Abstract
Argyranthemum frutescens, which belongs to the Anthemideae (Asteraceae), is widely cultivated as an ornamental plant. In this study, the complete chloroplast genome of A. frutescens was obtained based on the sequences generated by Illumina HiSeq. The chloroplast genome of A. frutescens was 149,626 base pairs (bp) in length, containing a pair of inverted repeats (IR, 24,510 bp) regions separated by a small single-copy (SSC, 18,352 bp) sequence and a large single-copy (LSC, 82,254 bp) sequence. The genome contained 132 genes, consisting of 85 coding DNA sequences, 37 tRNA genes, and 8 rRNA genes, with nineteen genes duplicated in the IR region. A comparison chloroplast genome analysis among ten species from the tribe of Anthemideae revealed that the chloroplast genome size varied, but the genome structure, gene content, and oligonucleotide repeats were highly conserved. Highly divergent regions, e.g., ycf1, trnK-psbK, petN-psbM intronic, were detected. Phylogenetic analysis supported Argyranthemum as a separate genus. The findings of this study will be helpful in the exploration of the phylogenetic relationships of the tribe of Anthemideae and contribute to the breeding improvement of A. frutescens.
1. Introduction
The chloroplasts of plants are self-replicating organelle that consists of their own genome, which encodes proteins that are essential for photosynthesis and different molecular processes [1,2]. The chloroplast genomes exhibit a highly conserved organization with a typical quadripartite structure that includes a pair of inverted repeats (IRs), separated by a large single-copy (LSC) region and a small single-copy (SSC) region among seed plants [3,4,5]. Beyond the conservation in the structure of the chloroplast genome, the differences, e.g., gene loss, sequence rearrangements, indels, and the expansion/contraction of the IR are found among species, even in individuals [6], which is valuable for plant evolution and taxonomic analysis [7,8]. In addition, chloroplast genomes are haploid in most angiosperms, and uni-parental inheritance [9,10] has made it a valuable resource for plant evolutionary and phylogenetic studies [11,12]. The complete chloroplast genomes have been demonstrated to be efficient, robust, and authentic in species discrimination and phylogenetic relationship resolution among species [5,10,13]. With the decreasing costs of next-generation sequencing, chloroplast genomes have been widely used to resolve the phylogenetic relationships of various plant species, e.g., in Amaranthus L. [14], chrysanthemums [5,15], and Vitis L. [16]. Comparative analyses of complete chloroplast genome could help us deepen penetrating insight into the evolution of chloroplast genomes and detect valuable polymorphic loci for phylogenetic analysis in plant [17,18].
Anthemideae belongs to Asteraceae, which contains 109 genera, including 1740 species [15]. There are abundant germplasm resources with excellent stress resistance, which is important for plant breeding, e.g., the improvement of cultivated chrysanthemums [19]. Although some progress has been made in the study of the relationship and geographical origin of some genera of Anthemideae based on morphology, cytology and molecular biology [20,21,22,23], the disputes about the relationship between the species and generic still existed due to the low sequence divergence of DNA markers and representative taxa across the family.
Argyranthemum is the largest endemic genus of the Atlantic oceanic islands in Anthemideae (Compositae) [20,24]. Taxonomic treatments recognized Argyranthemum as congeneric with Chrysanthemum [25]. Bremer and Humphries [20] considered Argyranthemum, Chrysanthemum, Heteranthemis, and Ismelia as a monophyletic group and treated as the subtribe Chrysantheminae. Molecular phylogenetic analyses based on ITS and cpDNA data supported Argyranthemum as a separate genus [26,27]. Thus, the phylogenetic position of Argyranthemum in the tribe remains uncertain. To provide more useful genetic data for resolving the phylogenetic relationship of Argyranthemum in Anthemideae, the complete genome sequence is a reliable resource.
To date, the genomic information of Argyranthemum is still lacking, hindering our understanding of its evolutionary history. Herein, the complete chloroplast genome was obtained from A. frutescens, a globally popular cultivated plant [28], on an Illumina HiSeq platform, and a comparison with nine other species in the tribe of Anthemideae was performed. The aims of this study were to: (1) characterize the chloroplast genome of A. frutescens and (2) explore the variation in the chloroplast genome among species of Anthemideae and determine the phylogenetic relationships of A. frutescens. This study provides useful genetic information to clarify the phylogenetic relationships of Argyranthemun and pave the way for the breeding and improvement of A. frutescens.
2. Materials and Methods
2.1. Sampling and Sequencing
The sample of A. frutescens was gathered from Shennongjia in the Hubei Province, China (31.26 N, 110.16 E), and was cultured in the nursery garden of Northeastern University, China. The voucher specimen was kept in the Herbarium of Northwest A&F University with the voucher number Zhao-2017–2. A modified CTAB method was used to isolated total genomic DNA from fresh leaves [29]. A Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 0.8% agarose gel electrophoresis were used to assess the quality and quantity of DNA. Paired-end libraries of 500 bp inserts was constructed using Illumina TruSeq Library Preparation Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instruction. The library was sequenced on an Illumina HiSeq 2500 platform in Novogene (Tiananjing, China), and paired-end reads of 2 × 150 base pairs (bp) were generated.
