Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds
Abstract
1. Introduction
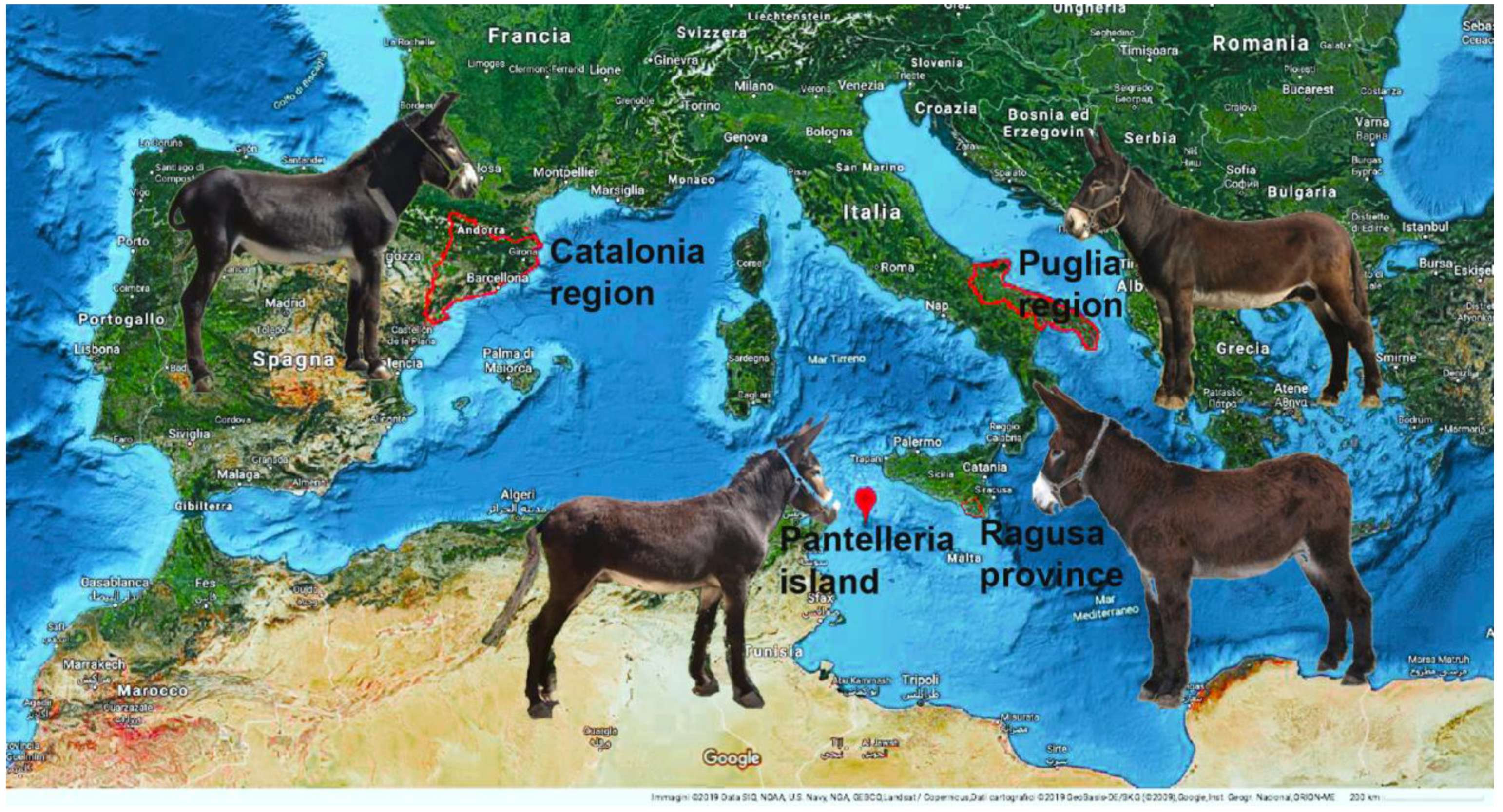
2. Materials and Methods
3. Results
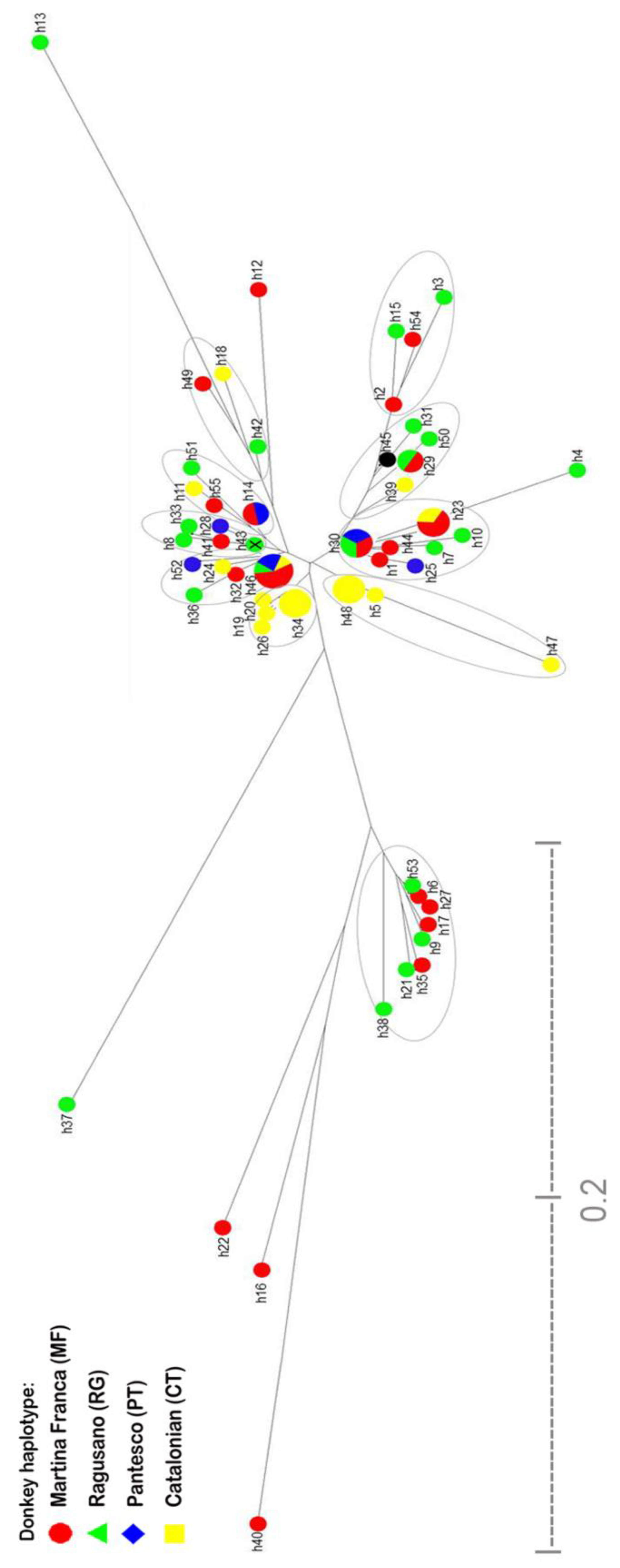
3.1. Breed Haplotype Analysis
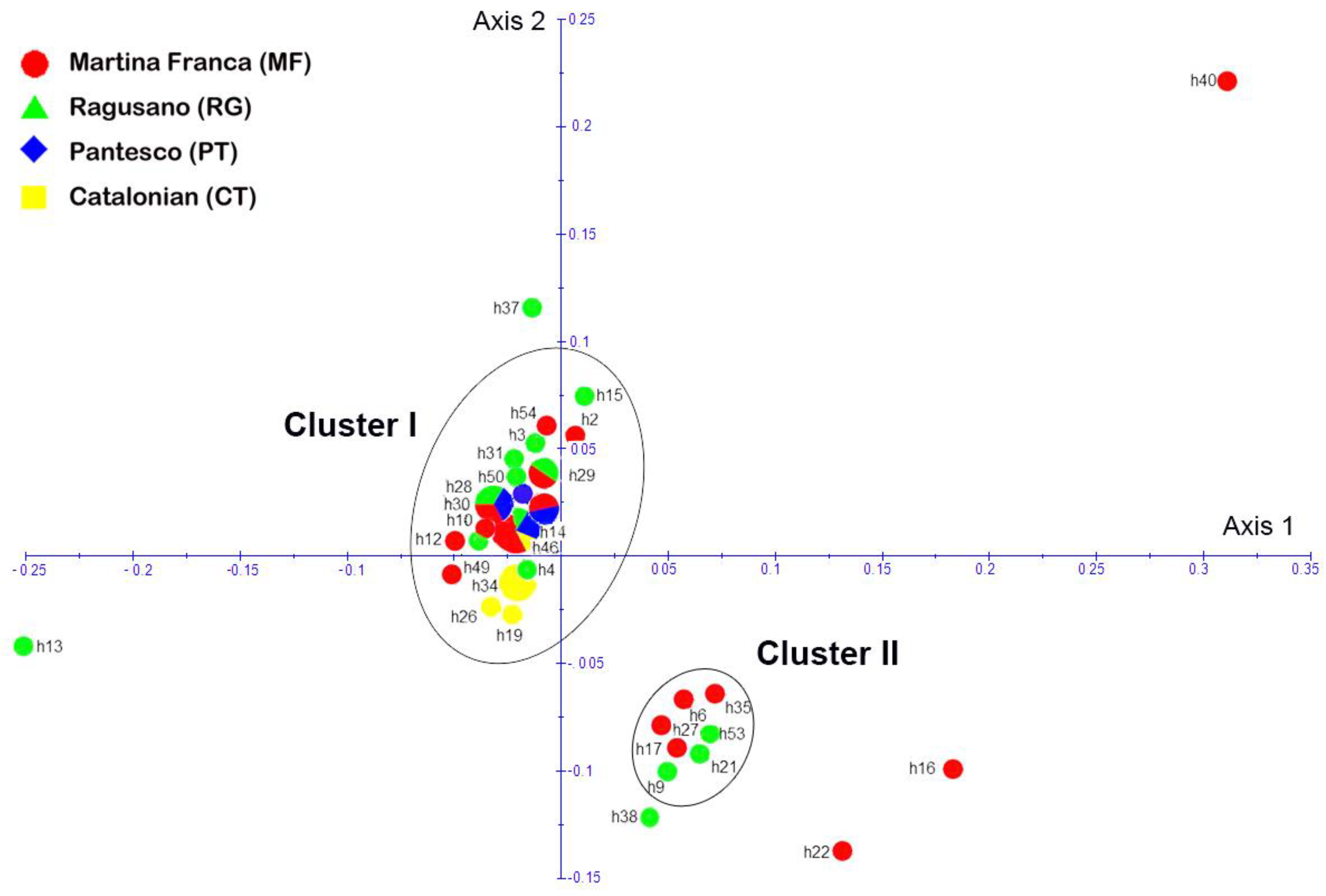
3.1.1. Population Analyses
3.1.2. Origin and Phylogenetic Relationships
4. Discussion
4.1. Breed Molecular Analysis
4.2. Population Analyses
4.3. Origin and Phylogenetic Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macfadden, B.J. Evolution. Fossil Horses—Evidence for Evolution. Science 2005, 307, 1728–1730. [Google Scholar] [CrossRef]
- Krüger, K.; Gaillard, C.; Stranzinger, G.; Rieder, S. Phylogenetic Analysis and Species Allocation of Individual Equids Using Microsatellite Data. J. Anim. Breed. Genet. 2005, 122 (Suppl. 1), 78–86. [Google Scholar] [CrossRef]
- Groves, C.; Grubb, P. Ungulate Taxonomy; The Johns Hopkins University Press: Baltimore, MD, USA, 2011; p. 336. [Google Scholar]
- Beja-Pereira, A.; England, P.R.; Ferrand, N.; Jordan, S.; Bakhiet, A.O.; Abdalla, M.A.; Mashkour, M.; Jordana, J.; Taberlet, P.; Luikart, G. African Origins of the Domestic Donkey. Science 2004, 304, 1781. [Google Scholar] [CrossRef]
- Kimura, B.; Marshall, F.B.; Chen, S.; Rosenbom, S.; Moehlman, P.D.; Tuross, N.; Sabin, R.C.; Peters, J.; Barich, B.; Yohannes, H.; et al. Ancient DNA from Nubian and Somali Wild Ass Provides Insights into Donkey Ancestry and Domestication. Proc. R. Soc. B Biol. Sci. 2011, 278, 50–57. [Google Scholar] [CrossRef]
- Rizzi, R.; Tullo, E.; Cito, A.M.; Caroli, A.; Pieragostini, E. Monitoring of Genetic Diversity in the Endangered Martina Franca Donkey Population. J. Anim. Sci. 2011, 89, 1304–1311. [Google Scholar] [CrossRef][Green Version]
- Piganoil, A.F.G. La Conquête Romaine; Edité par Félix Alcan: Paris, France, 1927. [Google Scholar]
- Rand, D.M. The Units of Selection on Mitochondrial DNA. Annu. Rev. Ecol. Syst. 2001, 32, 415–448. [Google Scholar] [CrossRef]
