Novel Technologies for Target Delivery of Therapeutics to the Placenta during Pregnancy: A Review
Abstract
1. Introduction
2. Targeting Strategies
2.1. Peptide-Mediated Placental Targeting
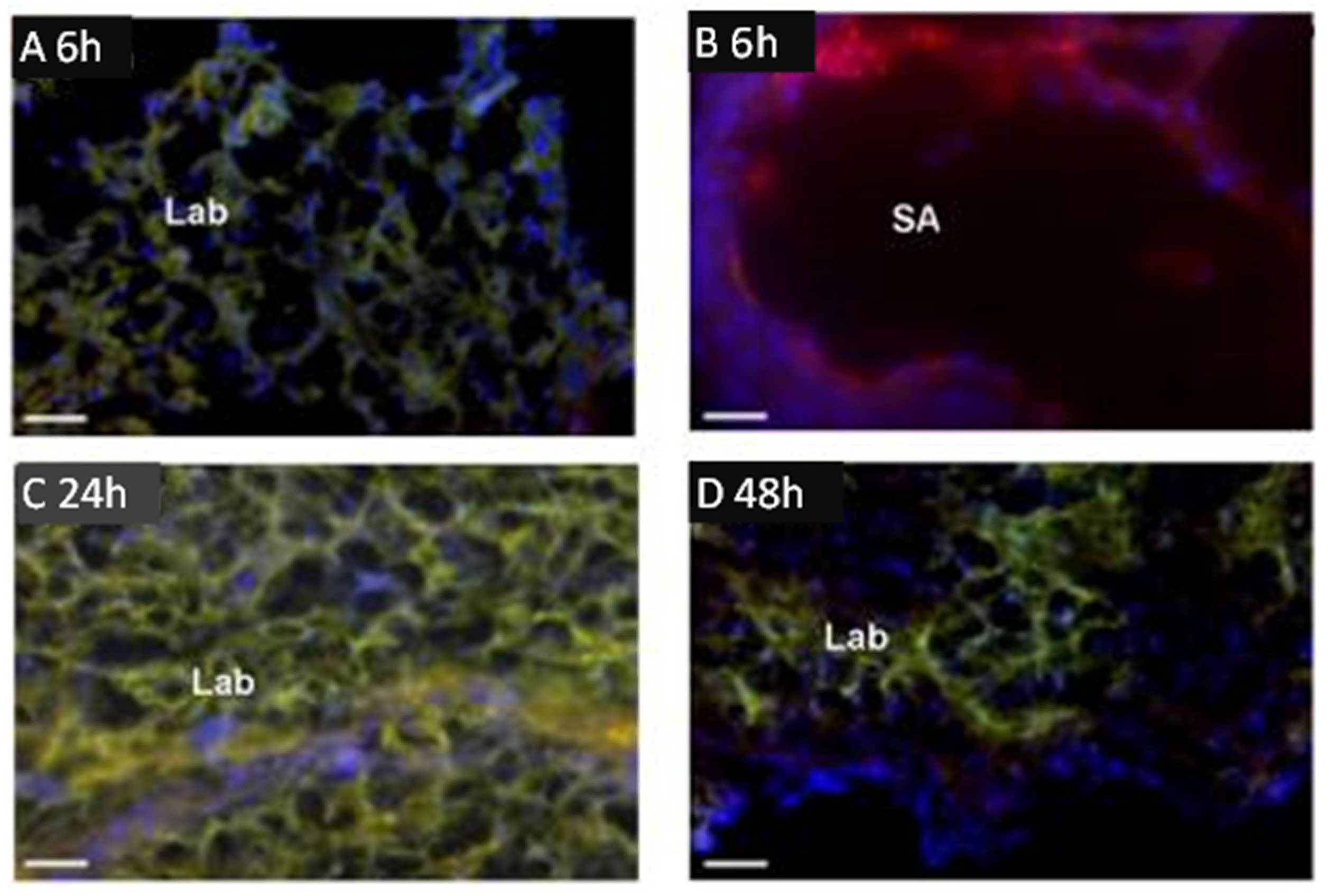
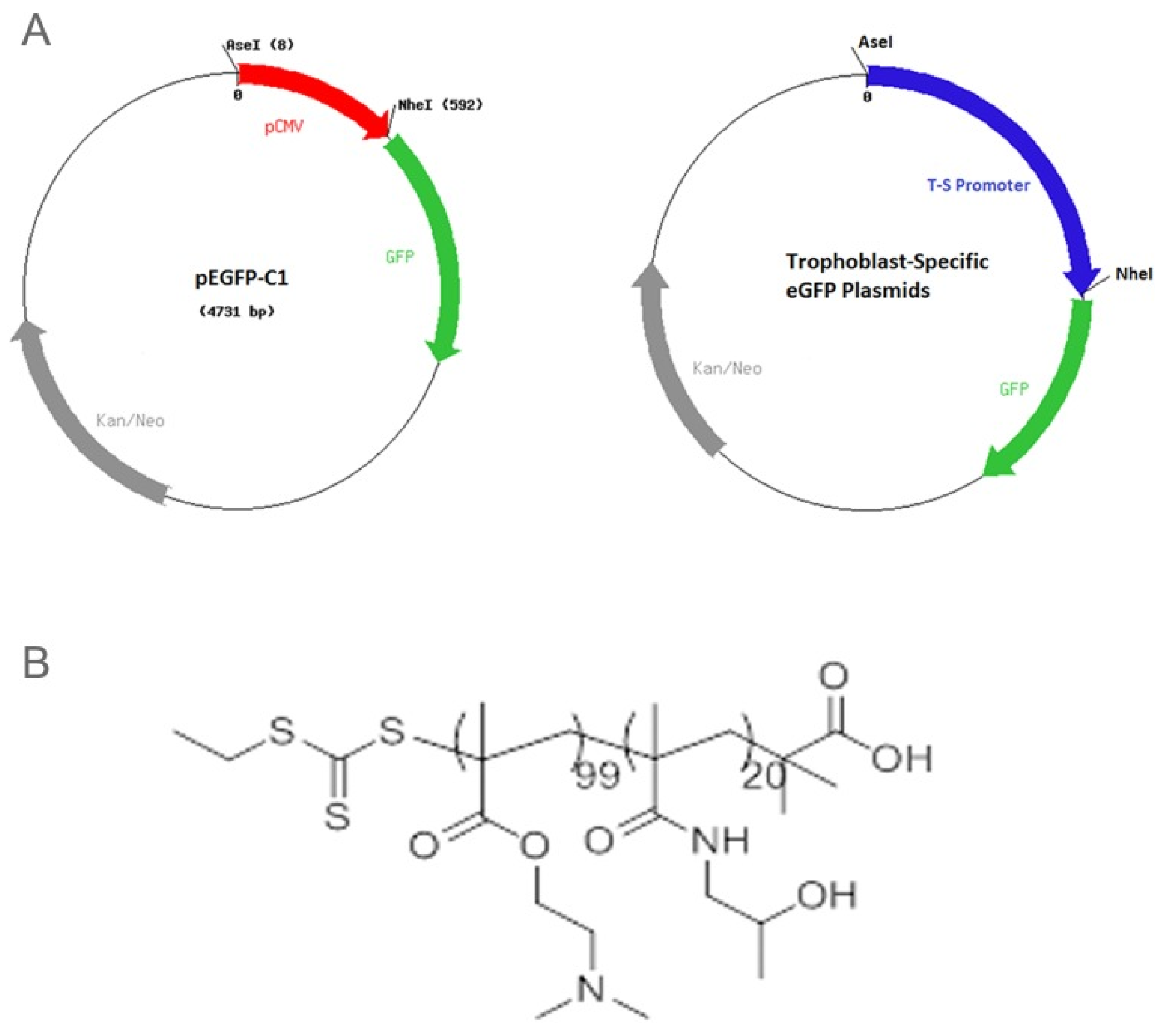
2.2. Trophoblast Targeted Nanoparticles
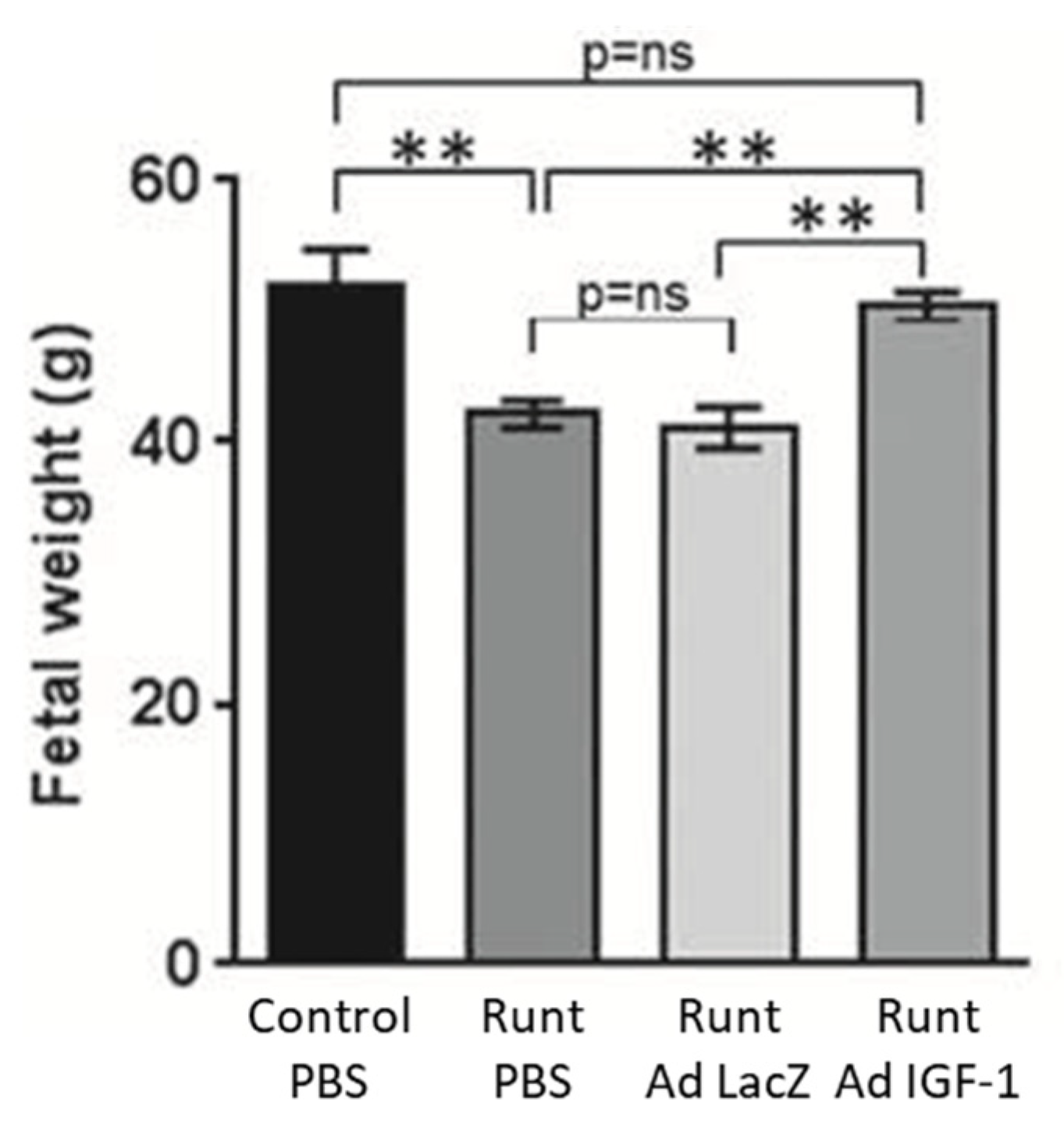
2.3. Adenovirus Mediated Intra-Placental Gene Therapy
2.4. Adenovirus Mediated Maternal Intrauterine Arterial Gene Therapy
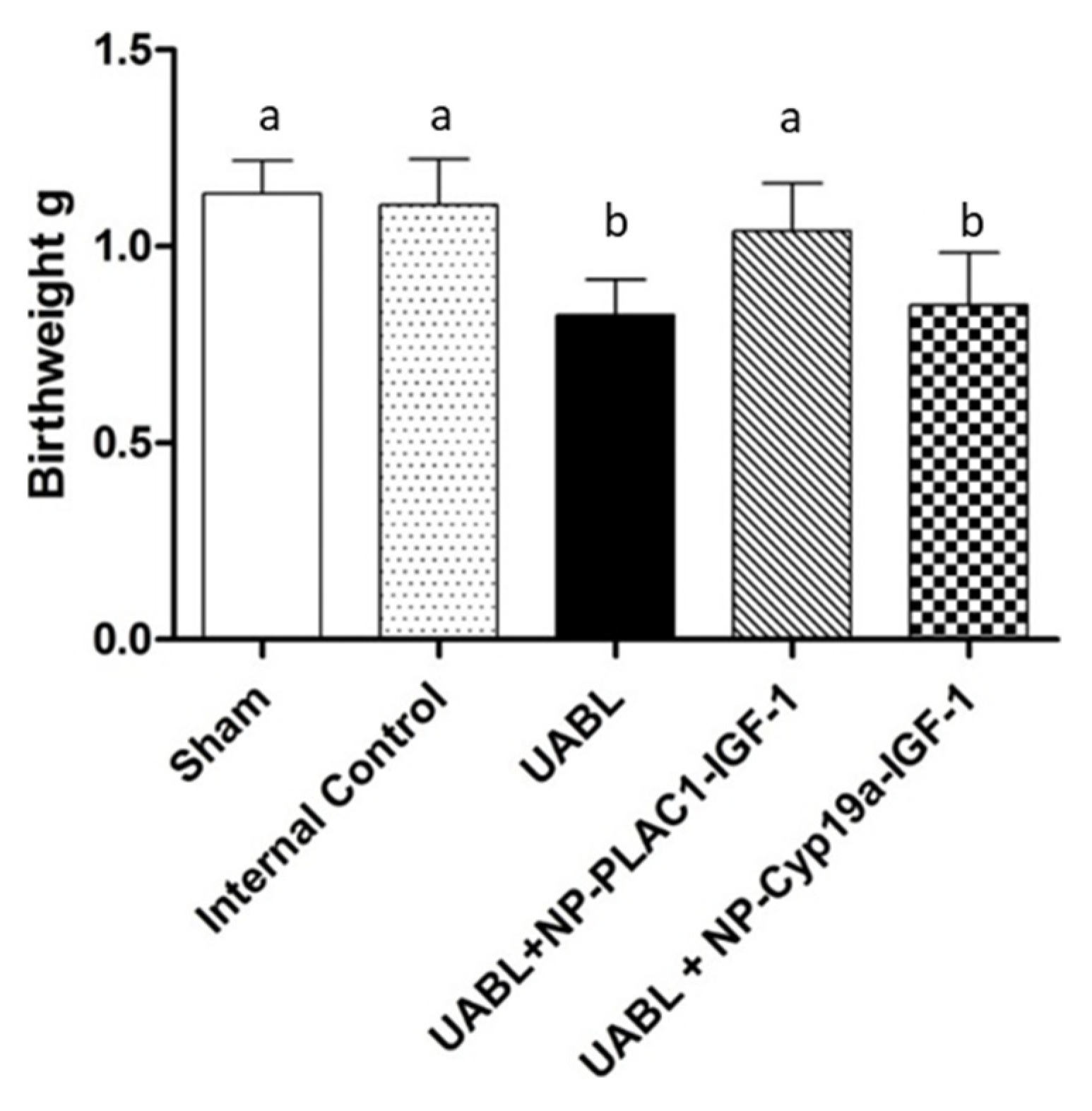
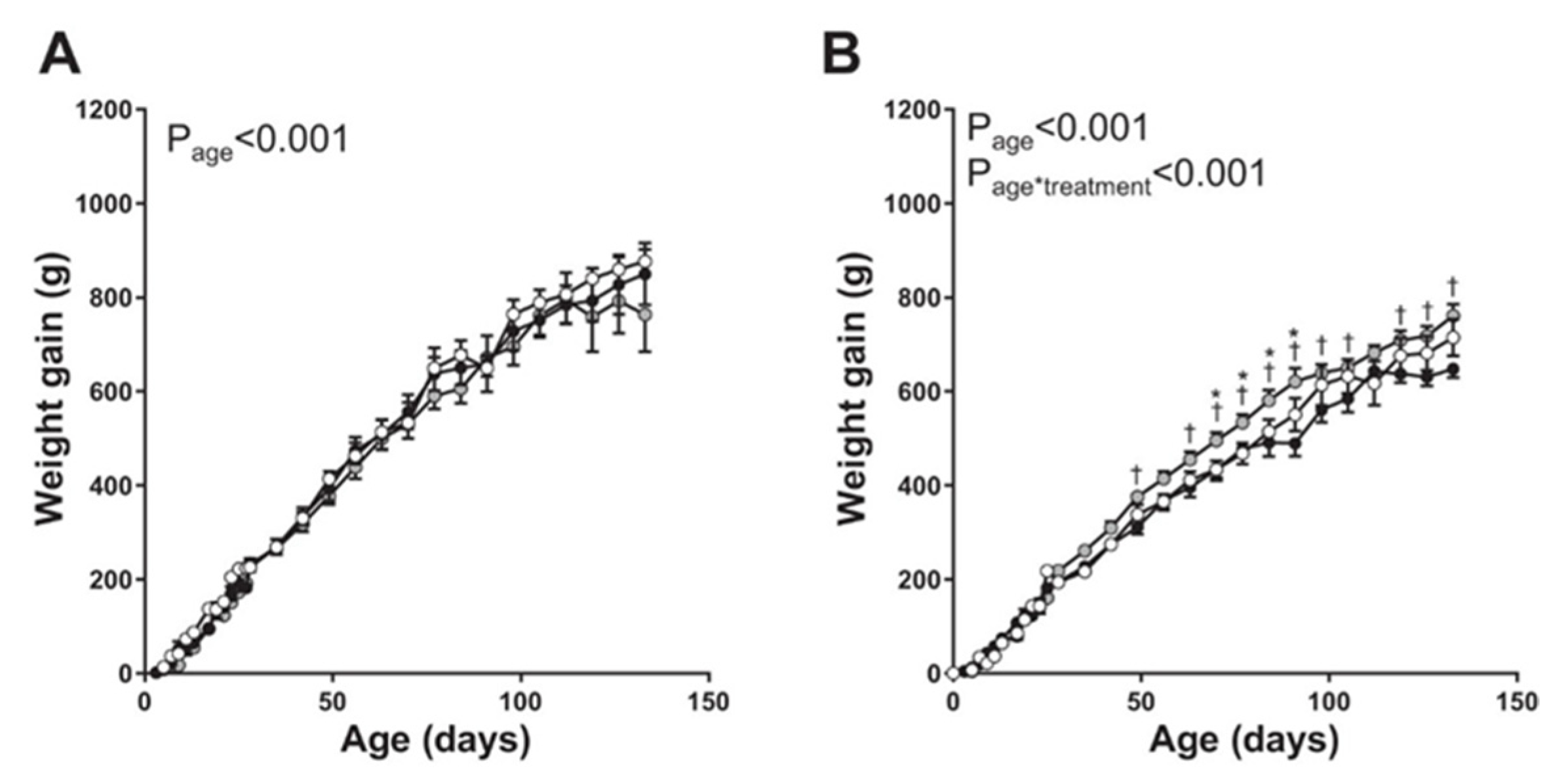
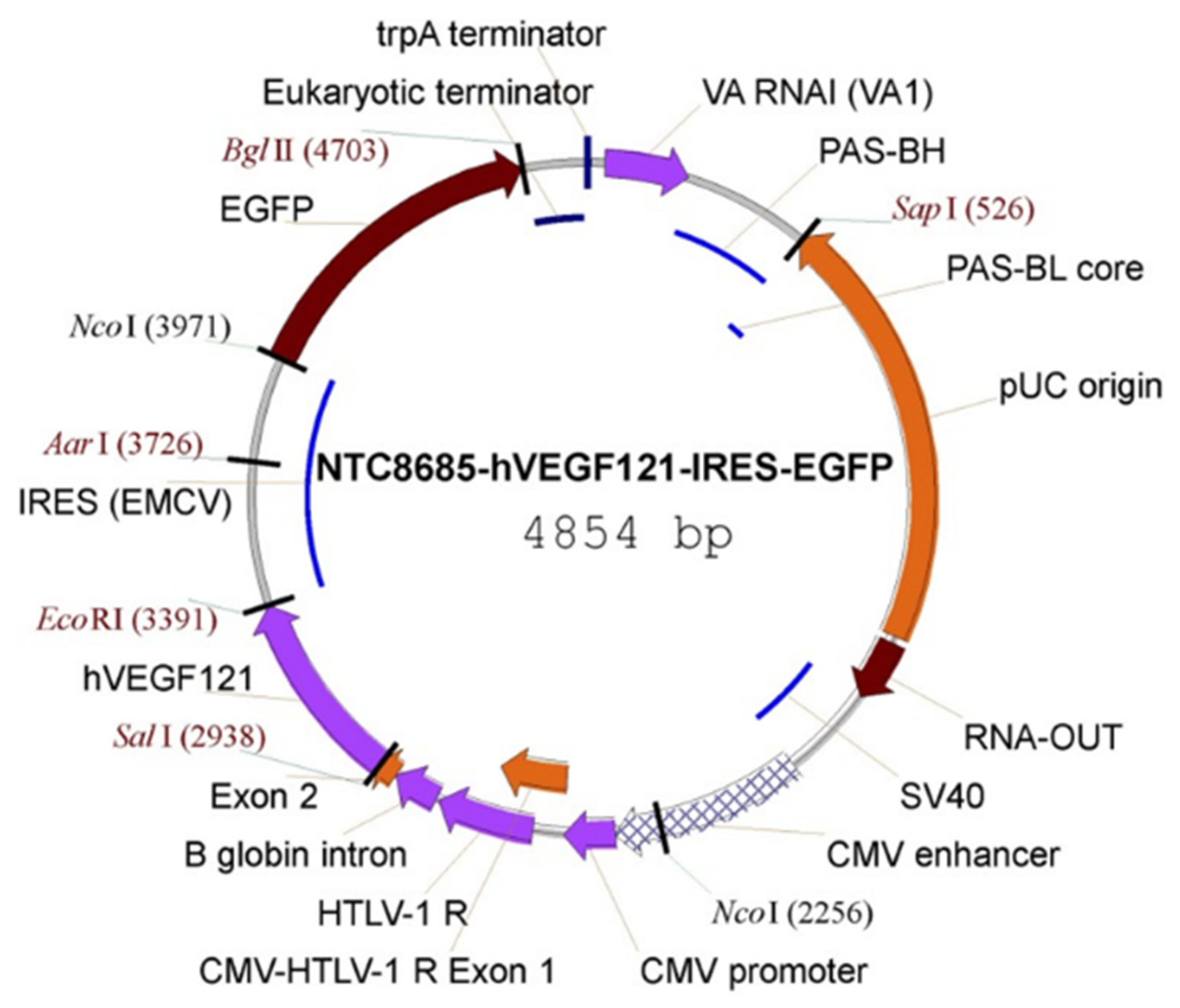
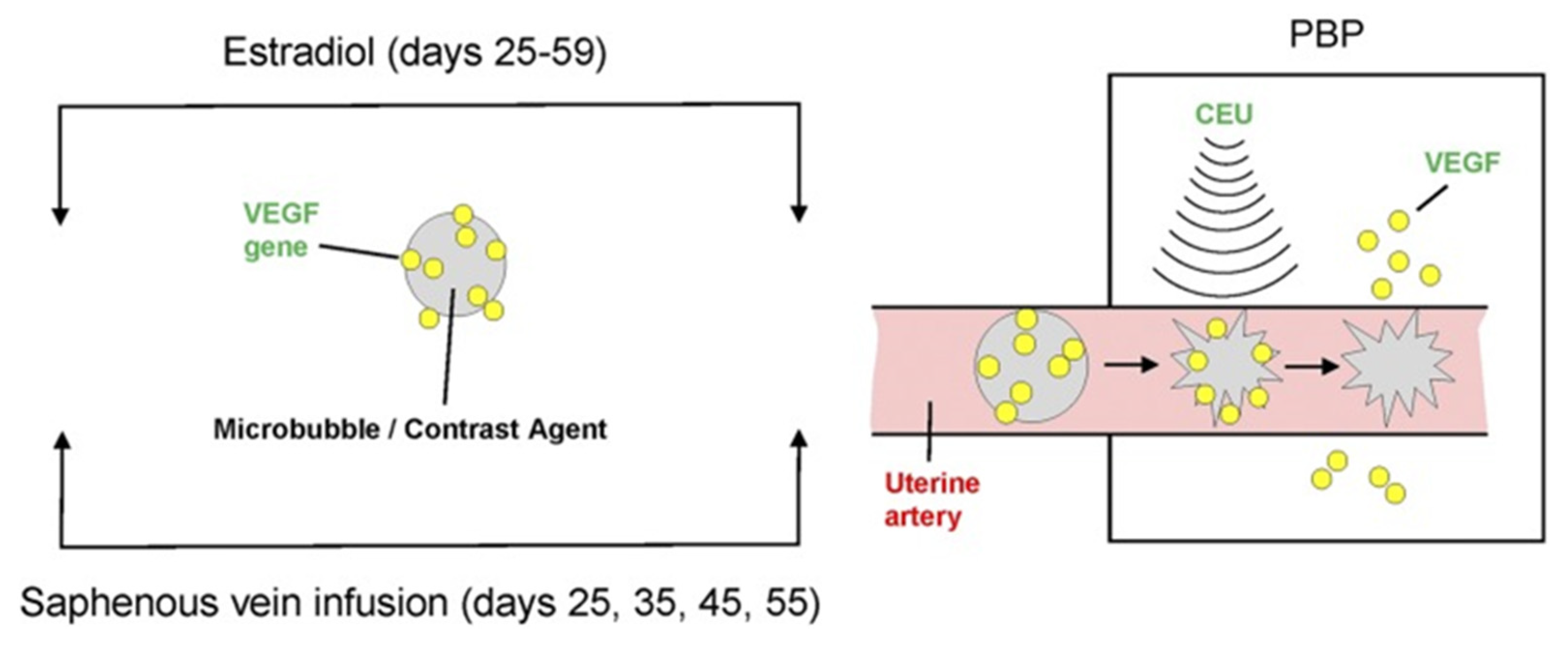
2.5. Targeted Placental VEGF Gene Therapy to Restore Uterine Artery Remodeling (UAR) in a Nonhuman Primate Model of Defective UAR
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamilton, W.J.; Boyd, J.D. Development of the human placenta in the first three months of gestation. J. Anat. 1960, 94, 297–328. [Google Scholar] [PubMed]
- Ramsey, E.M.; Houston, M.L.; Harris, J.W. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am. J. Obstet. Gynecol. 1976, 124, 647–652. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Dixon, G.; Robertson, W.B.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Enders, A.C.; King, B.F. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am. J. Anat. 1991, 192, 329–346. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V. Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin. Perinatol. 2014, 38, 131–132. [Google Scholar] [CrossRef]
- Friedman, A.M.; Cleary, K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014, 38, 177–182. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental origins of chronic disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Wen, X.; Triche, E.W.; Hogan, J.W.; Shenassa, E.D.; Buka, S.L. Association between placental morphology and childhood systolic blood pressure. Hypertension 2011, 57, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Regnault, T.R. In utero origins of adult insulin resistance and vascular dysfunction. Semin. Reprod. Med. 2011, 29, 211–224. [Google Scholar] [CrossRef]
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef]
