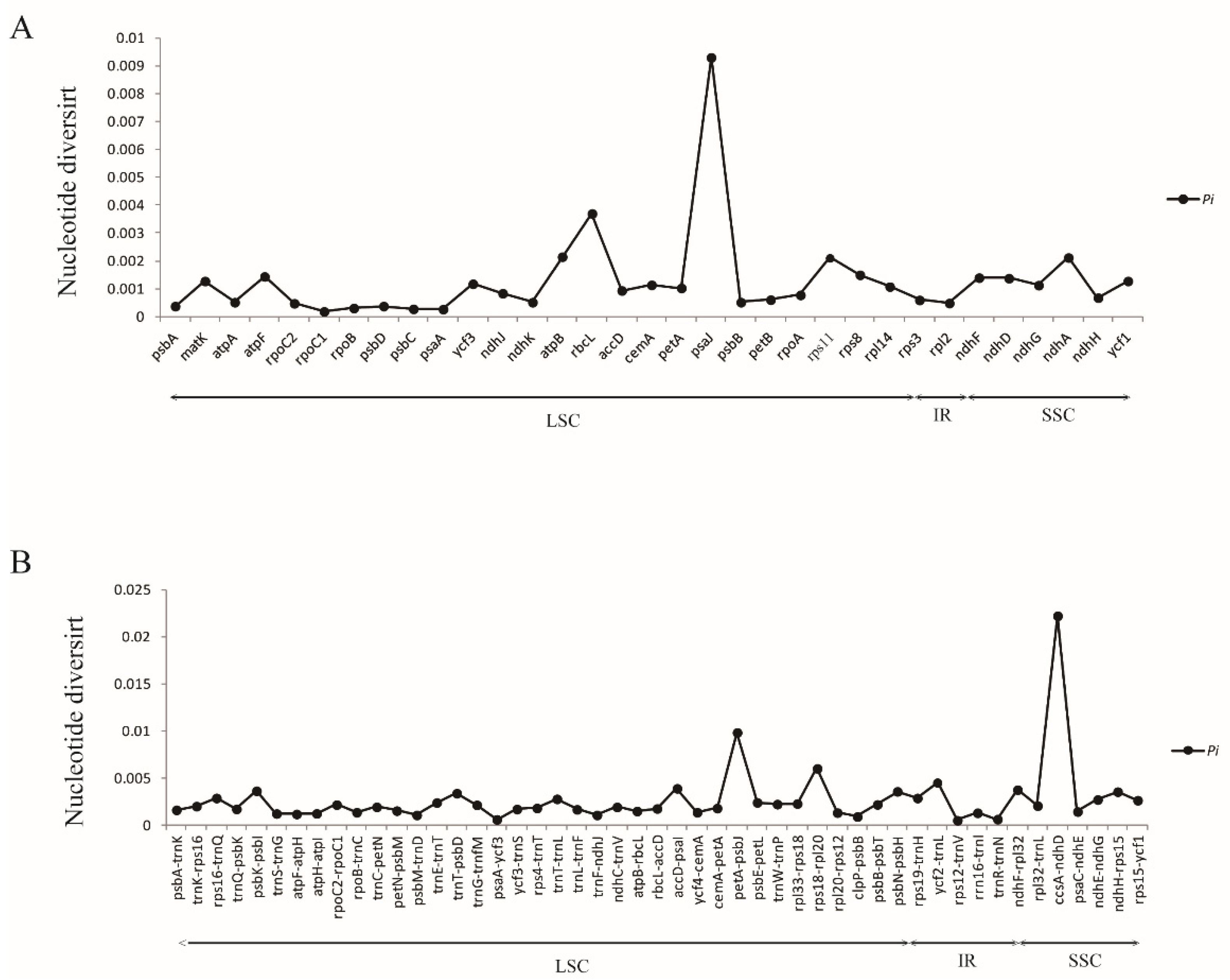
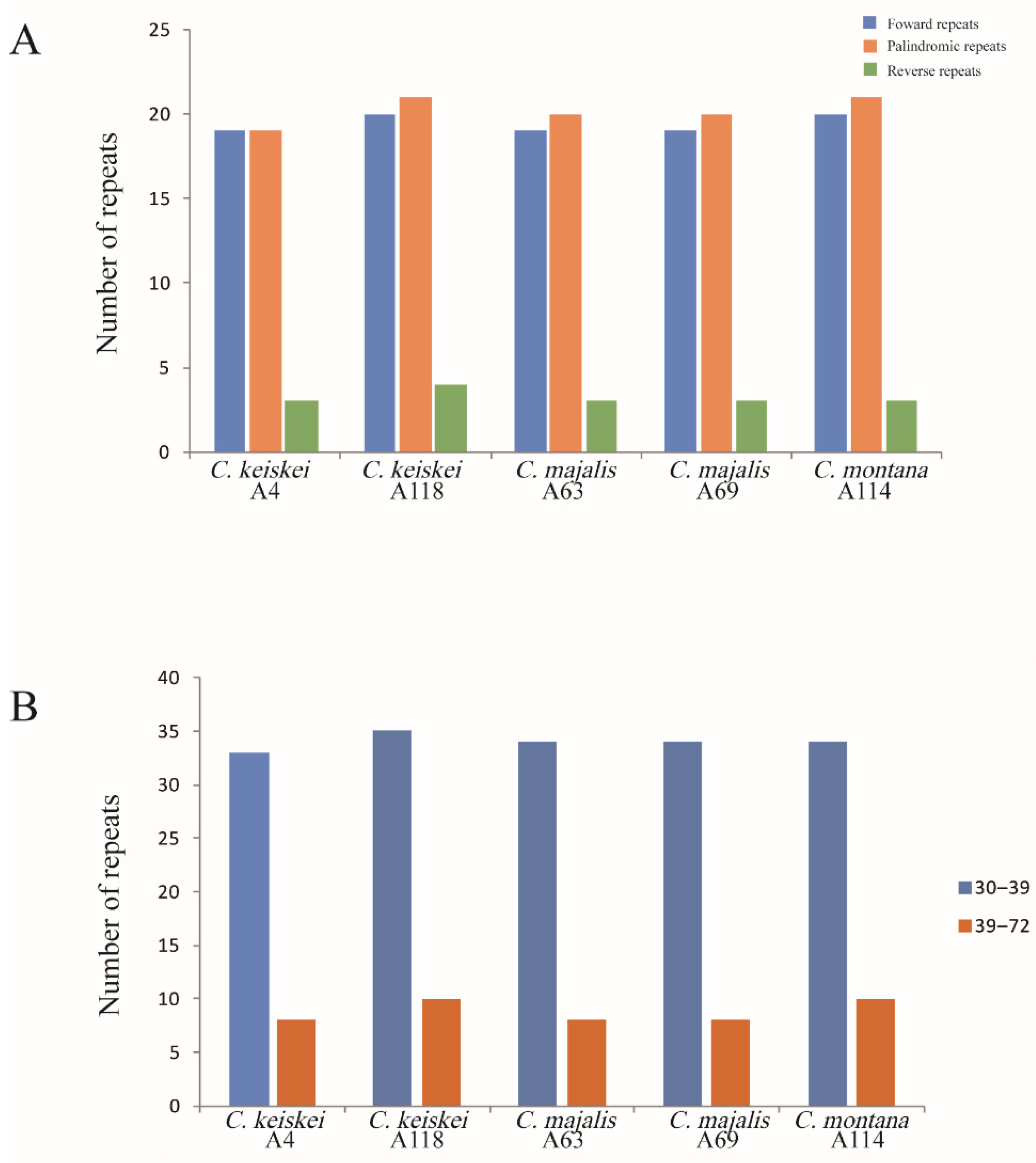
| Characteristics | C. keiskei (A4) | C. keiskei(A118) | C. montana (A114) | C. majalis (A63) | C. majalis (A69) |
|---|---|---|---|---|---|
| Location | China: Hebei | Japan: Fukushima | USA: Georgia | USA: Iowa | USA: Washington |
| Latitude (N) | 30°12′1″ | 35°43′6″ | 29°43′55″ | 30°44′27″ | 28°48′21″ |
| Longitude (E) | 120°71′6″ | 139°44′47″ | 121°5′10″ | 116°27′9″ | 120°54′47″ |
| Total cp DNA Size (bp) | 162,246 | 161,365 | 162,972 | 162,183 | 162,182 |
| LSC length (bp) | 85,432 | 85,183 | 85,521 | 85,419 | 85,418 |
| SSC length (bp) | 18,495 | 17,877 | 18,502 | 18,485 | 18,485 |
| IR length (bp) | 29,160 | 29,153 | 29,475 | 29,140 | 29,140 |
| Total GC content (%) | 37.9 | 37.9 | 37.9 | 37.9 | 37.9 |
| LSC | 35.6 | 35.7 | 35.7 | 35.6 | 35.6 |
| SSC | 31.4 | 31.6 | 31.4 | 31.4 | 31.4 |
| IR | 43.2 | 43.2 | 43.3 | 43.2 | 43.2 |
| Total number of genes | 133 | 133 | 133 | 133 | 133 |
| Protein-coding genes | 85 | 85 | 85 | 85 | 85 |
| rRNAs genes | 8 | 8 | 8 | 8 | 8 |
| tRNAs genes | 38 | 38 | 38 | 38 | 38 |
| Duplicated genes | 20 | 20 | 20 | 20 | 20 |
| Groups of Gene | Name of Genes |
|---|---|