- Clutton-Brock, J. Horse Power: A History of the Horse and the Donkey in Human Societies; Harvard University Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Bowling, A.T.; Del Valle, A.; Bowling, M. A Pedigree-Based Study of Mitochondrial D-Loop DNA Sequence Variation among Arabian Horses. Anim. Genet. 2000, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Newbold, J.E.; Potter, S.S.; Edgell, M.H. Maternal Inheritance of Mammalian Mitochondrial DNA. Nature 1974, 251, 536–538. [Google Scholar] [CrossRef]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid Evolution of Animal Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef]
- Wallace, D.C. Why Do We Still Have a Maternally Inherited Mitochondrial DNA? Insights from Evolutionary Medicine. Annu. Rev. Biochem. 2007, 76, 781–821. [Google Scholar] [CrossRef]
- Mirol, P.M.; Peral García, P.; Vega-Pla, J.L.; Dulout, F.N. Phylogenetic Relationships of Argentinean Creole Horses and Other South American and Spanish Breeds Inferred from Mitochondrial DNA Sequences. Anim. Genet. 2002, 33, 356–363. [Google Scholar] [CrossRef]
- Stoneking, M.; Sherry, S.T.; Redd, A.J.; Vigilant, L. New Approaches to Dating Suggest a Recent Age for the Human MtDNA Ancestor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 337, 167–175. [Google Scholar] [CrossRef]
- Vigilant, L.; Pennington, R.; Harpending, H.; Kocher, T.D.; Wilson, A.C. Mitochondrial DNA Sequences in Single Hairs from a Southern African Population. Proc. Natl. Acad. Sci. USA 1989, 86, 9350–9354. [Google Scholar] [CrossRef] [PubMed]
- Verginelli, F.; Capelli, C.; Coia, V.; Musiani, M.; Falchetti, M.; Ottini, L.; Palmirotta, R.; Tagliacozzo, A.; De Grossi Mazzorin, I.; Mariani-Costantini, R. Mitochondrial DNA from Prehistoric Canids Highlights Relationships between Dogs and South-East European Wolves. Mol. Biol. Evol. 2005, 22, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, A.; Carluccio, A.; Robbe, D.; Giulio, C.D.; Cellerino, A. The Companion Dog as a Unique Translational Model for Aging. Semin. Cell. Dev. Biol. 2017, 70, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Olivieri, A.; Soares, P.; Lancioni, H.; Hooshiar Kashani, B.; Perego, U.A.; Nergadze, S.G.; Carossa, V.; Santagostino, M.; Capomaccio, S.; et al. Mitochondrial Genomes from Modern Horses Reveal the Major Haplogroups That Underwent Domestication. Proc. Natl. Acad. Sci. USA 2012, 109, 2449–2454. [Google Scholar] [CrossRef]
- Cothran, E.G.; Juras, R.; Macijauskiene, V. Mitochondrial DNA D-Loop Sequence Variation among 5 Maternal Lines of the Zemaitukai Horse Breed. Genet. Mol. Biol. 2005, 28, 677–681. [Google Scholar] [CrossRef]
- Kavar, T.; Dovč, P. Domestication of the Horse: Genetic Relationships between Domestic and Wild Horses. Livest. Sci. 2008, 116, 1–14. [Google Scholar] [CrossRef]
- Lippold, S.; Matzke, N.J.; Reissmann, M.; Hofreiter, M. Whole Mitochondrial Genome Sequencing of Domestic Horses Reveals Incorporation of Extensive Wild Horse Diversity during Domestication. BMC Evol. Biol. 2011, 11, 328. [Google Scholar] [CrossRef]
- Cieslak, M.; Pruvost, M.; Benecke, N.; Hofreiter, M.; Morales, A.; Reissmann, M.; Ludwig, A. Origin and History of Mitochondrial DNA Lineages in Domestic Horses. PLoS ONE 2010, 5, e15311. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.M.; Zuccaro, A.; Criscione, A.; Marletta, D.; Bordonaro, S. Genetic Analysis of Sicilian Autochthonous Horse Breeds Using Nuclear and Mitochondrial DNA Markers. J. Hered. 2011, 102, 753–758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jansen, T.; Forster, P.; Levine, M.A.; Oelke, H.; Hurles, M.; Renfrew, C.; Weber, J.; Olek, K. Mitochondrial DNA and the Origins of the Domestic Horse. Proc. Natl. Acad. Sci. USA 2002, 99, 10905–10910. [Google Scholar] [CrossRef] [PubMed]
- Vilà, C.; Leonard, J.A.; Gotherstrom, A.; Marklund, S.; Sandberg, K.; Liden, K.; Wayne, R.K.; Ellegren, H. Widespread Origins of Domestic Horse Lineages. Science 2001, 291, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Kivisild, T.; Reidla, M.; Metspalu, E.; Rosa, A.; Brehm, A.; Pennarun, E.; Parik, J.; Geberhiwot, T.; Usanga, E.; Villems, R. Ethiopian Mitochondrial DNA Heritage: Tracking Gene Flow across and around the Gate of Tears. Am. J. Hum. Genet. 2004, 75, 752–770. [Google Scholar] [CrossRef]
