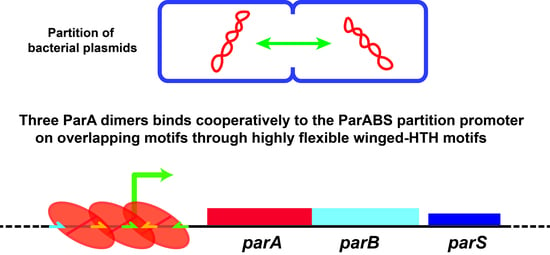
Three ParA Dimers Cooperatively Assemble on Type Ia Partition Promoters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Purification and Analytical Gel Filtration
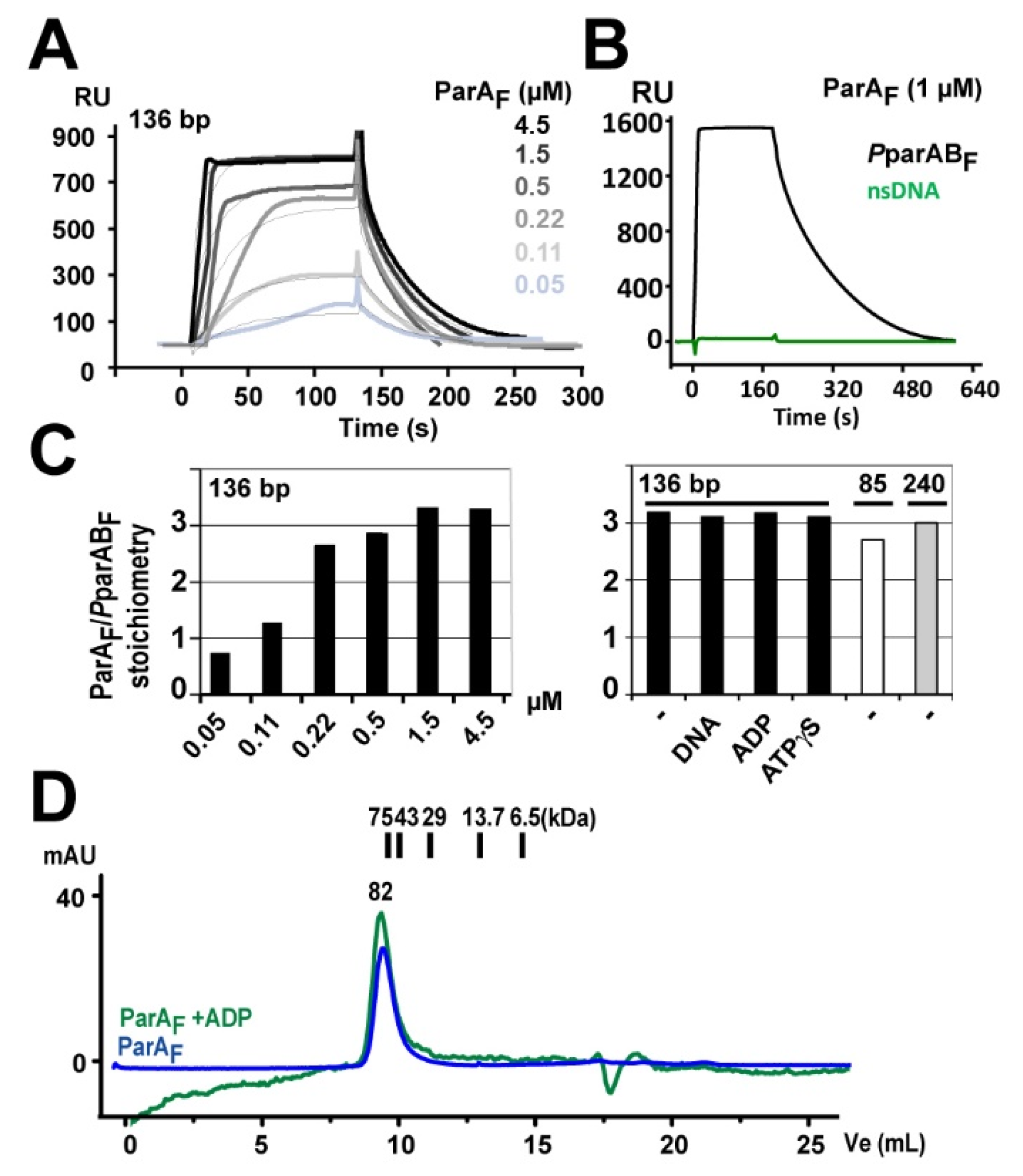
2.2. Surface Plasmon Resonance Assays
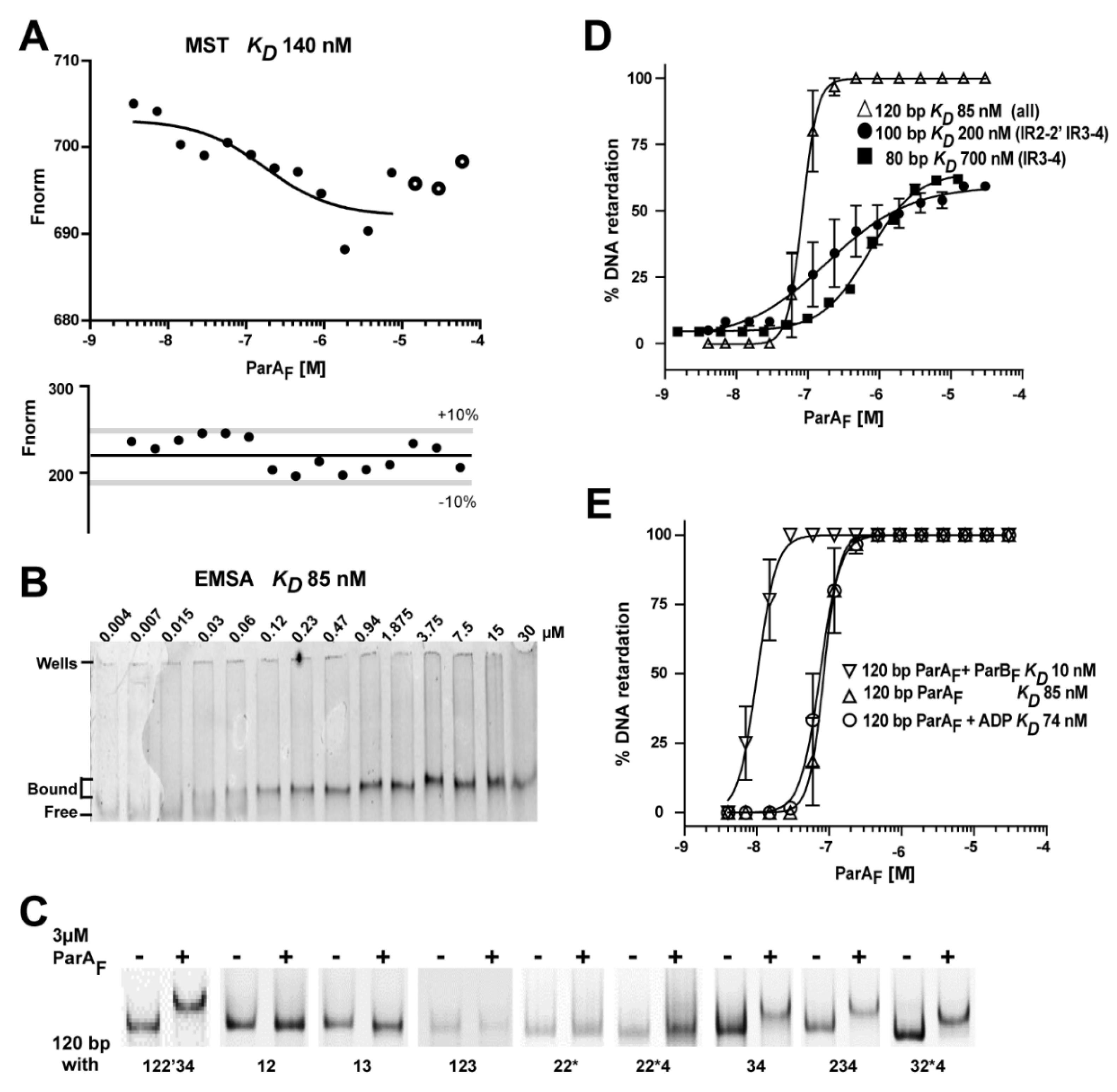
2.3. Electrophoretic Mobility Shift Assays
2.4. Microscale Thermophoresis Assays
