Comparative Transcriptome Analysis Reveals Sex-Based Differences during the Development of the Adult Parasitic Wasp Cotesia vestalis (Hymenoptera: Braconidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Staging of Wasp Pupae
2.2. Sample Collection
2.3. Illumina RNA-Seq Library Construction and Sequencing
2.4. Illumina Data Analysis
2.5. PacBio Library Construction and Sequencing
2.6. PacBio Data Analysis
2.7. LncRNA Identification and Function Analysis
2.8. Analysis of Differentially Expressed Genes
2.9. Function Annotation of Differentially Expressed Genes
2.10. Identification of Differential Alternative Splicing Events
2.11. RT-PCR Validation
2.12. Statistical Analysis and Data Presentation
3. Results
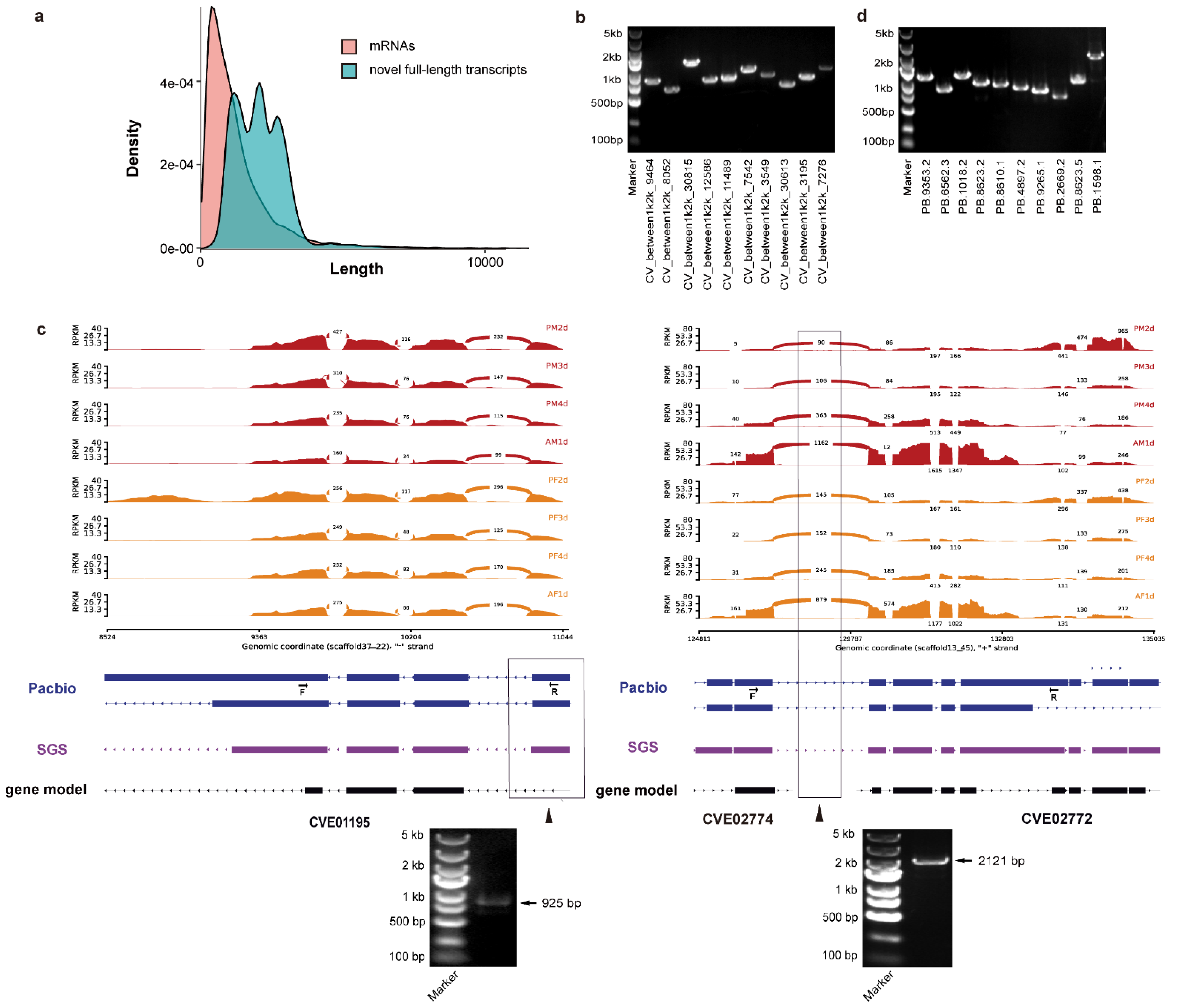
3.1. RNA-Seq Data Sequencing and Assembly
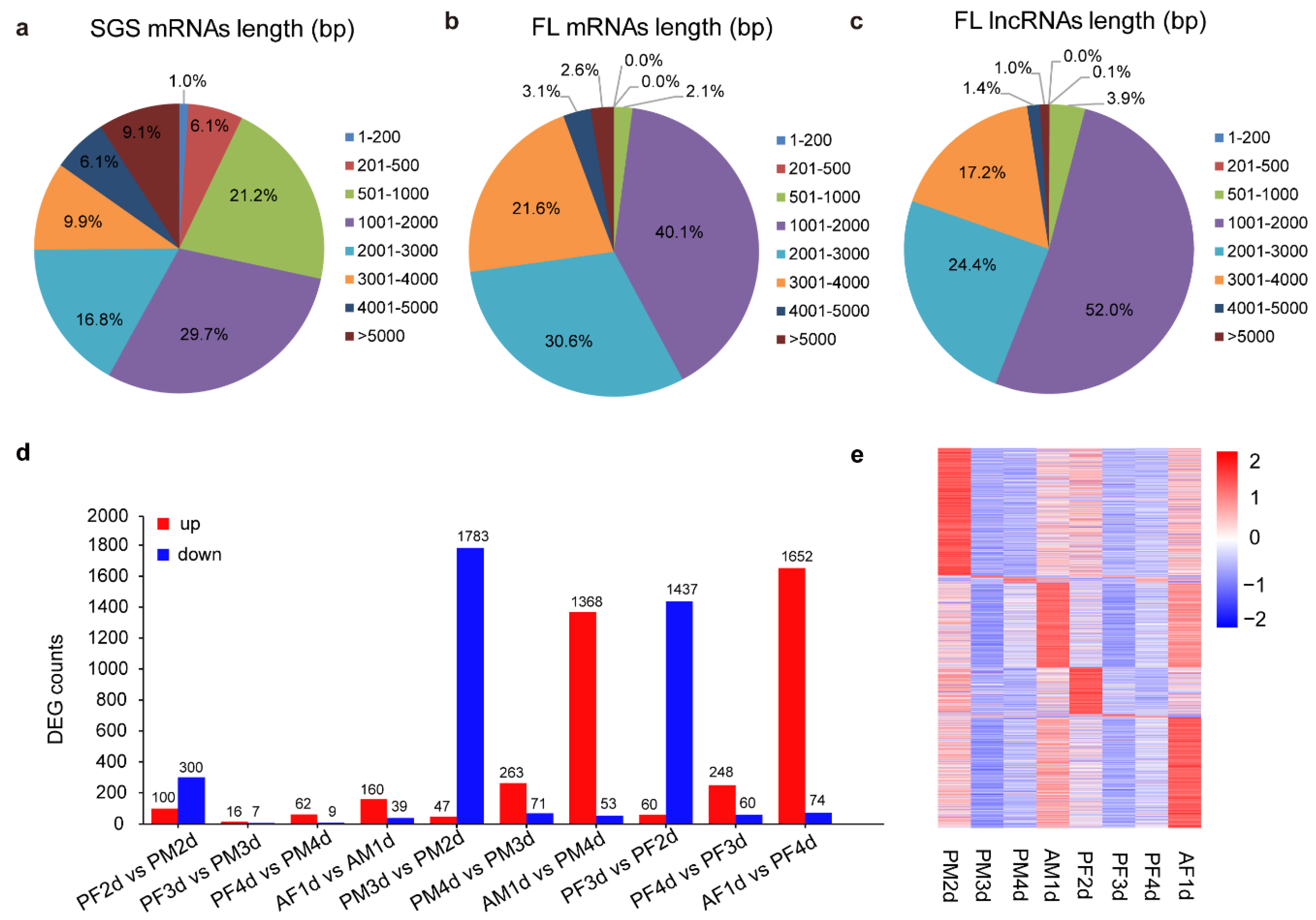
3.2. The Full-Length Sequencing by PacBio Iso-Seq
3.3. The Correction of C. vestalis Genome Annotation
3.4. LncRNA Identification, Expression Analysis, and Functional Prediction
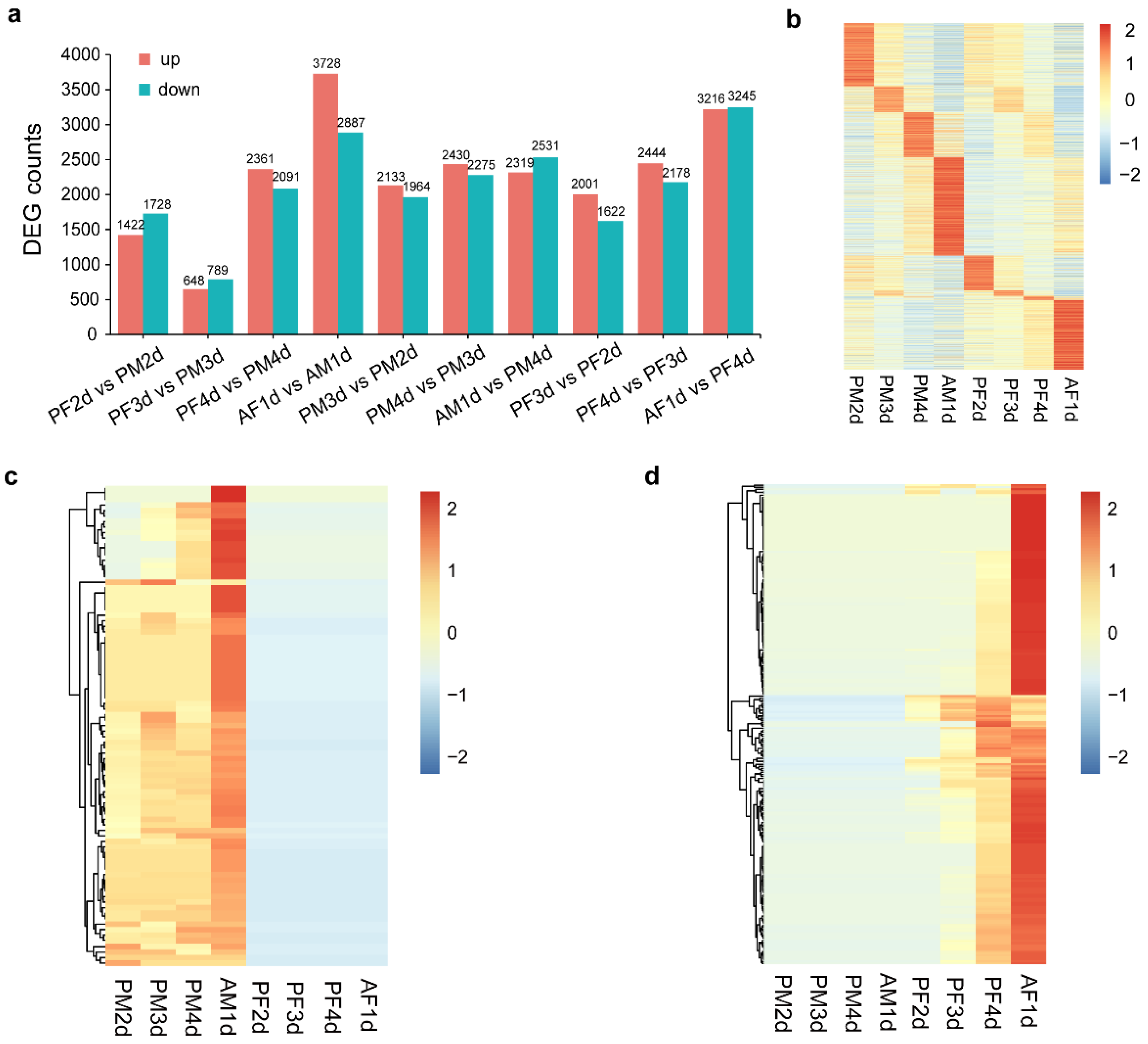
3.5. Analysis of Differentially Expressed Genes
3.6. GO and KEGG Enrichment of DEGs
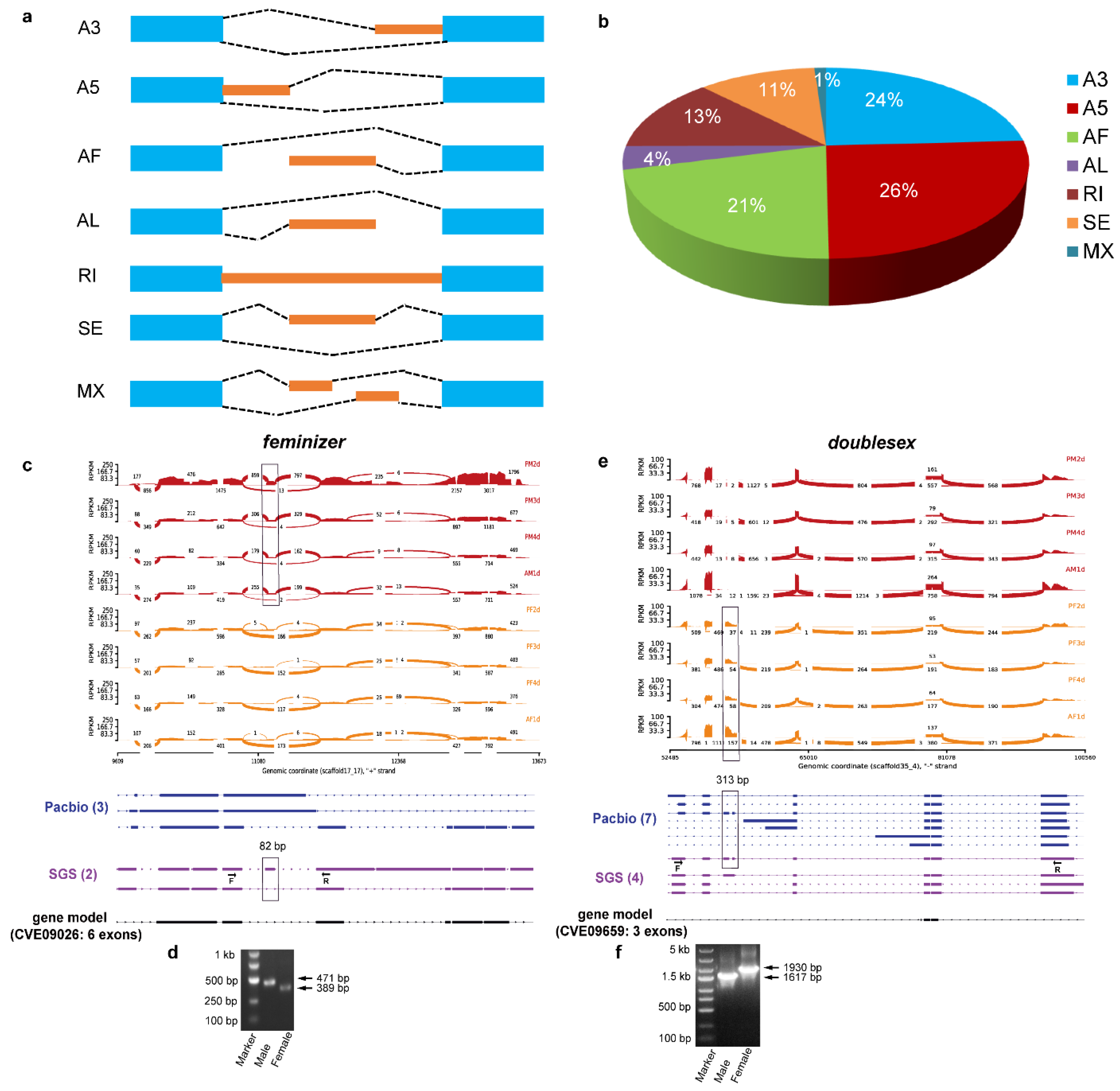
3.7. Identification of Alternative Splicing (AS) Events
3.8. Identification of Sex-Determining Genes and Their Transcripts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlesworth, B. The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 1996, 6, 149–162. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. The evolution of heteromorphic sex chromosomes. Biol. Rev. 1990, 65, 249–276. [Google Scholar] [CrossRef]
- Beye, M.; Hasselmann, M.; Fondrk, M.K.; Page, R.E., Jr.; Omholt, S.W. The Gene csd Is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 2003, 114, 419–429. [Google Scholar] [CrossRef]