2.2. Chloroplast Genome Assembly and Annotations
The raw sequencing data was filtered and trimmed using Trimmomatic v 0.32 [30] under default settings to remove adaptors and low-quality data. The quality of the filtered data was checked with FastQC. De novo and reference-guided methods were integrated to assemble the chloroplast genome as described by Ma et al. [5]. and Valcárcel and Wen [31]. The clean reads were initially de novo assembled into contigs using Velvet v.1.2.10 [32] implemented in Geneious 10.1.3 [33] with auto strategy. Furthermore, the reads were mapped to the chloroplast genome of Ismelia carinata downloaded from GenBank (NC_040110) using reference-guided assembly with default settings in Geneious. Then contigs obtained by de novo assembly mapped to the consensus sequence were obtained using the reference genome to check the errors or ambiguities resulting from either assembly method. The final chloroplast genome sequence of A. frutescens was obtained from the mapped contigs and annotated using GeSeq [34]. The start and stop codons of protein-coding genes and intron/exon boundaries were manually adjusted by comparison with other chloroplast genomes of Asteraceae from Genbank (e.g: NC020320, NC007977) using Geneious v.10.1.3. The annotated circular map was generated with the Organellar Genome DRAW tool (OGDRAW) [35] with default parameters and edited manually. The obtained chloroplasts genomes sequence of A. frutescens was deposited in the NCBI database (GenBank accession number OK585057)
2.3. Codon Usage and Putative RNA Editing Site
The relative synonymous codon usage (RSCU) of protein-coding genes was determined by CodonW1.4.2 [36] under default settings. Predictive RNA editors for the plant chloroplast (PREP-cp) was applied to explore the editing sites of putative RNA [37].
2.4. Chloroplast Genome Comparison
The chloroplast genome of nine species representing Anthemideae, including Ajania variifolia, Artemisia argyi, Leucanthemella linearis, Leucanthemum maximum, Chrysanthemum zawadskii, I. carinata, Crossostephium chinense isolate JPBB, Neopallasia pectinate, and Soliva sessilis, were downloaded from Genbank to compare with the chloroplast genome of A. frutescens for a deeper understanding of the intergeneric evolutionary relationships in species of the tribe of Anthemideae. MAFFT v5 [38] implemented in Geneious with default parameters was applied for pairing the sequence alignment of the chloroplast genomes. The complete chloroplast genome difference between A. frutescens and other species in the tribe of Anthemideae was determined with mVISTA in the shuffle-LAGAN mode [39]. The expansion and contraction of IRs junctions were performed by IRSCOPE [40].
2.5. Repeat Sequences and SSR Analysis
REPuter v1 (Bielefeld, Germany) [41] (https://bibiserv.cebitec.uni-bielefeld.de/reputer, accessed on 20 August 2022) was performed to detect long repetitive sequences in the cpDNA, including forward (F), palindromic (P), reverse (R), and complementary (C) repeats. The minimal repeat size was set to 20 bp; the hamming distance was set to 3 bp, and the sequence identity was >90%. Simple sequence repeats (SSRs) within chloroplast genomes of ten species were detected by MIcroSAtellite identification tool (Misa) [42] with a threshold of nine for mononucleotide; five for dinucleotide; four for trinucleotide; and three for tetranucleotide, pentanucleotide, and other nucleotide repeats.
2.6. Phylogenetic Analysis
The whole chloroplast genome sequences of 19 species in the tribe of Anthemideae were selected for phylogenetic analysis. Multiple-sequences alignment was performed using Geneious 10.1.3. The best-fit model was determined by MEGA11 [43]. A maximum likelihood (ML) tree was constructed with MEGA11 software using the model of GTR + G and 1000 bootstrap replicates, and Helianthus annuus was selected as the outgroup. All indels was excluded for ML analysis.
3. Results
3.1. Chloroplast Genome Assembly and Features of A. frutescens
Approximately 10 GB of raw data (2 × 150 bp in length) was generated on the Illumina HiSeq 2500. The chloroplast genome of A. frutescens was obtained by the integrated methods of de novo and reference-guided. The complete chloroplast genome of A. frutescens was 149,626 bp in length, with a typical quadripartite structure of seed plant chloroplast genomes. It comprised two inverted repeat regions (IRa/b, 24,510 bp) separated by a large single-copy region (LSC, 82,254 bp) and a small single-copy region (SSC, 18,352 bp) (Figure 1). The overall G + C content of the whole chloroplast, LSC, SSC, and IR regions is 37.5%, 35.6%, 30.9%, and 43.1%, respectively. The chloroplast genome contained 132 unique genes, including 85 protein-coding DNA sequences, 37 tRNA genes, and 8 rRNA genes. Seventeen genes were duplicated in the IR regions (Table 1). Nine of the unique genes and six of the tRNA genes contained a single intron. The genes coding clpP and ycf3 had two introns (Table 2). The rps12 was a trans-spliced gene with the first exon located in the LSC region, and other two exons located in IRs.
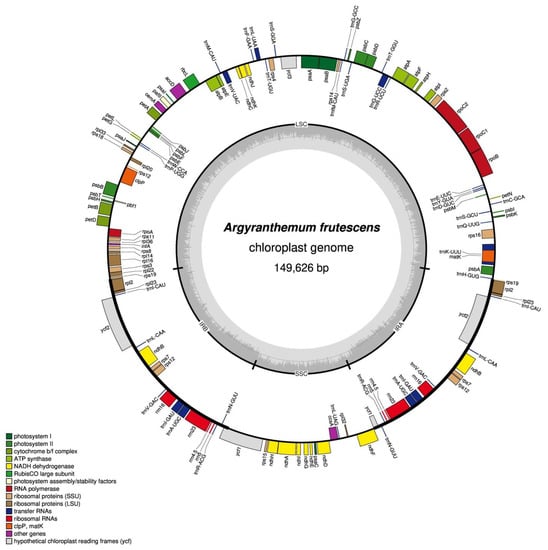
Figure 1.