2.5. Protein Melting Point Analysis
2.6. Modeling of ParAF Structure and Docking on DNA
2.7. Molecular Dynamics Simulation
3. Results
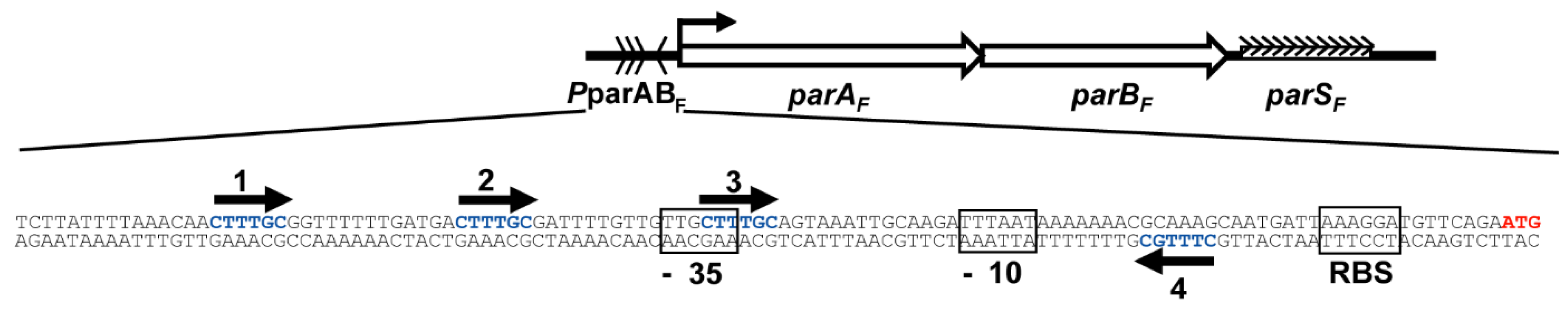