- Davis, E.F.; Newton, L.; Lewandowski, A.J.; Lazdam, M.; Kelly, B.A.; Kyriakou, T.; Leeson, P. Pre-eclampsia and offspring cardiovascular health: Mechanistic insights from experimental studies. Clin. Sci. 2012, 123, 53–72. [Google Scholar] [CrossRef]
- Lazdam, M.; de la Horra, A.; Diesch, J.; Kenworthy, Y.; Davis, E.; Lewandowski, A.J.; Szmigielski, C.; Shore, A.; Mackillop, L.; Kharbanda, R.; et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension 2012, 60, 1338–1345. [Google Scholar] [CrossRef]
- Gaillard, R.; Steegers, E.A.; Tiemeier, H.; Hofman, A.; Jaddoe, V.W. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: The generation R study. Circulation 2013, 128, 2202–2210. [Google Scholar] [CrossRef]
- Alsnes, I.V.; Vatten, L.J.; Fraser, A.; Bjorngaard, J.H.; Rich-Edwards, J.; Romundstad, P.R.; Asvold, B.O. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: Prospective and sibling studies in the HUNT study (Nord-Trondelag Health Study) in Norway. Hypertension 2017, 69, 591–598. [Google Scholar] [CrossRef]
- Yu, G.Z.; Leeson, P. Hypertension: Hypertension in pregnancy: A risk factor for the whole family? Nat. Rev. Nephrol. 2017, 13, 326–327. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Q.; Hu, R. Lasting effects of intrauterine exposure to preeclampsia on offspring and the underlying mechanism. AJP Rep. 2019, 9, e275–e291. [Google Scholar] [CrossRef] [PubMed]
- Breetveld, N.M.; Ghossein-Doha, C.; van Neer, J.; Sengers, M.J.J.M.; Geerts, L.; van Kuijk, S.M.J.; van Dijk, A.P.; van der Vlugt, M.J.; Heidema, W.M.; Brunner-La Rocca, H.P.; et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet. Gynecol. 2018, 52, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vikse, B.E.; Irgens, L.M.; Leivestad, T.; Skjaerven, R.; Iversen, B.M. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 2008, 359, 800–809. [Google Scholar] [CrossRef]