- Van Wilgenburg, E.; Driessen, G.; Beukeboom, L.W. Single locus complementary sex determination in Hymenoptera: An “unintelligent” design? Front. Zool. 2006, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Heimpel, G.E.; De Boer, J.G. Sex determination in the hymenoptera. Annu. Rev. Entomol. 2008, 53, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Fu, X.N.; Zhu, J.S. Juvenile hormone-regulated alternative splicing of the taiman gene primes the ecdysteroid response in adult mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, E7738–E7747. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Harrison, M.; Hammond, R.; Adams, S.; Gutierrez-Marcos, J.; Mallon, E. Alternative splicing associated with phenotypic plasticity in the bumble bee Bombus terrestris. Mol. Ecol. 2018, 27, 1036–1043. [Google Scholar] [CrossRef]
- Goralski, T.J.; Edström, J.E.; Baker, B.S. The sex determination locus transformer-2 of Drosophila encodes a polypeptide with similarity to RNA binding proteins. Cell 1989, 56, 1011–1018. [Google Scholar] [CrossRef]
- Verhulst, E.C.; van de Zande, L.; Beukeboom, L.W. Insect sex determination: It all evolves around transformer. Curr. Opin. Genet. Dev. 2010, 20, 376–383. [Google Scholar] [CrossRef]
- Saccone, G.; Salvemini, M.; Polito, L.C. The transformer gene of Ceratitis capitata: A paradigm for a conserved epigenetic master regulator of sex determination in insects. Genetics 2010, 139, 99–111. [Google Scholar] [CrossRef]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef]
- Flynn, R.; Chang, H.Y. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell 2014, 14, 752–761. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Xu, W.Y.; Wang, Y.Y.; Zhang, H.; Zhang, L.; Li, C.; Yao, S.; Huang, Z.X.; Huang, L.S.; Luo, D.X. LncRNA DNM3OS regulates GREM2 via miR-127-5p to suppress early chondrogenic differentiation of rat mesenchymal stem cells under hypoxic conditions. Cell. Mol. Biol. Lett. 2021, 26, 22. [Google Scholar] [CrossRef]