3.1. Three ParAF Dimers Bind to the Para Promoter Region
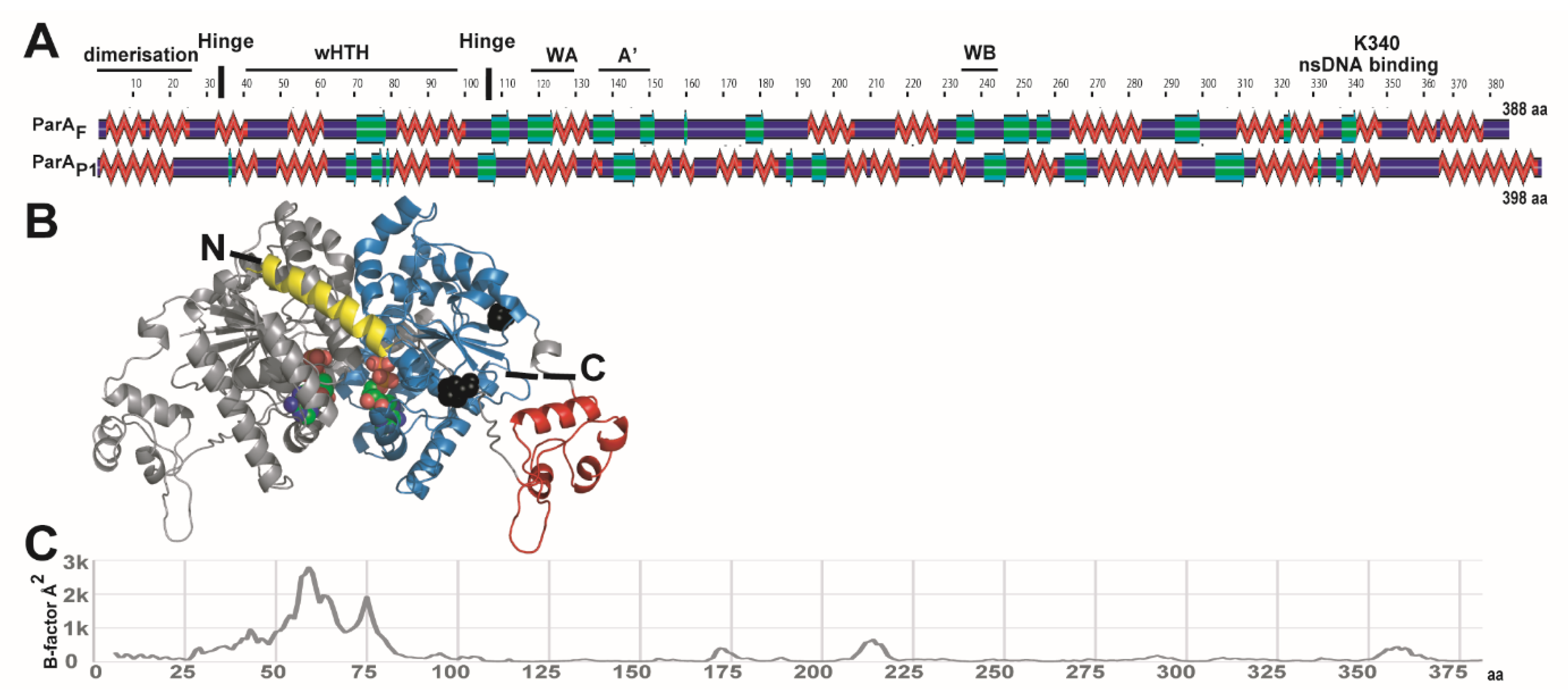
3.2. Modeling ParAF Structure from ParAP1
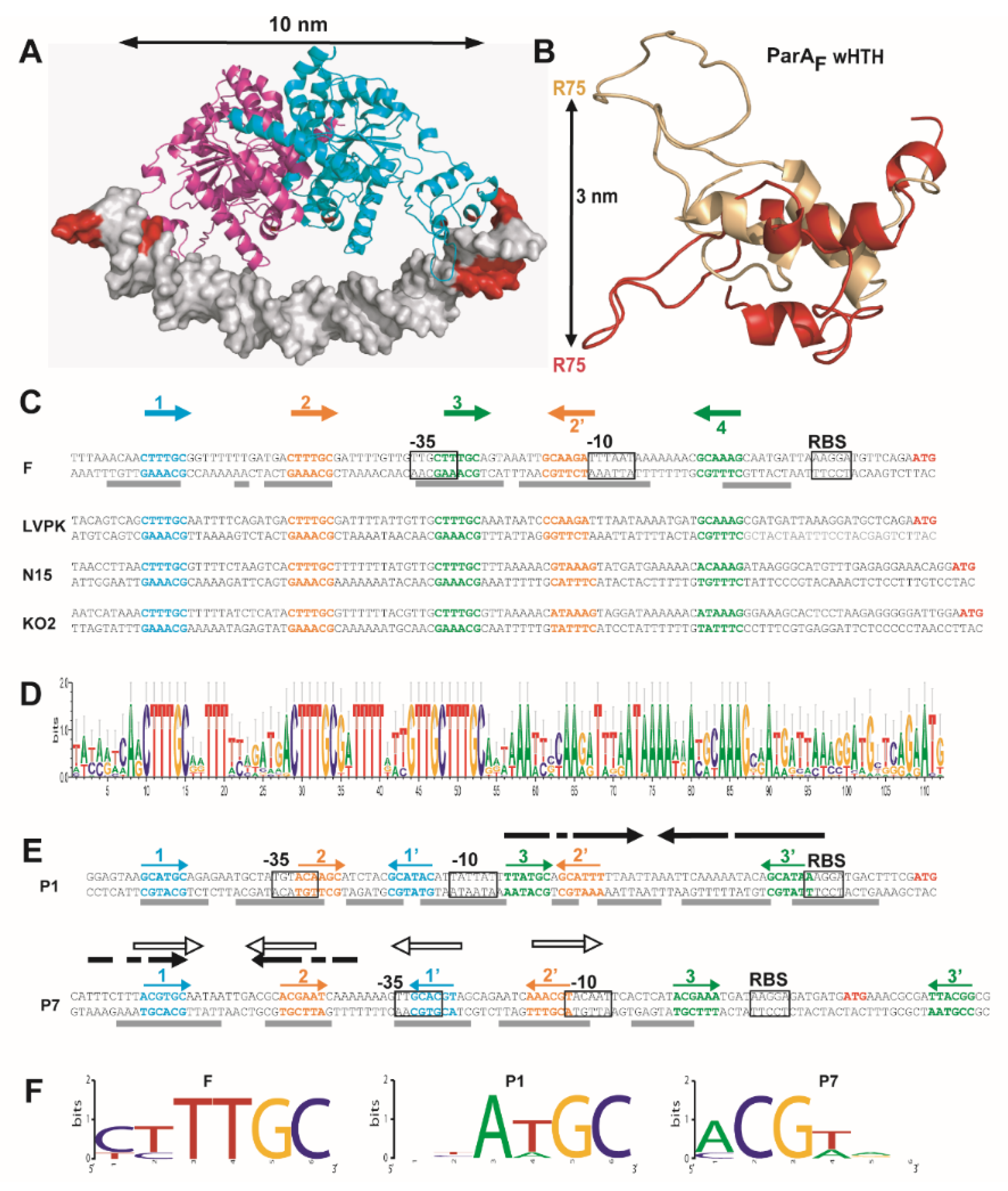
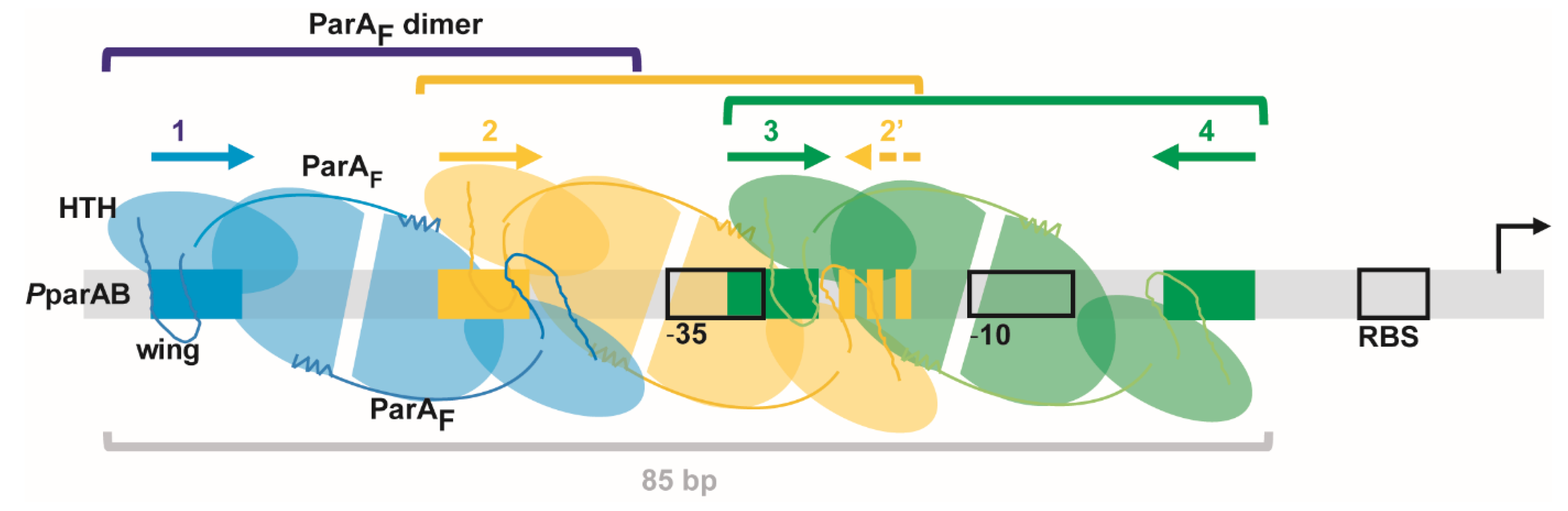
3.3. Overlapping Binding of Three ParAF Dimers in the Promoter Region
3.4. Is the Promoter Organization with Overlapping IR Motifs a Conserved Feature amongst Type Ia Plasmid Partition Operons?