- Simon-Tillaux, N.; Lecarpentier, E.; Tsatsaris, V.; Hertig, A. Sildenafil for the treatment of preeclampsia, an update: Should we still be enthusiastic? Nephrol. Dial. Transplant. 2019, 34, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Beards, F.; Jones, L.E.; Charnock, J.; Forbes, K.; Harris, L.K. Placental homing peptide-microRNA inhibitor conjugates for targeted enhancement of intrinsic placental growth signaling. Theranostics 2017, 7, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Fisk, N.M.; Atun, R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008, 5, e22. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Karim Rumi, M.A.; Soares, M.J. Review: Genetic manipulation of the rodent placenta. Placenta 2011, 32 (Suppl. 2), S130–S135. [Google Scholar] [CrossRef][Green Version]
- Fan, X.; Petitt, M.; Gamboa, M.; Huang, M.; Dhal, S.; Druzin, M.L.; Wu, J.C.; Chen-Tsai, Y.; Nayak, N.R. Transient, inducible, placenta-specific gene expression in mice. Endocrinology 2012, 153, 5637–5644. [Google Scholar] [CrossRef]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Pepe, G.J. Placental Endocrine Function and Hormone Action. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Academic Press: New York, NY, USA, 2015; pp. 1783–1834. [Google Scholar]
- Lu, F.; Longo, M.; Tamayo, E.; Maner, W.; Al-Hendy, A.; Anderson, G.D.; Hankins, G.D.; Saade, G.R. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am. J. Obstet. Gynecol. 2007, 196, 396–397. [Google Scholar] [CrossRef]
- Woods, A.K.; Hoffmann, D.S.; Weydert, C.J.; Butler, S.D.; Zhou, Y.; Sharma, R.V.; Davisson, R.L. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 2011, 57, 94–102. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ying, M.J.; Kapoun, A.M.; Shao, Q.; Kerr, I.; Lam, A.; O’Young, G.; Sannajust, F.; Stathis, P.; et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 2007, 50, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Bytautiene, E.; Lu, F.; Tamayo, E.H.; Betancourt, A.; Hankins, G.D.; Longo, M.; Saade, G.R. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am. J. Physiol Heart Circ. Physiol. 2011, 301, H1781–H1787. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.J.; Wallace, J.M.; Aitken, R.P.; Milne, J.S.; Martin, J.F.; Zachary, I.C.; Peebles, D.M.; David, A.L. Peri- and postnatal effects of prenatal adenoviral VEGF gene therapy in growth-restricted sheep. Biol. Reprod. 2016, 94, 142. [Google Scholar] [CrossRef]