- You, M.S.; Yue, Z.; He, W.Y.; Yang, X.H.; Yang, G.; Xie, M.; Zhan, D.L.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Ye, X.Q.; Shi, M.; Li, F.; Wang, Z.H.; Zhou, Y.N.; Gu, Q.J.; Wu, X.T.; Yin, C.L.; Guo, D.H.; et al. Parasitic insect-derived miRNAs modulate host development. Nat. Commun. 2018, 9, 2205. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A. Male-male sexual behavior in the parasitic wasp Psyttalia concolor. J. Insect Sci. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Sun, L.J.; Cao, D.P.; Walker, W.B.; Zhang, Y.Q.; Wang, G.R. Identification of candidate olfactory genes in Leptinotarsa decemlineata by antennal transcriptome analysis. Front. Ecol. Evol. 2015, 3, 60. [Google Scholar] [CrossRef]
- Liu, Y.P.; Du, L.X.; Zhu, Y.; Yang, S.Y.; Zhou, Q.; Wang, G.R.; Liu, Y. Identification and sex-biased profiles of candidate olfactory genes in the antennal transcriptome of the parasitoid wasp Cotesia vestalis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100657. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Xie, S.; Chen, J.N.; Wang, Z.H.; Yang, P.; Zhou, S.C.; Pang, L.; Li, F.; Shi, M.; Huang, J.H.; et al. Expression and functional characterization of odorant-binding protein genes in the endoparasitic wasp Cotesia vestalis. Insect Sci. 2020. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Z.Z.; Ye, X.Q.; Xie, H.Q.; Li, F.; Hu, X.X.; Wang, Z.H.; Yin, C.L.; Zhou, Y.N.; Gu, Q.J.; et al. The genomes of two parasitic wasps that parasitize the diamondback moth. BMC Genom. 2019, 20, 893. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shi, M.; Liu, P.C.; Huang, F.; Chen, X.X. Characterization of an IκB-like gene in Cotesia vestalis polydnavirus. Arch. Insect Biochem. Physiol. 2008, 68, 71–78. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.E.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Watanabe, C.K. GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Trincado, J.L.; Entizne, J.C.; Hysenaj, G.; Singh, B.; Skalic, M.; Elliott, D.J.; Eyras, E. SUPPA2: Fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 2018, 19, 40. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, M.; Ye, X.Q.; Li, F.; Wang, X.W.; Chen, X.X. Comparative transcriptome analysis of venom glands from Cotesia vestalis and Diadromus collaris, two endoparasitoids of the host Plutella xylostella. Sci. Rep. 2017, 7, 1298. [Google Scholar] [CrossRef]
- Chen, L.L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Gibb, E.; Brown, C.J.; Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 2011, 10, 38. [Google Scholar] [CrossRef]
- Lv, Y.D.; Liang, Z.K.; Ge, M.; Qi, W.C.; Zhang, T.F.; Lin, F.; Peng, Z.; Peng, Z.H. Genome-wide identification and functional prediction of nitrogen-responsive intergenic and intronic long non-coding RNAs in maize (Zea mays L.). BMC Genom. 2016, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wan, X.C.; Zhang, Y.L.; Kong, Z.; Lu, Y.L.; Sun, X.; Huang, Y.; Ji, C.N.; Li, D.J.; Luo, J.; et al. A novel androgen-reduced prostate-specific lncRNA, PSLNR, inhibits prostate-cancer progression in part by regulating the p53-dependent pathway. Prostate 2019, 79, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.J.; Yang, L.J.; Xiong, T.L.; Di, C.; Ma, D.H.; Wu, M.H.; Xue, Z.Y.; Zhang, X.D.; Long, L.; Zhang, W.M.; et al. Critical roles of long noncoding RNAs in Drosophila spermatogenesis. Genome Res. 2016, 26, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, T.T.; He, W.Y.; Shen, X.J.; Zhao, Q.; Bai, J.L.; You, M.S. Genome-wide identification and characterization of putative lncRNAs in the diamondback moth, Plutella xylostella (L.). Genomics 2018, 110, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Chen, H.Z.; Du, Y.; Zhou, D.D.; Geng, S.H.; Wang, H.P.; Wan, J.Q.; Xiong, C.L.; Zheng, Y.Z.; Guo, R. Genome-Wide Identification of Long Non-Coding RNAs and Their Regulatory Networks Involved in Apis mellifera ligustica Response to Nosema ceranae Infection. Insects 2019, 10, 245. [Google Scholar] [CrossRef]