Circular chloroplast genome of the A. frutescens. Genes presented inside the circle were transcribed counter-clockwise, and those drawn outside were transcribed clockwise. Color-coding represents different gene functional groups.

Table 1.
Chloroplast genome general features of A. frutescens.

Table 2.
Genes annotated in the chloroplast genome of A. frutescens.
3.2. Codon Usage and Putative RNA Editing Site
The complete chloroplast genome of A. frutescens comprised 25,751 codons among 85 protein-coding genes. Leucine was the richest amino acid with 10.76% (2770) occurrence, and cysteine was the least-common codon with 1.11% (286). Most of the codons ending with A or T were presented as RSCU > 1 (Table S1), suggesting that A or T were the preferred codon ending base.
Sixty-two putative RNA editing sites were detected in 23 CDS (Table S2). The majority of editing sites were predicted in the ndhB (10 sites) gene and preferred to produce leucine. In addition, the second base of the codon showed a higher probability of alteration. However, most of the synonymous codons were always altered at the third base as we know, which suggested that RNA editing avoided invalid editing to some extent (Table S1).
3.3. Repeat Sequences and Microsatellite Analysis
Long repetitive sequences and the SSR locus of the chloroplast genomes of A. frutescens and nine other species in the tribe of Anthemideae were analyzed. A total of 250 repeats were detected among these ten species, and F and P repeats were more abundant than C and R repeats. The total number and proportion of repeat types in 10 species showed similarity pattern, suggesting a similar evolutionary history and close relationship among species in the tribe of Anthemideae (Figure 2A). The most prevalent repeat units were in 26–30 bp, followed by 41–45 bp, while the length in 51–59 bp occurred less frequently (Figure 2B). Of which, twenty forward repeats, twenty-six palindromic repeats, three reverse repeats, and one complementary repeat were found in the A. frutescens chloroplast genome that was more similar to I. carinata. In A. frutescens, the longest forward repeat was 60 bp in length and was located in the IR region. In the LSC, IR, and SSC regions, 26, 8, and 15 repeats were presented, respectively.
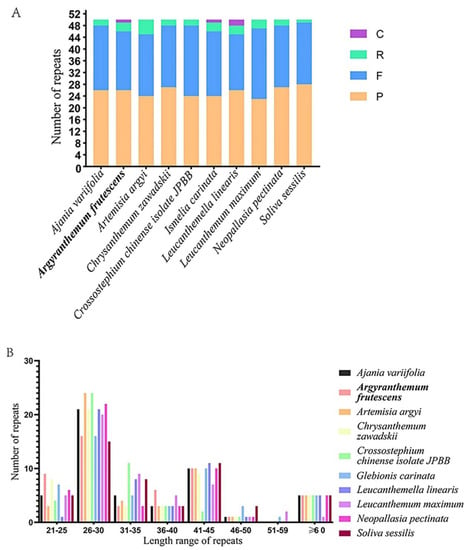
Figure 2.
The long repetitive sequences of 10 chloroplast genomes. (A) Number of identified repeats. F, forward repeats; P, palindromic repeats; R, reverse repeats; C, complementary repeats. (B) Number of different repeat types of different lengths in 10 chloroplast genomes.
A total of 1050 SSR loci were found across the chloroplast genomes of 10 species, with the shortest loci of 9 bp. L. maximum contained the highest number of SSRs (139), followed by L. linearis (114), C. chinense isolate JPBB (108), N. pectinata (108), A. frutescens (107), I. carinata (106), C. zawadskii (103), A. argyi (98), A. variifolia (95), and S. sessilis (72). In the chloroplast genome of the ten kinds of Anthemideae plants, mononucleotides were the prevalent SSR loci, which varied from 9 to 14 repeat units in length. Meanwhile, the abundance of pentanucleotide was the lowest. Additionally, A. frutescens and I. carinata contained the same number of trinucleotide (5), tetranucleotide (13) repeats, and pentanucleotide (1) (Figure 3), indicating a more recent evolutionary relationship between the two species. In all ten species, the SSRs were mainly composed of A and T, and mononucleotides repetition was the most prevalent in each chloroplast genome.
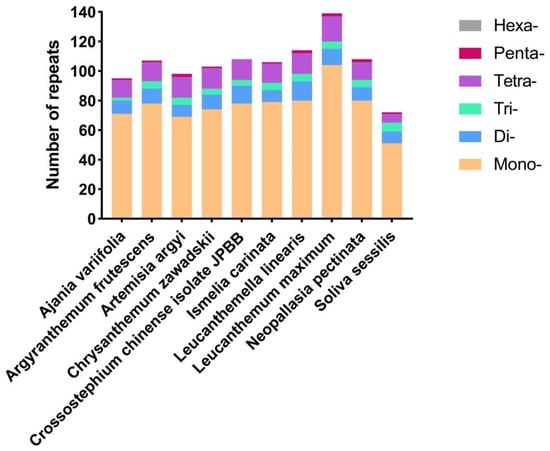
Figure 3.