- Rosario, F.J.; Sadovsky, Y.; Jansson, T. Gene targeting in primary human trophoblasts. Placenta 2012, 33, 754–762. [Google Scholar] [CrossRef][Green Version]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Ferretti, C.; Bruni, L.; Dangles-Marie, V.; Pecking, A.P.; Bellet, D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum. Reprod. Update 2007, 13, 121–141. [Google Scholar] [CrossRef]
- Donker, R.B.; Mouillet, J.F.; Chu, T.; Hubel, C.A.; Stolz, D.B.; Morelli, A.E.; Sadovsky, Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Lees, M.; Matthews, L.C.; Kimber, S.J.; Forbes, K.; Aplin, J.D. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J. Cell Sci. 2015, 128, 804–814. [Google Scholar] [CrossRef]
- Guo, L.; Tsai, S.Q.; Hardison, N.E.; James, A.H.; Motsinger-Reif, A.A.; Thames, B.; Stone, E.A.; Deng, C.; Piedrahita, J.A. Differentially expressed microRNAs and affected biological pathways revealed by modulated modularity clustering (MMC) analysis of human preeclamptic and IUGR placentas. Placenta 2013, 34, 599–605. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Y.; Liu, M.; Wang, Y.; Wang, H.; Li, Y.X.; Zhu, X.; Yao, Y.; Wang, H.; Qiao, J.; et al. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 2014, 63, 1276–1284. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Ondrackova, M.; Pirkova, P.; Kestlerova, A.; Novotna, V.; Hympanova, L.; Krofta, L. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015, 34, 437–457. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef]
- Farrokhnia, F.; Aplin, J.D.; Westwood, M.; Forbes, K. MicroRNA regulation of mitogenic signaling networks in the human placenta. J. Biol. Chem. 2014, 289, 30404–30416. [Google Scholar] [CrossRef] [PubMed]
- Keniry, A.; Oxley, D.; Monnier, P.; Kyba, M.; Dandolo, L.; Smits, G.; Reik, W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012, 14, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective targeting of a novel vasodilator to the uterine vasculature to treat Impaired uteroplacental perfusion in pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Espinoza, R.J.; Viricel, W.; Leblond, J.; Chissey, A.; Dhotel, H.; Roques, C.; Campiol, A.D.; Escriou, V.; et al. Liposomes as gene delivery vectors for human placental cells. Molecules 2018, 23, 1085. [Google Scholar] [CrossRef]