- Hong, F.; Mo, S.H.; Lin, X.Y.; Niu, J.Z.; Yin, J.; Wei, D. The PacBio Full-Length Transcriptome of the Tea Aphid as a Reference Resource. Front. Genet. 2020, 11, 558394. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.P.; Richards, E.H.; Isaac, R.E.; Edwards, J.P. Antibacterial and proteolytic activity in venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae). J. Insect Physiol. 2003, 49, 945–954. [Google Scholar] [CrossRef]
- Lin, H.H.; Cao, D.S.; Sethi, S.; Zeng, Z.; Chin, J.S.; Chakraborty, T.S.; Shepherd, A.K.; Nguyen, C.A.; Yew, J.Y.; Su, C.Y.; et al. Hormonal modulation of pheromone detection enhances male courtship success. Neuron 2016, 90, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for Insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- Burke, G.R.; Strand, M.R. Deep sequencing identifies viral and wasp genes with potential roles in replication of Microplitis demolitor bracovirus. J. Virol. 2012, 86, 3293–3306. [Google Scholar] [CrossRef]
- Wyler, T.; Lanzrein, B. Ovary development and polydnavirus morphogenesis in the parasitic wasp Chelonus inanitus. II. Ultrastructural analysis of calyx cell development, virion formation and release. J. Gen. Virol. 2003, 84, 1151–1163. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, W.M.; He, W.Y.; Li, Y.Y.; Li, Y.Q.; Li, T.P.; Vasseur, L.; You, M.S. Genome-wide profiling of the alternative splicing provides insights into development in Plutella xylostella. BMC Genom. 2019, 20, 463. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Chen, Z.J.; Wei, F.G.; Liu, Y.L.; Gao, L.Z. SMRT- and Illumina-based RNA-seq analyses unveil the ginsinoside biosynthesis and transcriptomic complexity in Panax notoginseng. Sci. Rep. 2020, 10, 15310. [Google Scholar] [CrossRef]