- Matisoo-Smith, E.; Robins, J. Mitochondrial DNA Evidence for the Spread of Pacific Rats through Oceania. Biol. Invasions 2009, 11, 1521–1527. [Google Scholar] [CrossRef]
- Xu, X.; Gullberg, A.; Arnason, U. The Complete Mitochondrial DNA (MtDNA) of the Donkey and MtDNA Comparisons among Four Closely Related Mammalian Species-Pairs. J. Mol. Evol. 1996, 43, 438–446. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y.; Liu, F.; Jiang, C.; Gao, Y. Mitochondrial Genome Sequence of the Tibetan Wild Ass (Equus Kiang). Mitochondrial DNA 2011, 22, 6–8. [Google Scholar] [CrossRef]
- Ciampolini, R.; Cecchi, F.; Mazzanti, E.; Ciani, E.; Tancredi, M.; De Sanctis, B. The Genetic Variability Analysis of the Amiata Donkey Breed by Molecular Data. Ital. J. Anim. Sci. 2007, 6, 78–80. [Google Scholar] [CrossRef]
- George, M., Jr.; Ryder, O.A. Mitochondrial DNA Evolution in the Genus Equus. Mol. Biol. Evol. 1986, 3, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Meadow, R.H. Equus africanus in Arabia. In Equids in the Ancient World; Uerpmann, H.P., Ed.; Ludwig Reichert Verlag: Wiesbaden, Germany, 1991; pp. 12–33. [Google Scholar]
- Vila, E. Data on equids from late fourth and third millennium sites in Northern Syria. In Equids in Time and Space; Mashkour, M., Ed.; Oxbow Books Ltd.: Oxford, UK, 2002; pp. 101–123. [Google Scholar]
- Rossel, S.; Marshall, F.; Peters, J.; Pilgram, T.; Adams, M.D.; O’Connor, D. Domestication of the Donkey: Timing, Processes, and Indicators. Proc. Natl. Acad. Sci. USA 2008, 105, 3715–3720. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhou, F.; Xiao, H.; Sha, T.; Wu, S.F.; Zhang, Y.P. Mitochondrial DNA Diversity and Population Structure of Four Chinese Donkey Breeds. Anim. Genet. 2006, 37, 427–429. [Google Scholar] [CrossRef]