- Valero, L.; Alhareth, K.; Gil, S.; Lecarpentier, E.; Tsatsaris, V.; Mignet, N.; Fournier, T.; Andrieux, K. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov. Today 2018, 23, 1099–1107. [Google Scholar] [CrossRef]
- Saunders, M. Transplacental transport of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 671–684. [Google Scholar] [CrossRef]
- Oberdorster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Moller, W. Health implications of nanoparticles. J. Nanopart. Res. 2006, 8, 543–562. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Fertsch, S.; Schmid, G.; Wenk, A.; Kreyling, W. Uptake of 1.4 nm versus 18 nm gold particles by secondary target organs is size dependent in control and pregnant rats after intratracheal or intravenous application. Proc. EuroNanoForum 2007, 2007, 19–21. [Google Scholar]
- Zhang, B.; Chen, Z.; Han, J.; Li, M.; Nayak, N.R.; Fan, X. Comprehensive evaluation of the effectiveness and safety of placenta-targeted drug delivery using three complementary methods. J. Vis. Exp. 2018, 139, 58219. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019, 20, 3642. [Google Scholar] [CrossRef]
- Abd, E.N.; Taylor, L.; Troja, W.; Owens, K.; Ayres, N.; Pauletti, G.; Jones, H. Development of non-viral, trophoblast-specific gene delivery for placental therapy. PLoS ONE 2015, 10, e0140879. [Google Scholar] [CrossRef]
- Wilson, R.L.; Owens, K.; Sumser, E.K.; Fry, M.V.; Stephens, K.K.; Chuecos, M.; Carrillo, M.; Schlabritz-Loutsevitch, N.; Jones, H.N. Nanoparticle mediated increased insulin-like growth factor 1 expression enhances human placenta syncytium function. Placenta 2020, 93, 1–7. [Google Scholar] [CrossRef]
- Breyer, B.; Jiang, W.; Cheng, H.; Zhou, L.; Paul, R.; Feng, T.; He, T.C. Adenoviral vector-mediated gene transfer for human gene therapy. Curr. Gene Ther. 2001, 1, 149–162. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Keswani, S.; Katz, A.B.; Kozin, E.; Zoltic, P.W. Crombleholme TM Intraplacental gene transfer of IGF-1 corrects intrauterine growth restriction in the rabbit model. Mol. Ther. 2004, 9, S23. [Google Scholar]