Simple-sequence repeats (SSRs) in ten different Anthemideae chloroplast genomes. Mono- represents mononucleotide SSRs, and Di- represents dinucleotide SSRs, etc. The vertical axis represents the number of different SSR types.
3.4. Genome Comparison and Boundaries between SC and IR Regions
The length of the chloroplast genome in ten plants varied considerably and ranged from 149,626 bp in A. frutescens to 151,865 bp in L. maximum. The whole genome exhibited a high degree of synteny and the conservation of structure and gene order (Figure S1),which indicated that the chloroplast genomes in tribe of Anthemideae were conserved. The variation among the 10 chloroplast genomes according to the analysis of mVISTA revealed that genes located in coding regions were more conserved than those in intergenic regions. Eleven highly polymorphic regions were detected including ndhF, ycf1, ccsA, and ycf2 genes and trnK-psbK, petN-psbM, atpI-atpH, trnT-psbD, ycf3-rps4, psbE-petL, and trnL-ndhF intergenic regions (Figure S1). These regions might be potential molecular markers for phylogenetic analyses of the tribe Anthemideae.
The boundaries of IRs and SCs among the ten species from the tribe of Anthemideae were compared and showed a high resemblance. The junction between the LSC and IRa occurred within pseudo rps19 with most part of rps19 gene located in the IRa region, as is typical within angiosperms. Compared to the other nine species, the rps19 in the IRa of L. maximum was slightly contrasted (by about 28 bp) (Figure 4). The junction between SSC and IRb occurred within the ycf1 gene, which was found in most land plants. The length of ycf1 varied apparently in different species and crossed over SSC and IRb. The junction of the SSC and IRa occurred between ndhF and pseudo ycf1. The pseudo ycf1 did not extend to SSC in all species except for A. frutescens, and the ndhF located in SSC was 25–76 bp away from the junction in all species except for S. sessilis.
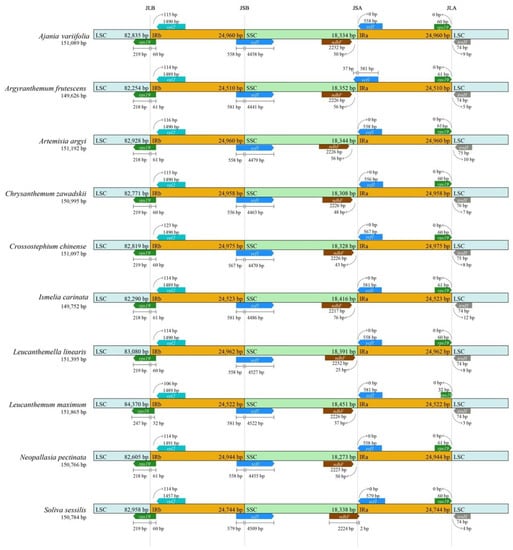
Figure 4.
The junctions of IR/SC comparison among 10 species in the tribe of Anthemideae. Arrows indicated the distance of the gene to the junctions, and the I-shaped line represented the length of the gene on either side of the junctions.
3.5. Phylogenetic Analysis
The phylogenetic position of A. frutescens was determined based on the complete chloroplast genome sequences of 19 species within the Anthemideae tribe, with H. annuus as outgroup. No heteroplasmy was detected among the chloroplast genome sequences we compared. The ML tree showed that all of the species in the tribe of Anthemideae were grouped into a monophyletic group with high support value. A. frutescens was clustered with I. carinata into a subclade with 100% supported value and sister with L. maximum, which was consistent with the taxonomic status (Figure 5). All species from the same genus were grouped into the same clade, which indicates that the chloroplast genome has a good species discrimation potential.
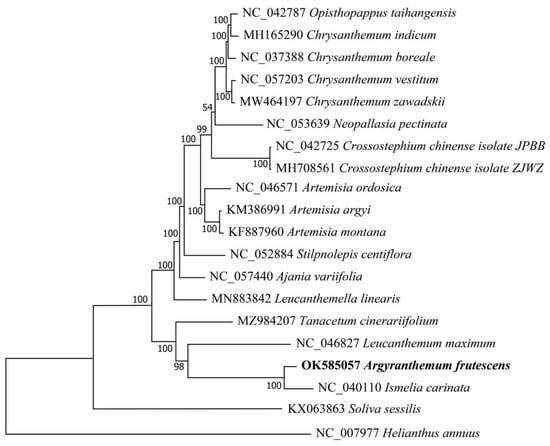
Figure 5.
Maximum likelihood (ML) tree of 19 species in different genera of Anthemideae based on the whole chloroplast genomes.
4. Discussion
In the present study, the entire chloroplast genome of A. frutescens was sequenced and assembled, and the comparison of the chloroplast genome between A. frutescens and nine other species in the tribe of Anthemideae was performed. The chloroplast genome of A. frutescens exhibited a classfic quadripartite structure like other seed plants [44]. The GC content of A. frutescens varied across different regions, and IR had the highest GC content (43.1%), as previously reported [45,46,47,48], suggesting high GC content (43.04%) increasing IR region stability and maintaining its structure in the cp genome evolution.