- Kefena, E.; Dessie, T.; Tegegne, A.; Beja-Pereira, A.; Yusuf Kurtu, M.; Rosenbom, S.; Han, J.L. Genetic Diversity and Matrilineal Genetic Signature of Native Ethiopian Donkeys (Equus asinus) Inferred from Mitochondrial DNA Sequence Polymorphism. Livest. Sci. 2014, 167, 73–79. [Google Scholar] [CrossRef]
- Matassino, D.; Cecchi, F.; Ciani, F.; Incoronato, C.; Occidente, M.; Santoro, L.; Ciampolini, R. Genetic Diversity and Variability in Two Italian Autochthonous Donkey Genetic Types Assessed by Microsatellite Markers. Ital. J. Anim. Sci. 2014, 13, 3028. [Google Scholar] [CrossRef]
- Bordonaro, S.; Guastella, A.M.; Criscione, A.; Zuccaro, A.; Marletta, D. Genetic Diversity and Variability in Endangered Pantesco and Two Other Sicilian Donkey Breeds Assessed by Microsatellite Markers. Sci. World J. 2012, 2012, 648427. [Google Scholar] [CrossRef]
- Colli, L.; Perrotta, G.; Negrini, R.; Bomba, L.; Bigi, D.; Zambonelli, P.; Verini Supplizi, A.; Liotta, L.; Ajmone-Marsan, P. Detecting Population Structure and Recent Demographic History in Endangered Livestock Breeds: The Case of the Italian Autochthonous Donkeys. Anim. Genet. 2013, 44, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Scimone, C.; Geraci, C.; Schiavo, G.; Utzeri, V.J.; Chiofalo, V.; Fontanesi, L. Next Generation Semiconductor Based Sequencing of the Donkey (Equus asinus) Genome Provided Comparative Sequence Data against the Horse Genome and a Few Millions of Single Nucleotide Polymorphisms. PLoS ONE 2015, 10, e0131925. [Google Scholar] [CrossRef]
- Cozzi, M.C.; Valiati, P.; Cherchi, R.; Gorla, E.; Prinsen, R.T.M.M.; Longeri, M.; Bagnato, A.; Strillacci, M.G. Mitochondrial DNA Genetic Diversity in Six Italian Donkey Breeds (Equus Asinus). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2018, 29, 409–418. [Google Scholar] [CrossRef]