- Jones, H.; Crombleholme, T.; Habli, M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta 2014, 35, 132–138. [Google Scholar] [CrossRef]
- Jones, H.N.; Crombleholme, T.; Habli, M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS ONE 2013, 8, e74632. [Google Scholar] [CrossRef]
- Keswani, S.G.; Balaji, S.; Katz, A.B.; King, A.; Omar, K.; Habli, M.; Klanke, C.; Crombleholme, T.M. Intraplacental gene therapy with Ad-IGF-1 corrects naturally occurring rabbit model of intrauterine growth restriction. Hum. Gene Ther. 2015, 26, 172–182. [Google Scholar] [CrossRef]
- Alsaied, T.; Omar, K.; James, J.; Hinton, R.; Crombleholme, T.; Habli, M. Fetal origins of adult cardiac disease: A novel approach to prevent fetal growth restriction induced cardiac dysfunction using insulin like growth factor. Pediatr. Res. 2017, 81, 919–925. [Google Scholar] [CrossRef]
- Carr, D.J.; Wallace, J.M.; Aitken, R.P.; Milne, J.S.; Mehta, V.; Martin, J.F.; Zachary, I.C.; Peebles, D.M.; David, A.L. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum. Gene Ther. 2014, 25, 375–384. [Google Scholar] [CrossRef]
- Mehta, V.; Abi-Nader, K.N.; Peebles, D.M.; Benjamin, E.; Wigley, V.; Torondel, B.; Filippi, E.; Shaw, S.W.; Boyd, M.; Martin, J.; et al. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A(165) in the uterine arteries of pregnant sheep. Gene Ther. 2012, 19, 925–935. [Google Scholar] [CrossRef] [PubMed]
- David, A.L.; Torondel, B.; Zachary, I.; Wigley, V.; Abi-Nader, K.; Mehta, V.; Buckley, S.M.; Cook, T.; Boyd, M.; Rodeck, C.H.; et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 2008, 15, 1344–1350. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rossi, C.A.; Ginsberg, Y.; White, A.; Hristova, M.; Sebire, N.J.; Martin, J.; Zachary, I.C.; Peebles, D.M.; David, A.L. Perinatal and long-term effects of maternal uterine artery adenoviral VEGF-A165 gene therapy in the growth-restricted guinea pig fetus. Am. J. Physiol Regul. Integr. Comp. Physiol. 2018, 315, R344–R353. [Google Scholar] [CrossRef]