3.5. The Winged-HTH Domain Is Highly Flexible
3.6. The IR3-4 Motif Is Sufficient to Nucleate the Binding of Three ParAF Dimers
3.7. ADP Stabilizes ParAF but Does Not Increase Its Affinity to PparABF
3.8. ParBF Increases ParAF Binding Affinity to the Promoter Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | amino acid |
| ADP | adenosine di-phosphate |
| ATP | adenosine tri-phosphate |
| bp | base pair |
| btn | biotinylated |
| EMSA | electrophoretic mobility shift assay |
| IR | inverted repeat |
| MD | molecular dynamics |
| nsDNA | non-specific DNA |
| RU | response unit |
| SPR | surface plasmon resonance |
References
- Baxter, J.C.; Funnell, B.E. Plasmid partition mechanisms. Microbiol. Spectr. 2014, 2, PLAS-0023-2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouet, J.Y.; Funnell, B.E. Plasmid localization and partition in Enterobacteriaceae. EcoSal Plus 2019, 8, ESP-0003-2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, T.A.; Butler, P.J.; Lowe, J. Bacterial chromosome segregation: Structure and DNA binding of the Soj dimer-a conserved biological switch. EMBO J. 2005, 24, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Hester, C.M.; Lutkenhaus, J. Soj (ParA) DNA binding is mediated by conserved arginines and is essential for plasmid segregation. Proc. Natl. Acad. Sci. USA 2007, 104, 20326–20331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouet, J.Y.; Ah-Seng, Y.; Benmeradi, N.; Lane, D. Polymerization of SopA partition ATPase: Regulation by DNA binding and SopB. Mol. Microbiol. 2007, 63, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Castaing, J.P.; Bouet, J.Y.; Lane, D. F plasmid partition depends on interaction of SopA with non-specific DNA. Mol. Microbiol. 2008, 70, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Mori, Y.; Ichinose, C.; Niki, H.; Ogura, T.; Kato, A.; Hiraga, S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J. Biol. Chem. 1989, 264, 15535–15541. [Google Scholar] [CrossRef]
- Gerdes, K.; Moller-Jensen, J.; Bugge Jensen, R. Plasmid and chromosome partitioning: Surprises from phylogeny. Mol. Microbiol. 2000, 37, 455–466. [Google Scholar] [CrossRef]
- Friedman, S.A.; Austin, S.J. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid 1988, 19, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Hayes, F.; Radnedge, L.; Davis, M.A.; Austin, S.J. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol. Microbiol. 1994, 11, 249–260. [Google Scholar] [CrossRef]
- Biek, D.P.; Strings, J. Partition functions of mini-F affect plasmid DNA topology in Escherichia coli. J. Mol. Biol. 1995, 246, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Yates, P.; Lane, D.; Biek, D.P. The F plasmid centromere, sopC, is required for full repression of the sopAB operon. J. Mol. Biol. 1999, 290, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.J.; Yarmolinsky, M. Effects of the P1 plasmid centromere on expression of P1 partition genes. J. Bacteriol. 2002, 184, 4857–4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunham, T.D.; Xu, W.; Funnell, B.E.; Schumacher, M.A. Structural basis for ADP-mediated transcriptional regulation by P1 and P7 ParA. EMBO J. 2009, 28, 1792–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radnedge, L.; Youngren, B.; Davis, M.; Austin, S. Probing the structure of complex macromolecular interactions by homolog specificity scanning: The P1 and P7 plasmid partition systems. EMBO J. 1998, 17, 6076–6085. [Google Scholar] [CrossRef] [PubMed]