The codon usage bias in the plant chloroplast genome has been widely used to explore the evolutionary feature of many plant species [49,50]. Synonym codons for specific amino acids of genes was preferentially selected instead of random;y distributed [51,52]. The amino acid usage-frequency analysis in this study revealed that leucine was the most abundant amino acid and that cysteine was the least-occurrent codon. Codons ended with A or T were preferred in A. frutescens, which was consistent with reported genomes [53,54,55,56]. The RNA editing sites generally resulted in amino acid changes, which were useful for understanding the function of the translated proteins [57]. Most editing changes in the A. frutescens cp genome were C-to-U (88.4%) events. The majority of editing sites were predicted in the ndhB (10 sites) gene and preferred to produce leucine, which was consistent with previous reports [57,58], suggesting Leu may have a crucial role in the A. frutescens cp genome.
The repeat sequences and SSRs, which were widely distributed in cp genomes related to the genome rearrangement and recombination, are informative source for developing markers in population genetics and evolutionary studies [59]. The total number and proportion of repeat types in 10 species showed a similarity pattern (Figure 2A), suggesting a similar evolutionary history and a close relationship among species in the tribe of Anthemideae. Most of the SSRs were mononucleotides in the ten chloroplast genomes of Anthemideae plants and were mainly composed of A and T, as reported in previous studies of other Asteraceae [22,60]. The polymorphic long-repeat sequences and SSRs detected in our study might provide useful information for pursuing the evolution of species in the tribe Anthemideae in the future.
The whole plastome of A. frutescens and nine other species in the tribe of Anthemideae exhibited highly collinearity and was conservative in structure and gene order, similar to the previously reported plastid genome of Blumea species of Asteroideae [17]. However, the length varied considerably among ten species (Figure 4). Previous studies suggested that the change in genome size is mainly due to the length change of IR and SSC regions [61,62]. However the cp genome sizes in Chrysanthemum boreale and the cotton genus were found to be related to the length variation of LSC regions [21,63], whereas the size of cp genome in duckweeds was due to IR regions [64]. Compared to the other eight species, A. frutescens and I. carinata have the shorter length among ten species due to the shorter length of LSC and IR region (Figure 5). These findings suggest that the main reason for the change in chloroplast genome size may depend on the species.
The variation among the 10 chloroplast genomes according to the analysis of mVISTA revealed that genes located in coding regions were more conserved than those in intergenic regions, which was similar with the results in other plants [65,66]. The coding genes including ndhF, ycf1, ccsA, and ycf2 genes were found to be relatively divergent as previous studies [67]. The relatively high variation of ycf1 have been reported in the genus of chrysanthemums [5,15]. Seven highly polymorphic regions in intergenic regions were also detected. These regions might be promising genetic markers for phylogenetic analyses of the tribe Anthemideae.
The variable pattern of the contraction and expansion of the inverse region in the plastid genome of plants are related to the size change and the reset of the four regions (LSC/IRB/SSC/IRA) boundaries. Minor differences were found to be presented in the boundaries of IRs and SCs among ten species of Anthemideae, which is consistent with the report of a previous study [46]. The variation in four region boundaries of A. frutescens was similar to I. carinata, supporting a close relationship between these two species.
The phylogenetic relationship among different genera in the Anthemideae has been explored based on chloroplast single-sequence data [25,26,27]. However, the position of genus Argyranthemum is still unclear. Phylogenetic analysis showed that all of the species in the tribe of Anthemideae were grouped into a monophyletic clade with 100% bootstrap support. A. frutescens was sister with I. carinata, supporting a close relationship between Argyranthemum and Ismelia, which was consistent with previous studies based on morphological and molecular evidence [23,24]. However, A. frutescens was not closely related with Chrysanthemum, supported it as a separate genus from Chrysanthemum. The species of the genera Opisthopappus and Chrysanthemum were grouped into a clade with 100% support value, supporting their close relationship that was consistant the AFLP results [68]. The genera of Opisthopappus and Tanacetum have been classified into the same subtribe of Tanacetinae, according to the morphology of pollen [69]; however, in this phylogenetic tree, the species of genus Opisthopappus was not closely related to Tanacetum. A broader taxon sampling and integration with other molecular makers, e.g., nuclear genes, need to be used to explore the phylogenetic relationship of tribe Anthemideae.
5. Conclusions
The complete chloroplast genomes of A. frutescens displayed the typical quadripartite structure of a land plant. A comparison chloroplast genome analysis among ten species from the tribe of Anthemideae revealed that the organization and gene order are highly conserved. Some repeated sequences, SSR loci, and highly variable regions were detected, which will be a potential molecular marker for phylogenetic analyses of the tribe Anthemideae. Phylogenetic analysis supported Argyranthemum as a separate genus. The findings of this study improve our understanding of the internal structure of the chloroplast genome of the tribe Anthemideae and are valuable for the future breeding, environmental adaptation, and hybridization of A. frutescens.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes13101720/s1, Figure S1: Sequence identity plots of the 10 Anthemideae chloroplast genomes generated by mVISTA. The vertical and horizontal axes in the figure represent the consistency degree of the sequences from 50% to 100% and the sequence length, respectively. Table S1: Codon usage in chloroplast genome of A. frutescens. Table S2: Putative RNA Editing Sites of A. frutescens chloroplast genome.
Author Contributions
Y.Z. and D.Q. extracted the DNA, assembled the sequence, processed the data, performed bioinformatic analyses, and drafted the manuscript. Y.M. conceptualized and designed the study and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 31872710).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are openly available in the GenBank of NCBI at https://www.ncbi.nlm.nih.gov/, accessed on 20 August 2022), reference number (OK585057).
