Molecular Cloning and Functional Characterization of Three 5-HT Receptor Genes (HTR1B, HTR1E, and HTR1F) in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Primers, Peptides, and Antibodies
2.2. Animals and Tissues
2.3. Reverse Transcription (RT) and Quantitative Real-Time PCR
2.4. Phylogenetic Analysis
2.5. Rapid Amplification of 5′- and 3′-cDNA Ends (RACE) and Construction of Plasmids
2.6. Functional Characterization of Chicken HTR1 Receptors
2.7. Western Blot
2.8. Effect of 5-HT, CP94253, BRL54443 and LY344864 on PRL Secretion in Cultured Chick Pituitary Cells
2.9. Data Analysis
3. Results
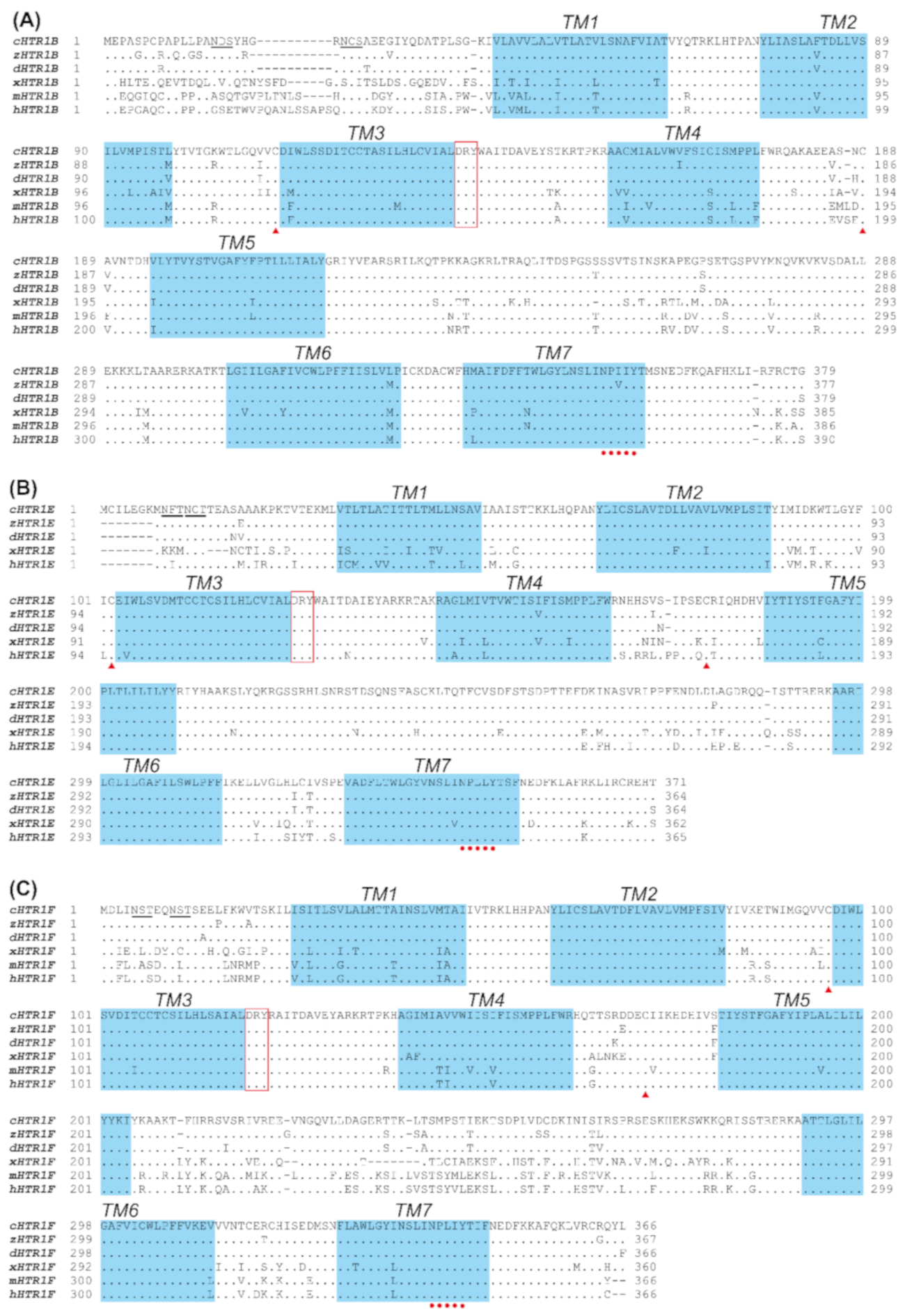
3.1. Molecular Cloning of HTR1B, HTR1E, and HTR1F in Chickens
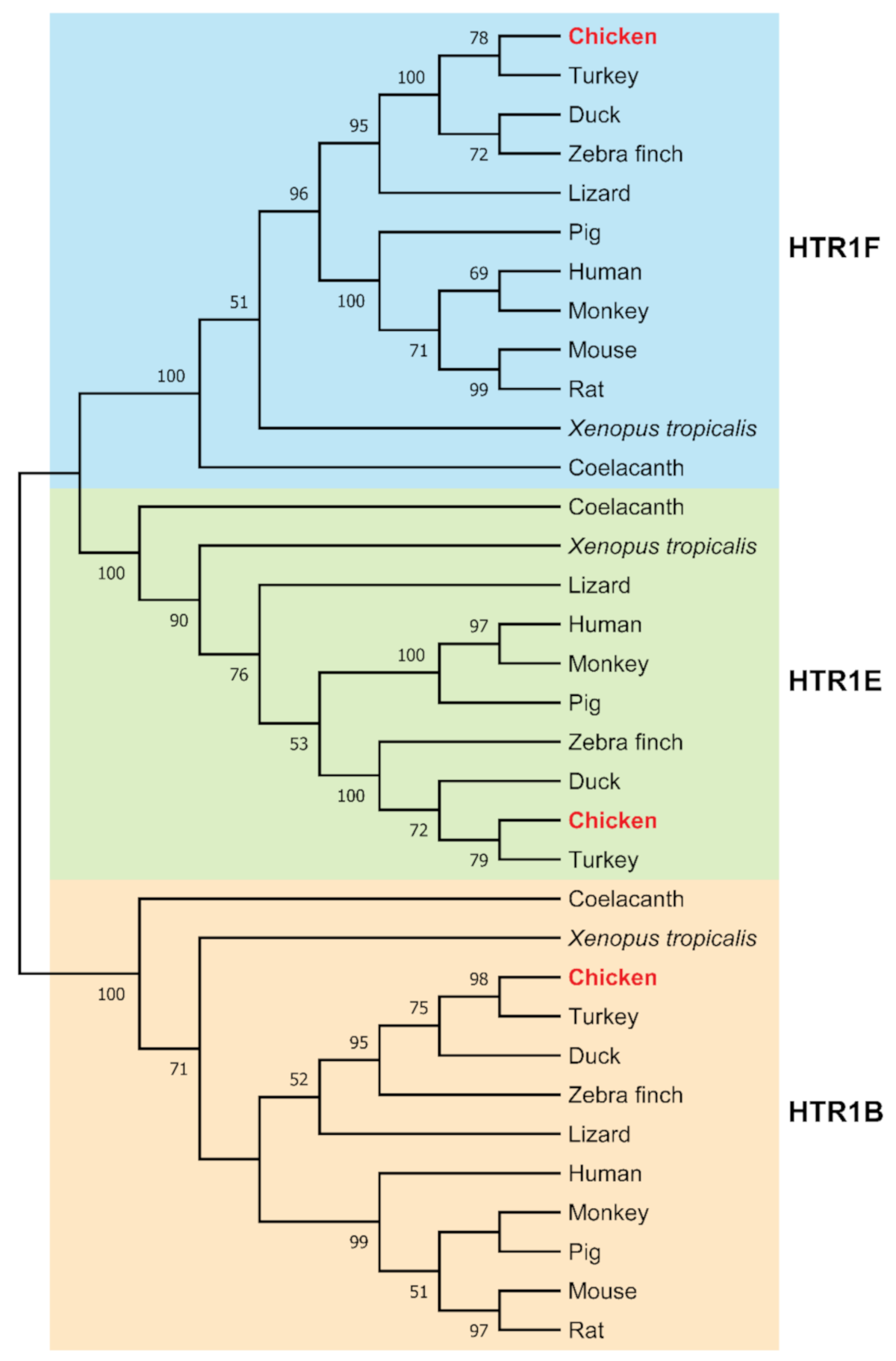
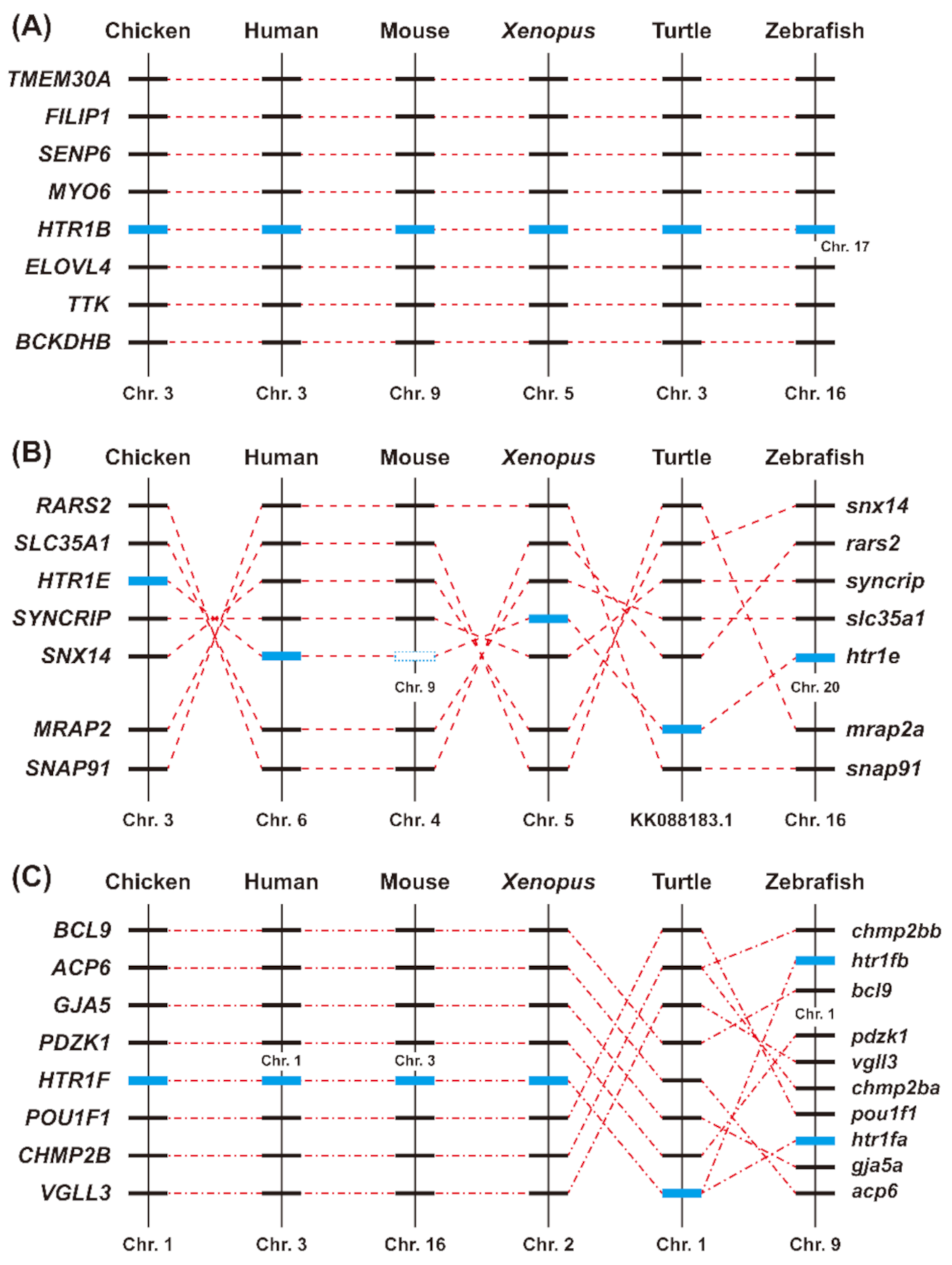