- Ravin, N.V.; Rech, J.; Lane, D. Mapping of functional domains in F plasmid partition proteins reveals a bipartite SopB-recognition domain in SopA. J. Mol. Biol. 2003, 329, 875–889. [Google Scholar] [CrossRef]
- Bouet, J.Y.; Krisch, H.M.; Louarn, J.M. Ndd, the bacteriophage T4 protein that disrupts the Escherichia coli nucleoid, has a DNA binding activity. J. Bacteriol. 1998, 180, 5227–5230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeles, A.L.; Friedman, S.A.; Austin, S.J. Partition of unit-copy miniplasmids to daughter cells: III. The DNA sequence and functional organization of the P1 partition region. J. Mol. Biol. 1985, 185, 261–272. [Google Scholar] [CrossRef]
- Funnell, B.E. Mini-P1 plasmid partitioning: Excess ParB protein destabilizes plasmids containing the centromere parS. J. Bacteriol. 1988, 170, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Lemonnier, M.; Bouet, J.Y.; Libante, V.; Lane, D. Disruption of the F plasmid partition complex in vivo by partition protein SopA. Mol. Microbiol. 2000, 38, 493–505. [Google Scholar] [CrossRef]
- Komai, M.; Umino, M.; Hanai, R. Mode of DNA binding by SopA protein of Escherichia coli F plasmid. J. Biochem. 2011, 149, 455–461. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.J.; Olson, W.K. 3DNA: A software package for the analysis, rebuilding and visualization of three-dimensional nucleic acid structures. Nucleic Acids Res. 2003, 31, 5108–5121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emekli, U.; Schneidman-Duhovny, D.; Wolfson, H.J.; Nussinov, R.; Haliloglu, T. HingeProt: Automated prediction of hinges in protein structures. Proteins 2008, 70, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. IUPred: Web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callebaut, I.; Courvalin, J.C.; Worman, H.J.; Mornon, J.P. Hydrophobic cluster analysis reveals a third chromodomain in the Tetrahymena Pdd1p protein of the chromo superfamily. Biochem. Biophys. Res. Commun. 1997, 235, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meagher, K.L.; Redman, L.T.; Carlson, H.A. Development of polyphosphate parameters for use with the AMBER force field. J. Comput. Chem. 2003, 24, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- DeLano, W.L.; Ultsch, M.H.; de Vos, A.M.; Wells, J.A. Convergent solutions to binding at a protein-protein interface. Science 2000, 287, 1279–1283. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.J.; Funnell, B.E. The P1 plasmid partition protein ParA. A role for ATP in site-specific DNA binding. J. Biol. Chem. 1994, 269, 29908–29913. [Google Scholar] [CrossRef]
- Pratto, F.; Cicek, A.; Weihofen, W.A.; Lurz, R.; Saenger, W.; Alonso, J.C. Streptococcus pyogenes pSM19035 requires dynamic assembly of ATP-bound ParA and ParB on parS DNA during plasmid segregation. Nucleic Acids Res. 2008, 36, 3676–3689. [Google Scholar] [CrossRef]
- Wu, H.M.; Crothers, D.M. The locus of sequence-directed and protein-induced DNA bending. Nature 1984, 308, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.D.; Stephens, R.M. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.J.; Funnell, B.E. Modulation of the P1 plasmid partition protein ParA by ATP, ADP, and P1 ParB. J. Biol. Chem. 1997, 272, 15286–15292. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Chang, H.Y.; Lai, Y.C.; Pan, C.C.; Tsai, S.F.; Peng, H.L. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 2004, 337, 189–198. [Google Scholar] [CrossRef]