Conflicts of Interest
There are no conflict of interest to declare.
References
- Jensen, P.; Leister, D. Chloroplast evolution, structure and functions. F1000 Prime Rep. 2014, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, B.; Yang, H.; Li, Y.; Yang, Q.; Lu, Y.; Gao, Y. Sequencing and Analysis of Chrysanthemum carinatum Schousb and Kalimeris indica. The Complete Chloroplast Genomes Reveal Two Inversions and rbcL as Barcoding of the Vegetable. Molecules 2018, 23, 1358. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. The Molecular Biology of Plastids; Academic Press: Pittsburgh, PA, USA, 1991; CHAPTER 2-Plastid chromosomes: Structure and evolution. [Google Scholar]
- Jansen, R.K.; Raubeson, L.A.; Boore, J.L.; Depamphilis, C.W.; Chumley, T.W.; Haberle, R.C.; Wyman, S.K.; Alverson, A.J.; Peery, R.; Herman, S.J.; et al. Methods for Obtaining and Analyzing Whole Chloroplast Genome Sequences. Methods Enzymol. 2005, 395, 348–384. [Google Scholar] [PubMed]
- Ma, Y.; Zhao, L.; Zhang, W.; Zhang, Y.; Xing, X.; Duan, X.; Hu, J.; Harris, A.J.; Liu, P.; Dai, S.; et al. Origins of cultivars of Chrysanthemum—Evidence from the chloroplast genome and nuclear LFY gene. J. Syst. Evol. 2020, 58, 925–944. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Li, L.; Gao, L.; Xu, J.; Yang, M. Structural and Comparative Analysis of the Complete Chloroplast Genome of Pyrus hopeiensis—“Wild Plants with a Tiny Population”—and Three Other Pyrus Species. Int. J. Mol. Sci. 2018, 19, 3262. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Biggs, P.J.; Matthews, P.J.; Collins, L.J.; Hendy, M.D.; Lockhart, P.J. Mutational dynamics of aroid chloroplast genomes. Genome Biol. Evol. 2012, 4, 1316–1323. [Google Scholar] [CrossRef]
- Song, W.; Chen, Z.; He, L.; Feng, Q.; Zhang, H.; Du, G.; Shi, C.; Wang, S. Comparative chloroplast genome analysis of wax gourd (Benincasa hispida) with three Benincaseae Species, revealing evolutionary dynamic patterns and phylogenetic implications. Genes 2022, 13, 461. [Google Scholar] [CrossRef]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; Depamphilis, C.W.; Leebens-Mack, J.; Müller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K.; et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 19369–19374. [Google Scholar] [CrossRef]
- Zhang, N.; Erickson, D.L.; Ramachandran, P.; Ottesen, A.R.; Timme, R.E.; Funk, V.A.; Luo, Y.; Handy, S.M. An analysis of Echinacea chloroplast genomes: Implications for future botanical identification. Sci. Rep. 2017, 7, 216. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Landis, J.; Deng, T.; Meng, A.; Sun, H.; Peng, Y.; Wang, H.; Sun, Y. Plastome phylogenomic analysis of Torreya (Taxaceae). J. Syst. Evol. 2019, 57, 607–615. [Google Scholar] [CrossRef]
- Wang, H.; Moore, M.; Barrett, R.; Landrein, S.; Sakaguchi, S.; Maki, M.; Weng, H.; Wang, H. Plastome phylogenomic insights into the Sino–Japanese biogeography of Diabelia (Caprifoliaceae). J. Syst. Evol. 2020, 58, 972–987. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Linh Giang, V.N.; Waminal, N.E.; Park, H.S.; Kim, N.H.; Jang, W.; Lee, J.; Yang, T.J. Comprehensive comparative analysis of chloroplast genomes from seven Panax species and development of an authentication system based on species-unique single nucleotide polymorphism markers. J. Ginseng Res. 2018, 44, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, E.; Odeny, D.A.; Coetzee, M.; Berger, D.K.; Rees, D. Application of chloroplast phylogenomics to resolve species relationships within the plant genus Amaranthus. J. Mol. Evol. 2018, 86, 216–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liao, B.; Ostevik, K.L.; Zhou, H.; Wang, F.; Wang, B.; Xia, H. The Maternal donor of Chrysanthemum cultivars revealed by comparative analysis of the chloroplast genome. Front. Plant Sci. 2022, 13, 923442. [Google Scholar] [CrossRef]
- Wen, J.; Herron, S.A.; Yang, X.; Liu, B.B.; Zuo, Y.J.; Harris, A.J.; Kalburgi, Y.; Johnson, G.; Zimmer, E.A. Nuclear and chloroplast sequences resolve the enigmatic origin of the concord grape. Front. Plant Sci. 2020, 11, 263. [Google Scholar] [CrossRef]
- Mehmood, F.; Rahim, A.; Heidari, P.; Ahmed, I.; Poczai, P. Comparative plastome analysis of Blumea, with implications for genome evolution and phylogeny of Asteroideae. Ecol. Evol. 2021, 11, 7810–7826. [Google Scholar]
- Henriquez, C.L.; Ahmed, I.; Carlsen, M.M.; Zuluaga, A.; Croat, T.B.; McKain, M.R. Molecular evolution of chloroplast genomes in Monsteroideae (Araceae). Planta 2020, 251, 72. [Google Scholar] [CrossRef]