- Aranguren-Mendez, J.; Beja-Pereira, A.; Avellanet, R.; Dzama, K.; Jordana, J. Mitochondrial DNA variation and genetic relationships in Spanish donkey breeds (Equus asinus). J. Anim. Breed. Genet. 2004, 121, 319–330. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for Inferring Very Large Phylogenies by Using the Neighbor-Joining Method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Tamura, K.; Battistuzzi, F.U.; Billing-Ross, P.; Murillo, O.; Filipski, A.; Kumar, S. Estimating Divergence Times in Large Molecular Phylogenies. Proc. Natl. Acad. Sci. USA 2012, 109, 19333–19338. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 1–4. [Google Scholar] [CrossRef]
- Cecchi, F.; Ciampolini, R.; Ciani, E.; Matteoli, B.; Mazzanti, E.; Tancredi, M.; Presciuttini, S. Demographic Genetics of the Endangered Amiata Donkey Breed. Ital. J. Anim. Sci. 2006, 5, 387–391. [Google Scholar] [CrossRef]
- Navas, F.J.; Jordana, J.; León, J.M.; Barba, C.; Delgado, J.V. A Model to Infer the Demographic Structure Evolution of Endangered Donkey Populations. Animal 2017, 11, 2129–2138. [Google Scholar] [CrossRef]
- Cinar Kul, B.; Bilgen, N.; Akyuz, B.; Ertugrul, O. Molecular Phylogeny of Anatolian and Cypriot Donkey Populations Based on Mitochondrial DNA and Y-Chromosomal STRs. Ank. Univ. Vet. Fak. Derg. 2016, 63, 143–149. [Google Scholar]
- Gutiérrez, J.P.; Marmi, J.; Goyache, F.; Jordana, J. Pedigree Information Reveals Moderate to High Levels of Inbreeding and a Weak Population Structure in the Endangered Catalonian Donkey Breed. J. Anim. Breed. Genet. 2005, 122, 378–386. [Google Scholar] [CrossRef]
- Ivanković, A.; Ramljak, J.; Konjačić, M.; Kelava, N.; Dovč, P.; Mijić, P. Mitochondrial D-Loop Sequence Variation among Autochthonous Horse Breeds in Croatia. Czech J. Anim. Sci. 2009, 54, 101–111. [Google Scholar] [CrossRef]
- Stanisic, L.J.; Aleksic, J.M.; Dimitrijevic, V.; Simeunovic, P.; Glavinic, U.; Stevanovic, J.; Stanimirovic, Z. New Insights into the Origin and the Genetic Status of the Balkan Donkey from Serbia. Anim. Genet. 2017, 48, 580–590. [Google Scholar] [CrossRef]
- Aranguren-Méndez, J.; Jordana, J.; Gomez, M. Genetic Diversity in Spanish Donkey Breeds Using Microsatellite DNA Markers. Genet. Sel. Evol. 2001, 33, 433. [Google Scholar] [CrossRef]