| Ribosomal RNAs | rrn16(×2), rrn23(×2), rrn4.5(×2), rrn5(×2) |
| Transfer RNAs | trnA-UGC(×2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-CAU, trnH-GUG, trnI-CAU(×2), trnI-GAU(×2), trnK-UUU, trnL-CAA(×2), trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU(×2), trnP-UGG, trnQ-UUG, trnR-ACG(×2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(×2), trnV-UAC, trnW-CCA, trnY-GUA |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT |
| Cytochrome | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT |
| ATP synthase | atpA, atpF a, atpH, atpI, atpE, atpB |
| Rubisco | brick |
| NADH dehydrogenase | ndhJ, ndhK, ndhC, ndhB a (×2), ndhF, ndhD, ndhE, ndhG, ndhI, ndhA a, ndhH |
| ATP-dependent protease subunit P | clpP b |
| Chloroplast translational initiation factor | infA |
| Chloroplast envelope membrane protein | cemA |
| Large units | rpl33, rpl20, rpl36, rpl14, rpl16 a, rpl2, rpl2 a (×2), rpl23(×2), rpl32 |
| Small units | rps16 a, rps2, rps14, rps4, rps18, rps12 b (×2), rps11, rps8, rps19, rps3, rps7 (×2), rps15 |
| RNA polymerase | rpoC2, rpoC1 a, rpoB, rpoA |
| Miscellaneous proteins | matK, aacD, ccsA |
| Hypothetical proteins and conserved reading frame | ycf1, ycf2(×2), ycf3 b, ycf4 |