- de Boer, J.G.; Ode, P.J.; Rendahl, A.K.; Vet, L.; Whitfield, J.B.; Heimpel, G.E. Experimental support for multiple-locus complementary sex determination in the parasitoid Cotesia vestalis. Genetics 2008, 180, 1525–1535. [Google Scholar] [CrossRef][Green Version]
- Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003, 72, 291–336. [Google Scholar] [CrossRef]
- Shukla, J.N.; Nagaraju, J. Doublesex: A conserved downstream gene controlled by diverse upstream regulators. J. Genet. 2010, 89, 341–356. [Google Scholar] [CrossRef]
- Nguantad, S.; Chumnanpuen, P.; Thancharoen, A.; Vongsangnak, W.; Sriboonlert, A. Identification of potential candidate genes involved in the sex determination cascade in an aquatic firefly, Sclerotia aquatilis (Coleoptera, Lampyridae). Genomics 2020, 112, 2590–2602. [Google Scholar] [CrossRef]
- Lin, J.; He, J.Y.; Liang, A.W.; Wang, F.H. Transcriptome profiling and dimorphic expression of sex-related genes in fifth-instar nymphs of Sogatella furcifera, an important rice pest. Genomics 2020, 112, 1105–1111. [Google Scholar] [CrossRef]
- Hasselmann, M.; Gempe, T.; Schiøtt, M.; Nunes-Silva, C.G.; Otte, M.; Beye, M. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 2008, 454, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Vasconcelos, M.L.; Ruta, V.; Luo, S.; Wong, A.; Demir, E.; Flores, J.; Balonze, K.; Dickson, B.J.; Axel, R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 2008, 452, 473–477. [Google Scholar] [CrossRef]
- Clyne, J.D.; Miesenböck, G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 2008, 133, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Clynen, E.; Ciudad, L.; Belles, X.; Piulachs, M.-D. Conservation of fruitless’ role as master regulator of male courtship behaviour from cockroaches to flies. Dev. Genes Evol. 2011, 221, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Meier, N.; Käppeli, S.C.; Niessen, M.H.; Billeter, J.C.; Goodwin, S.F.; Bopp, D. Genetic control of courtship behavior in the housefly: Evidence for a conserved bifurcation of the sex-determining pathway. PLoS ONE 2013, 8, e62476. [Google Scholar] [CrossRef]