- Ivankovic, A.; Kavar, T.; Caput, P.; Mioc, B.; Pavic, V.; Dovc, P. Genetic Diversity of Three Donkey Populations in the Croatian Coastal Region. Anim. Genet. 2002, 33, 169–177. [Google Scholar] [CrossRef]
- Pérez-Pardal, L.; Grizelj, J.; Traoré, A.; Cubric-Curik, V.; Arsenos, G.; Dovenski, T.; Marković, B.; Fernández, I.; Cuervo, M.; Álvarez, I.; et al. Lack of Mitochondrial DNA Structure in Balkan Donkey Is Consistent with a Quick Spread of the Species after Domestication. Anim. Genet. 2014, 45, 144–147. [Google Scholar] [CrossRef]
- Bjørnstad, G.; Røed, K.H. Evaluation of Factors Affecting Individual Assignment Precision Using Microsatellite Data from Horse Breeds and Simulated Breed Crosses. Anim. Genet. 2002, 33, 264–270. [Google Scholar] [CrossRef]
- Toro, M.A.; Barragán, C.; Ovilo, C. Estimation of Genetic Variability of the Founder Population in a Conservation Scheme Using Microsatellites. Anim. Genet. 2003, 34, 226–228. [Google Scholar] [CrossRef]

| Breed | n | NHap | SNPs | π |
|---|---|---|---|---|
| Martina Franca (MF) | 27 | 22(5 s) | 13 | 0.162 ± 0.022 |
| Ragusano (RG) | 22 | 22(3 s) | 15 | 0.132 ± 0.028 |
| Pantesco (PT) | 8 | 6(3 s) | 1 | 0.025 ± 0.001 |
| Catalonian (CT) | 19 | 13(4 s) | 2 | 0.038 ± 0.009 |
| crossbreed | 1 | 1 | 1 | |
| ALL | 77 | 56 | 33 | 0.128 |
| SNP | Martina Franca (MF) | Catalano (CT) | Pantesco (PT) | Ragusano (RG) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | Reference | Mutation | Samples (n) | % | Samples (n) | % | Samples (n) | % | Samples (n) | % |
| 15719 | C | T | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 |
| 15699 | C | T | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15663 | A | G | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15653 | C | T | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15646 | A | G | 2 | 7 | 0 | 0 | 0 | 0 | 1 | 5 |
| 15645 | G | A | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15622 | A | G | 4 | 14 | 0 | 0 | 0 | 0 | 2 | 9 |