- Sheppard, M.; Spencer, R.N.; Ashcroft, R.; David, A.L. Ethics and social acceptability of a proposed clinical trial using maternal gene therapy to treat severe early-onset fetal growth restriction. Ultrasound Obstet. Gynecol. 2016, 47, 484–491. [Google Scholar] [CrossRef]
- Spencer, R.; Ambler, G.; Brodszki, J.; Diemert, A.; Figueras, F.; Gratacós, E.; Hansson, S.R.; Hecher, K.; Huertas-Ceballos, A.; Marlow, N.; et al. EVERREST prospective study: A 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth 2017, 17, 43. [Google Scholar] [CrossRef]
- Bonagura, T.W.; Pepe, G.J.; Enders, A.C.; Albrecht, E.D. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 2008, 149, 5078–5087. [Google Scholar] [CrossRef]
- Bonagura, T.W.; Babischkin, J.S.; Aberdeen, G.W.; Pepe, G.J.; Albrecht, E.D. Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and α1β1 and α5β1 integrins. Endocrinology 2012, 153, 2897–2906. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Babischkin, J.S.; Aberdeen, G.W.; Burch, M.G.; Pepe, G.J. Maternal systemic vascular dysfunction in a primate model of defective uterine spiral artery remodeling. Am. J. Physiol Heart Circ. Physiol. 2021, 320, H1712–H1723. [Google Scholar] [CrossRef]
- Turan, O.; Babischkin, J.S.; Aberdeen, G.W.; Turan, S.; Harman, C.; Pepe, G.J.; Albrecht, E.D. B-flow/Spatio temporal image correlation M-mode: A novel ultrasound method that detects a decrease in spiral artery luminal diameter in the first trimester in a primate model of impaired spiral artery remodeling. Ultrasound Obstet. Gynecol. 2021. ahead of print. [Google Scholar] [CrossRef]
- Babischkin, J.S.; Aberdeen, G.W.; Lindner, J.R.; Bonagura, T.W.; Pepe, G.J.; Albrecht, E.D. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology 2019, 160, 1492–1505. [Google Scholar] [CrossRef]
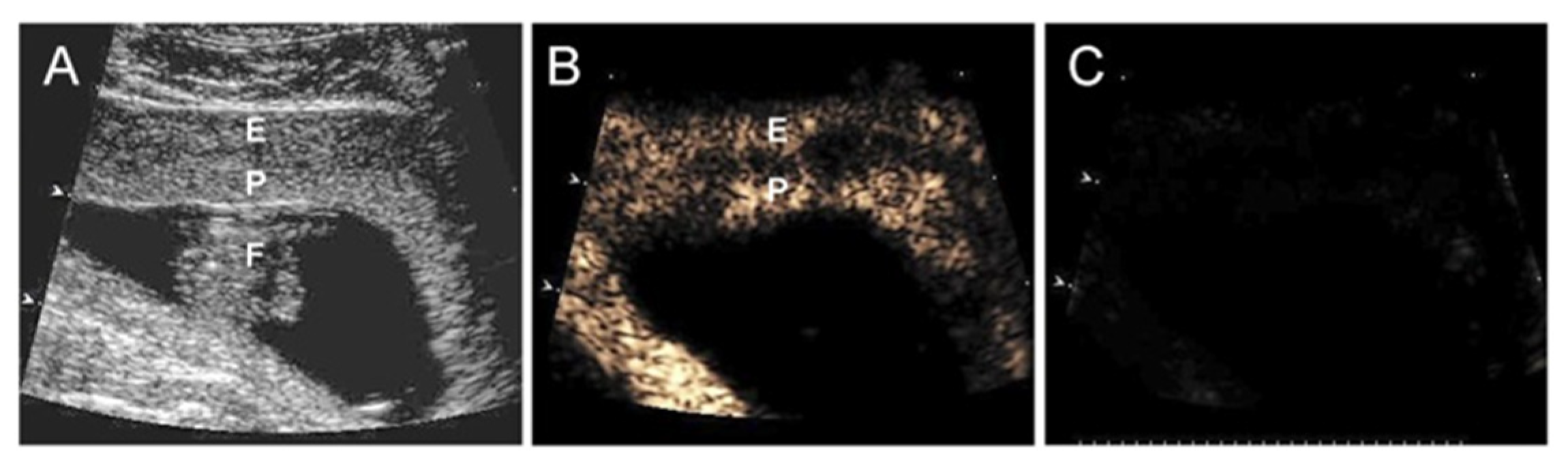
- Roberts, V.H.; Lo, J.O.; Salati, J.A.; Lewandowski, K.S.; Lindner, J.R.; Morgan, T.K.; Frias, A.E. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am. J. Obstet. Gynecol. 2016, 214, 369.e1–369.e8. [Google Scholar] [CrossRef]
- Roberts, V.H.; Frias, A.E. Contrast-enhanced ultrasound for the assessment of placental development and function. Biotechniques 2020, 69, 392–399. [Google Scholar] [CrossRef]