- Ravin, N.; Lane, D. Partition of the linear plasmid N15: Interactions of N15 partition functions with the sop locus of the F plasmid. J. Bacteriol. 1999, 181, 6898–6906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casjens, S.R.; Gilcrease, E.B.; Huang, W.M.; Bunny, K.L.; Pedulla, M.L.; Ford, M.E.; Houtz, J.M.; Hatfull, G.F.; Hendrix, R.W. The pKO2 linear plasmid prophage of Klebsiella oxytoca. J. Bacteriol. 2004, 186, 1818–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, E.; Bouet, J.Y.; Funnell, B.E. Probing the ATP-binding site of P1 ParA: Partition and repression have different requirements for ATP binding and hydrolysis. EMBO J. 2001, 20, 4901–4911. [Google Scholar] [CrossRef] [Green Version]
- Bouet, J.Y.; Funnell, B.E. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 1999, 18, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Ah-Seng, Y.; Lopez, F.; Pasta, F.; Lane, D.; Bouet, J.Y. Dual role of DNA in regulating ATP hydrolysis by the SopA partition protein. J. Biol. Chem. 2009, 284, 30067–30075. [Google Scholar] [CrossRef] [Green Version]
- Aravind, L.; Anantharaman, V.; Balaji, S.; Babu, M.M.; Iyer, L.M. The many faces of the helix-turn-helix domain: Transcription regulation and beyond. FEMS Microbiol. Rev. 2005, 29, 231–262. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Lee, J.; Zeng, W. Molecular insights into DNA binding and anchoring by the Bacillus subtilis sporulation kinetochore-like RacA protein. Nucleic Acids Res. 2016, 44, 5438–5449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, M.P.; Galkin, V.E.; Yu, X.; Stasiak, A.Z.; Stasiak, A.; Waldor, M.K.; Egelman, E.H. ParA2, a Vibrio cholerae chromosome partitioning protein, forms left-handed helical filaments on DNA. Proc. Natl. Acad. Sci. USA 2010, 107, 4590–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.A.; Martin, K.A.; Austin, S.J. Biochemical activities of the ParA partition protein of the P1 plasmid. Mol. Microbiol. 1992, 6, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.C.; Waples, W.G.; Funnell, B.E. Nonspecific DNA binding by P1 ParA determines the distribution of plasmid partition and repressor activities. J. Biol. Chem. 2020, 295, 17298–17309. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.G.; Havey, J.C.; Ing, L.L.; Wong, E.O.; Waples, W.G.; Funnell, B.E. Dissection of the ATPase active site of P1 ParA reveals multiple active forms essential for plasmid partition. J. Biol. Chem. 2013, 288, 17823–17831. [Google Scholar] [CrossRef] [Green Version]
| Tm (°C) | |
|---|---|
| ParAF | 44.5 +/− 0.4 |
| ParAF + PparABF | 45.1 +/− 0.2 |
| ParAF + ADP | 46.1 +/− 0.1 |
| ParAF + PparABF + ADP | 45.8 +/− 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudsocq, F.; Salhi, M.; Barbe, S.; Bouet, J.-Y. Three ParA Dimers Cooperatively Assemble on Type Ia Partition Promoters. Genes 2021, 12, 1345. https://doi.org/10.3390/genes12091345
Boudsocq F, Salhi M, Barbe S, Bouet J-Y. Three ParA Dimers Cooperatively Assemble on Type Ia Partition Promoters. Genes. 2021; 12(9):1345. https://doi.org/10.3390/genes12091345
Chicago/Turabian StyleBoudsocq, François, Maya Salhi, Sophie Barbe, and Jean-Yves Bouet. 2021. "Three ParA Dimers Cooperatively Assemble on Type Ia Partition Promoters" Genes 12, no. 9: 1345. https://doi.org/10.3390/genes12091345
APA StyleBoudsocq, F., Salhi, M., Barbe, S., & Bouet, J.-Y. (2021). Three ParA Dimers Cooperatively Assemble on Type Ia Partition Promoters. Genes, 12(9), 1345. https://doi.org/10.3390/genes12091345