- Hao, D.-C.; Song, Y.; Xiao, P.; Zhong, Y.; Wu, P.; Xu, L. The genus Chrysanthemum: Phylogeny, biodiversity, phytometabolites, and chemodiversity. Front. Plant Sci. 2022, 13, 973197. [Google Scholar] [CrossRef]
- Bremer, K.; Humphries, C.J. Generic monograph of the Asteraceae-Anthemideae. Bull. Nat. Hist. Mus. Lond. (Bot.) 1993, 23, 71–177. [Google Scholar]
- Francisco-Ortega, J.; Santos-Guerra, A.; Hines, A.; Jansen, R. Molecular evidence for a Mediterranean origin of the Macaronesian endemic genus Argyranthemum (Asteraceae). Am. J. Bot. 1997, 84, 1595–1613. [Google Scholar] [CrossRef]
- Lin, N.; Zhang, X.; Deng, T.; Zhang, J.; Meng, A.; Wang, H.; Sun, H.; Sun, Y. Plastome sequencing of Myripnois dioica and comparison within Asteraceae. Plant Divers. 2019, 41, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Jung, J.A.; Kim, J.S.; Won, S.Y. A comparative analysis of the complete chloroplast genomes of three Chrysanthemum boreale strains. PeerJ 2020, 8, e9448. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Kuronuma, T.; Ando, M.; Katsuoka, H.; Inaba, Z.; Watanabe, H. Development of the sequence-characterized amplified region (SCAR) marker for distinction of intergeneric hybrids between Argyranthemum frutescens (L.) Sch. Bip. and Rhodanthemum gayanum (Cross. & Durieu) B.H. Wilcox, K. Bremer & Humphries. Plant Biotechnol. 2020, 37, 77–81. [Google Scholar] [PubMed]
- Borgen, L. Embryology and achene morphology in endemic Canarian species of Chrysanthemum (L.) Hoffm. subgenus Argyranthemum (Webb) Harling. Nor. J. Botany. 1972, 19, 149–170. [Google Scholar]
- Francisco-ortega, J.; Jansen, R.K.; Crawford, D.J.; Santos-guerra, A. Chloroplast DNA evidence for intergeneric relationships of the Macaronesian endemic genus Argyranthemum (Asteraceae). Syst. Bot. 1995, 20, 413–422. [Google Scholar] [CrossRef]
- Francisco-ortega, J.; Jansen, R.K. Origin and evolution of Argyranthemum (Asteraceae: Anthemideae) in Macaronesia. In Molecular Evolution and Adaptive Radiation; Givnish, T., Sytsma, K., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 407–431. [Google Scholar]
- Hu, J.; Jin, Q.; Ma, Y. AfLFY, a LEAFY homolog in Argyranthemum frutescens, controls flowering time and leaf development. Sci. Rep. 2020, 10, 1616. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, S. Genomic DNA extraction from chrysanthemum using high salt CTAB method. Biotechnol. Bull. 2009, 7, 90–93. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Valcárcel, V.; Wen, J. Chloroplast phylogenomic data support Eocene amphi-Pacific early radiation for the Asian Palmate core Araliaceae. J. Syst. Evol. 2019, 57, 547–560. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, C.J.; Wang, Y.; Liu, W.L.; Yin, X.R.; Li, X.; Chen, M.; Chen, K.S. Codon usage patterns in Chinese bayberry (Myrica rubra) based on RNA-Seq data. BMC Genom. 2013, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P. The PREP suite: Predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009, 37, W253–W259. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, S.; Yin, Y.; Zhang, J.; Yin, X.; Liang, C.; Wang, Z.; Huang, B.; Liu, Y.; Xiao, S.; et al. Complete chloroplast genome sequence and phylogenetic analysis of Aster tataricus. Molecules 2018, 23, 2426. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, A.R.; Waqas, M.; Kang, S.M.; Khan, M.A.; Lee, S.M.; Lee, I.J. Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front. Plant Sci. 2016, 7, 843. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhao, X.; Zhou, J.; Huo, Y.; Ding, Y.; Yuan, Z. Thecomplete chloroplast genomes of Punica granatum and a comparison with other species in Lythraceae. Int. J. Mol. Sci. 2019, 20, 2886. [Google Scholar] [CrossRef]
- Mehmood, F.; Abdullah, S.I.; Ahmed, I.; Waheed, M.T.; Mirza, B. Characterization of Withania somnifera chloroplast genome and its comparison with other selected species of Solanaceae. Genomics 2020, 112, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L.; Zhao, W.; Xu, J.; Li, Y.; Zhang, X.; Shen, X.; Wu, M.; Hou, X. Complete chloroplast genome sequence and phylogenetic analysis of Paeonia ostii. Molecules 2018, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Cai, Z.; Xia, G.; Wang, M. Synonymous codon usage bias is correlative to intron number and shows disequilibrium among exons in plants. BMC Genom. 2013, 14, 56. [Google Scholar] [CrossRef]
- Wang, L.; Xing, H.; Yuan, Y.; Wang, X.; Saeed, M.; Tao, J.; Feng, W.; Zhang, G.; Song, X.; Sun, X. Genome-wide analysis of codon usage bias in four sequenced cotton species. PLoS ONE 2018, 13, e0194372. [Google Scholar] [CrossRef]