- Bekeredjian, R.; Chen, S.; Frenkel, P.A.; Grayburn, P.A.; Shohet, R.V. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation 2003, 108, 1022–1026. [Google Scholar] [CrossRef]
- Leong-Poi, H.; Kuliszewski, M.A.; Lekas, M.; Sibbald, M.; Teichert-Kuliszewska, K.; Klibanov, A.L.; Stewart, D.J.; Lindner, J.R. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ. Res. 2007, 101, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Belcik, J.T.; Qi, Y.; Kaufmann, B.A.; Xie, A.; Bullens, S.; Morgan, T.K.; Bagby, S.P.; Kolumam, G.; Kowalski, J.; Oyer, J.A.; et al. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J. Am. Coll. Cardiol. 2012, 60, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Belcik, T.; Qi, Y.; Morgan, T.K.; Champaneri, S.A.; Taylor, S.; Davidson, B.P.; Zhao, Y.; Klibanov, A.L.; Kuliszewski, M.A.; et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc. Imaging 2012, 5, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Wu, M.D.; Cigarroa, G.; Belcik, J.T.; Ammi, A.; Moccetti, F.; Lindner, J.R. Influence of DNA-microbubble coupling on contrast ultrasound-mediated gene transfection in muscle and liver. J. Am. Soc. Echocardiogr. 2016, 29, 812–818. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Pepe, G.J. Regulation of uterine spiral artery remodeling: A Review. Reprod. Sci. 2020, 27, 1932–1942. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.J.; Albrecht, E.D. Novel Technologies for Target Delivery of Therapeutics to the Placenta during Pregnancy: A Review. Genes 2021, 12, 1255. https://doi.org/10.3390/genes12081255
Pepe GJ, Albrecht ED. Novel Technologies for Target Delivery of Therapeutics to the Placenta during Pregnancy: A Review. Genes. 2021; 12(8):1255. https://doi.org/10.3390/genes12081255
Chicago/Turabian StylePepe, Gerald J., and Eugene D. Albrecht. 2021. "Novel Technologies for Target Delivery of Therapeutics to the Placenta during Pregnancy: A Review" Genes 12, no. 8: 1255. https://doi.org/10.3390/genes12081255
APA StylePepe, G. J., & Albrecht, E. D. (2021). Novel Technologies for Target Delivery of Therapeutics to the Placenta during Pregnancy: A Review. Genes, 12(8), 1255. https://doi.org/10.3390/genes12081255