| PacBio | SGS | Combination | ||||
|---|---|---|---|---|---|---|
| Type | Genes | Events | Genes | Events | Genes | Events |
| A3 | 412 | 644 (13%) | 1803 | 2222 (33%) | 2092 | 2866 (24%) |
| A5 | 496 | 793 (16%) | 1874 | 2247 (33%) | 2265 | 3040 (26%) |
| AF | 327 | 1889 (38%) | 472 | 615 (9%) | 792 | 2504 (21%) |
| AL | 63 | 289 (6%) | 140 | 186 (3%) | 194 | 475 (4%) |
| RI | 542 | 930 (18%) | 523 | 582 (9%) | 1065 | 1512 (13%) |
| SE | 296 | 425 (8%) | 781 | 904 (13%) | 973 | 1329 (11%) |
| MX | 45 | 73 (1%) | 52 | 57 (1%) | 97 | 130 (1%) |
| Total | 1331 | 5043 | 4188 | 6813 | 4819 | 11,856 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, P.; Xie, S.; Shi, M.; Huang, J.; Wang, Z.; Chen, X. Comparative Transcriptome Analysis Reveals Sex-Based Differences during the Development of the Adult Parasitic Wasp Cotesia vestalis (Hymenoptera: Braconidae). Genes 2021, 12, 896. https://doi.org/10.3390/genes12060896
Zhou Y, Yang P, Xie S, Shi M, Huang J, Wang Z, Chen X. Comparative Transcriptome Analysis Reveals Sex-Based Differences during the Development of the Adult Parasitic Wasp Cotesia vestalis (Hymenoptera: Braconidae). Genes. 2021; 12(6):896. https://doi.org/10.3390/genes12060896
Chicago/Turabian StyleZhou, Yuenan, Pei Yang, Shuang Xie, Min Shi, Jianhua Huang, Zhizhi Wang, and Xuexin Chen. 2021. "Comparative Transcriptome Analysis Reveals Sex-Based Differences during the Development of the Adult Parasitic Wasp Cotesia vestalis (Hymenoptera: Braconidae)" Genes 12, no. 6: 896. https://doi.org/10.3390/genes12060896
APA StyleZhou, Y., Yang, P., Xie, S., Shi, M., Huang, J., Wang, Z., & Chen, X. (2021). Comparative Transcriptome Analysis Reveals Sex-Based Differences during the Development of the Adult Parasitic Wasp Cotesia vestalis (Hymenoptera: Braconidae). Genes, 12(6), 896. https://doi.org/10.3390/genes12060896