| 15600 | A | G | 29 | 100 | 19 | 100 | 8 | 100 | 17 | 77 |
| 15599 | C | T | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15581 | A | G | 6 | 21 | 11 | 58 | 0 | 0 | 4 | 18 |
| 15570 | A | G | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15524 | C | DEL | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 9 |
| 15504 | T | C | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15491 | C | T | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15485 | G | A | 6 | 21 | 0 | 0 | 0 | 0 | 4 | 18 |
| 15474 | C | T | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 9 |
| Breed | Mean within Groups |
| Martina Franca (MF) | 0.289 |
| Ragusano (RG) | 0.521 |
| Pantesco (PT) | 0.044 |
| Catalonian (CT) | 0.020 |
| Mean between Groups | |
| MF × CT | 0.162 |
| MF × RG | 0.387 |
| MF × PT | 0.158 |
| RG × CT | 0.276 |
| RG × PT | 0.278 |
| CT × PT | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzatenta, A.; Vignoli, M.; Caputo, M.; Vignola, G.; Tamburro, R.; De Sanctis, F.; Roig, J.M.; Bucci, R.; Robbe, D.; Carluccio, A. Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds. Genes 2021, 12, 1109. https://doi.org/10.3390/genes12081109
Mazzatenta A, Vignoli M, Caputo M, Vignola G, Tamburro R, De Sanctis F, Roig JM, Bucci R, Robbe D, Carluccio A. Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds. Genes. 2021; 12(8):1109. https://doi.org/10.3390/genes12081109
Chicago/Turabian StyleMazzatenta, Andrea, Massimo Vignoli, Maurizio Caputo, Giorgio Vignola, Roberto Tamburro, Francesco De Sanctis, Jordi Mirò Roig, Roberta Bucci, Domenico Robbe, and Augusto Carluccio. 2021. "Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds" Genes 12, no. 8: 1109. https://doi.org/10.3390/genes12081109
APA StyleMazzatenta, A., Vignoli, M., Caputo, M., Vignola, G., Tamburro, R., De Sanctis, F., Roig, J. M., Bucci, R., Robbe, D., & Carluccio, A. (2021). Maternal Phylogenetic Relationships and Genetic Variation among Rare, Phenotypically Similar Donkey Breeds. Genes, 12(8), 1109. https://doi.org/10.3390/genes12081109