| Pseudogenes | Ψycf1, ΨinfA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.-X.; Chang, X.; Gao, J.; Wu, X.; Wu, J.; Qi, Z.-C.; Wang, R.-H.; Yan, X.-L.; Li, P. Evolutionary Comparison of the Complete Chloroplast Genomes in Convallaria Species and Phylogenetic Study of Asparagaceae. Genes 2022, 13, 1724. https://doi.org/10.3390/genes13101724
Lu Q-X, Chang X, Gao J, Wu X, Wu J, Qi Z-C, Wang R-H, Yan X-L, Li P. Evolutionary Comparison of the Complete Chloroplast Genomes in Convallaria Species and Phylogenetic Study of Asparagaceae. Genes. 2022; 13(10):1724. https://doi.org/10.3390/genes13101724
Chicago/Turabian StyleLu, Qi-Xiang, Xiao Chang, Jing Gao, Xue Wu, Jing Wu, Zhe-Chen Qi, Rui-Hong Wang, Xiao-Ling Yan, and Pan Li. 2022. "Evolutionary Comparison of the Complete Chloroplast Genomes in Convallaria Species and Phylogenetic Study of Asparagaceae" Genes 13, no. 10: 1724. https://doi.org/10.3390/genes13101724
APA StyleLu, Q.-X., Chang, X., Gao, J., Wu, X., Wu, J., Qi, Z.-C., Wang, R.-H., Yan, X.-L., & Li, P. (2022). Evolutionary Comparison of the Complete Chloroplast Genomes in Convallaria Species and Phylogenetic Study of Asparagaceae. Genes, 13(10), 1724. https://doi.org/10.3390/genes13101724