- Sorimachi, K. Codon evolution in double-stranded organelle DNA: Strong regulation of homonucleotides and their analog alternations. Nat. Sci. 2010, 2, 846–854. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Zhang, C.; Guo, X.; Liu, Q.; Wang, K. Complete chloroplast genome of Camellia japonica genome structures, comparativeand phylogenetic analysis. PLoS ONE 2019, 14, e0216645. [Google Scholar]
- Saina, J.K.; Li, Z.Z.; Gichira, A.W.; Liao, Y.Y. The complete chloroplast genome sequence of tree of Heaven (Ailanthus altissima (Mill.) (Sapindales: Simaroubaceae), an important pantropical tree. Int. J. Mol. Sci. 2018, 19, 929. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Wang, J.; Lei, W.; Gao, J.; Qiu, X.; Wang, J. The Complete chloroplast genome sequences of the medicinal plant Forsythia suspensa (Oleaceae). Int. J. Mol. Sci. 2017, 18, 2288. [Google Scholar] [CrossRef]
- Gichira, A.W.; Avoga, S.; Li, Z.; Hu, G.; Wang, Q.; Chen, J. Comparative genomics of 11 complete chloroplast genomes of Senecioneae (Asteraceae) species: DNA barcodes and phylogenetics. Bot. Stud. 2019, 60, 17. [Google Scholar] [CrossRef]
- Meng, D.; Xiaomei, Z.; Wenzhen, K.; Xu, Z. Detecting useful genetic markers and reconstructing the phylogeny of an important medicinal resource plant, Artemisia selengensis, based on chloroplast genomics. PLoS ONE 2019, 14, e0211340. [Google Scholar] [CrossRef] [PubMed]
- Pinard, D.; Myburg, A.A.; Mizrachi, E. The plastid and mitochondrial genomes of Eucalyptus grandis. BMC Genom. 2019, 20, 132. [Google Scholar] [CrossRef]
- Rabah, S.O.; Lee, C.; Hajrah, N.H.; Makki, R.M.; Alharby, H.F.; Alhebshi, A.M.; Sabir, J.; Jansen, R.K.; Ruhlman, T.A. Plastome sequencing of ten nonmodel crop species uncovers a large insertion of mitochondrial DNA in Cashew. Plant Genome. 2017, 10. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, T.; Su, X.; Wang, G.; Zhang, X.; Guo, Q.; Cao, F. Structural characterization and comparative analysis of the chloroplast genome of Ginkgo biloba and other gymnosperms. J. For. Res. 2020, 32, 765–778. [Google Scholar] [CrossRef]
- Sablok, G.; Amiryousefi, A.; He, X.; Hyvönen, J.; Poczai, P. Sequencing the plastid genome of giant ragweed (Ambrosia trifida, Asteraceae) from a herbarium specimen. Front. Plant Sci. 2019, 10, 218. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Waqas, M.; Kang, S.M.; Yun, B.W.; Lee, I.J. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Sci. Rep. 2017, 7, 7556. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tang, P.; Li, Z.; Li, D.; Liu, Y.; Huang, H. The first complete chloroplast genome sequences in Actinidiaceae: Genome structure and comparative analysis. PLoS ONE 2015, 10, e0129347. [Google Scholar] [CrossRef]
- Chen, Z.; Grover, C.E.; Li, P.; Wang, Y.; Nie, H.; Zhao, Y.; Wang, M.; Liu, F.; Zhou, Z.; Wang, X.; et al. Molecular evolution of the plastid genome during diversification of the cotton genus. Mol. Phylogenetics Evol. 2017, 112, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fang, Y.; Guo, L.; Li, Z.; He, K.; Zhao, Y.; Zhao, H. Phylogenic study of Lemnoideae (duckweeds) through complete chloroplast genomes for eight accessions. PeerJ 2017, 5, e4186. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cui, Y.; Wang, Q.; Xu, Z.; Wang, Y.; Lin, Y.; Song, J.; Yao, H. Identification and phylogenetic analysis of five Crataegus species (Rosaceae) based on complete chloroplast genomes. Planta 2021, 254, 14. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Yang, H.; Lan, J.; Zhang, X.; Zhang, F.; Yang, J.; Chen, S. Comparative analysis of chloroplast genomes reveals phylogenetic relationships and intraspecific variation in the medicinal plant Isodon rubescens. PLoS ONE 2022, 17, e0266546. [Google Scholar] [CrossRef] [PubMed]
- Neubig, K.M.; Whitten, W.M.; Carlsward, B.S.; Blanco, M.A.; Endara, L.; Williams, N.H.; Moore, M. Phylogenetic utility of ycf1 in orchids: A plastid gene more variable than matK. Plant Syst. Evol. 2009, 277, 75–84. [Google Scholar] [CrossRef]
- Zhao, H.B.; Chen, F.D.; Chen, S.M.; Wu, G.S.; Guo, W.M. Molecular phylogeny of Chrysanthemum, Ajania and its allies (Anthemideae, Asteraceae) as inferred from nuclear ribosomal ITS and chloroplast trnL-F IGS sequences. Plant Syst. Evol. 2010, 284, 153–169. [Google Scholar] [CrossRef]
- Zhao, H.B. Phylogeny of Tribe Anthemideae (Asteraceae) from East Asia and Intergeneric cross between Dendranthema×grandiflorum (Ramat.) Kitam. and Ajania Pcifica (Nakai) K. Bremer & Humphries. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).