3.2. Phylogenetic Analysis and Synteny Analysis of HTR1B, HTR1E, and HTR1F in Vertebrates
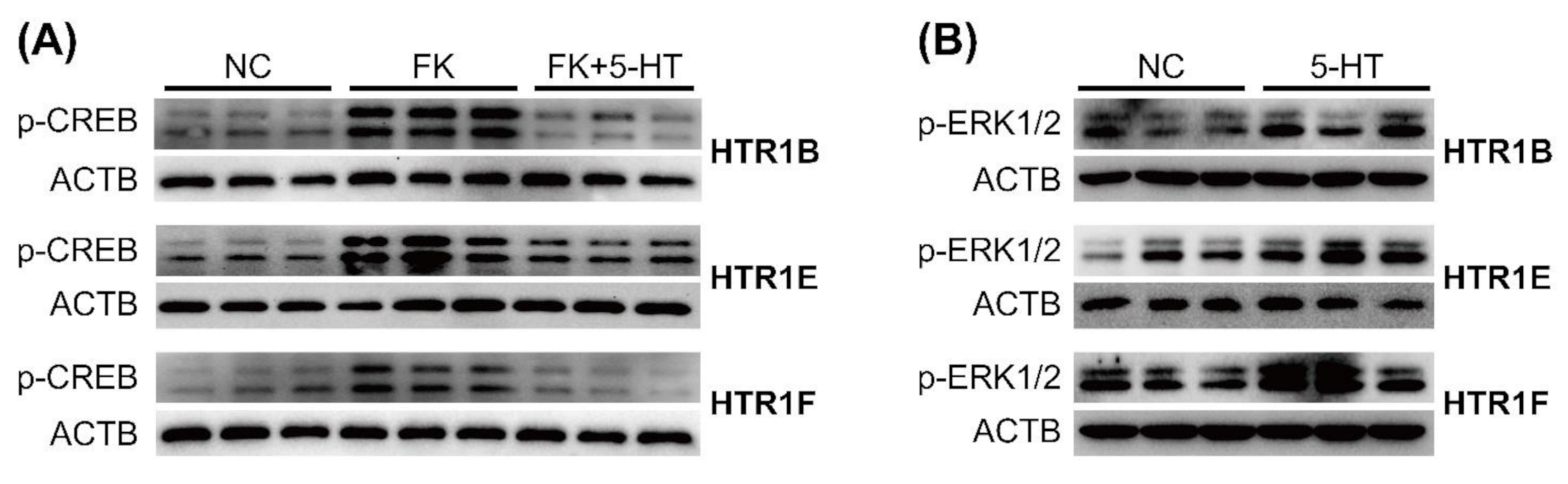
3.3. Functional Characterization of HTR1B, HTR1E, and HTR1F in Cultured HEK293 Cells
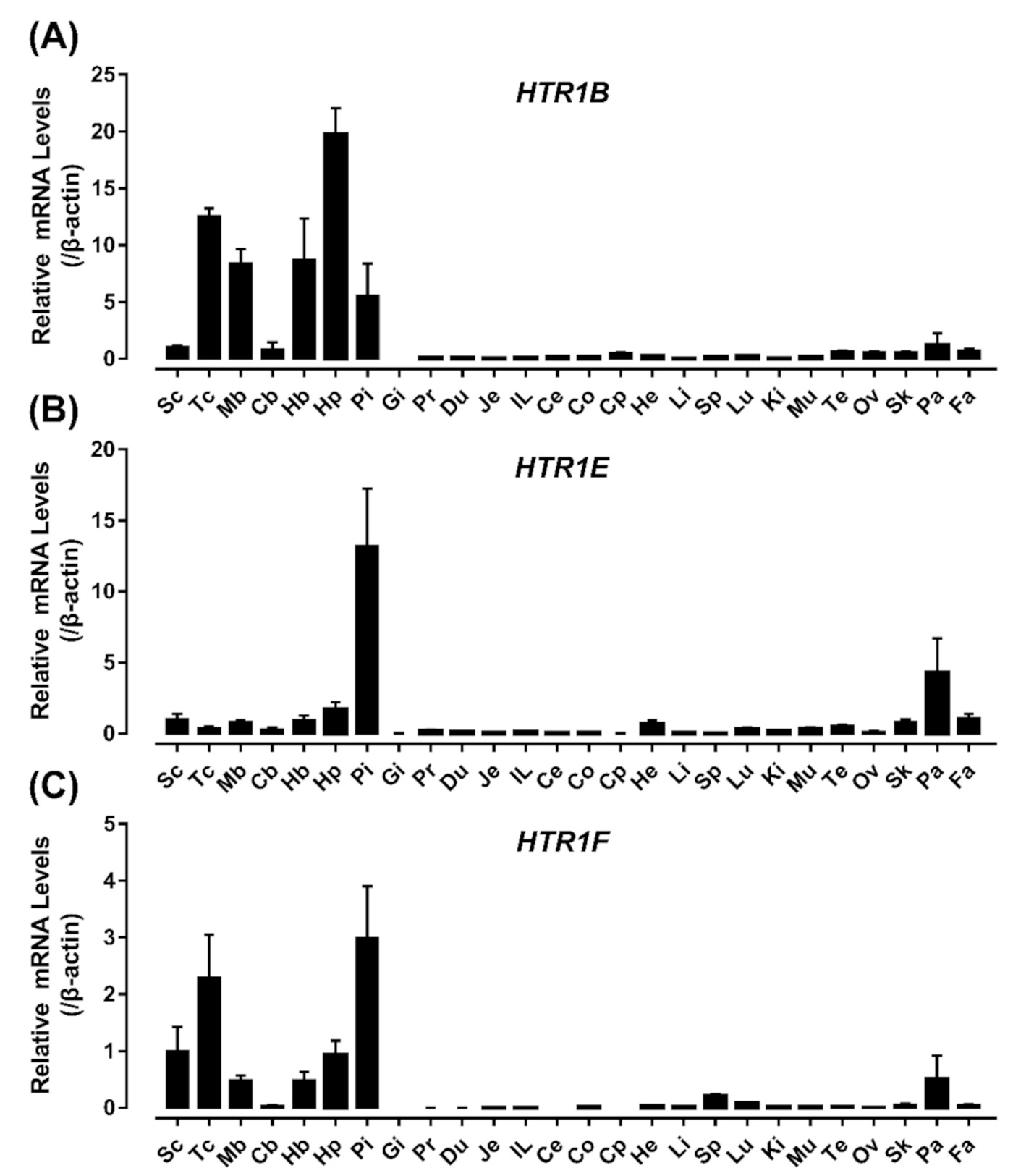
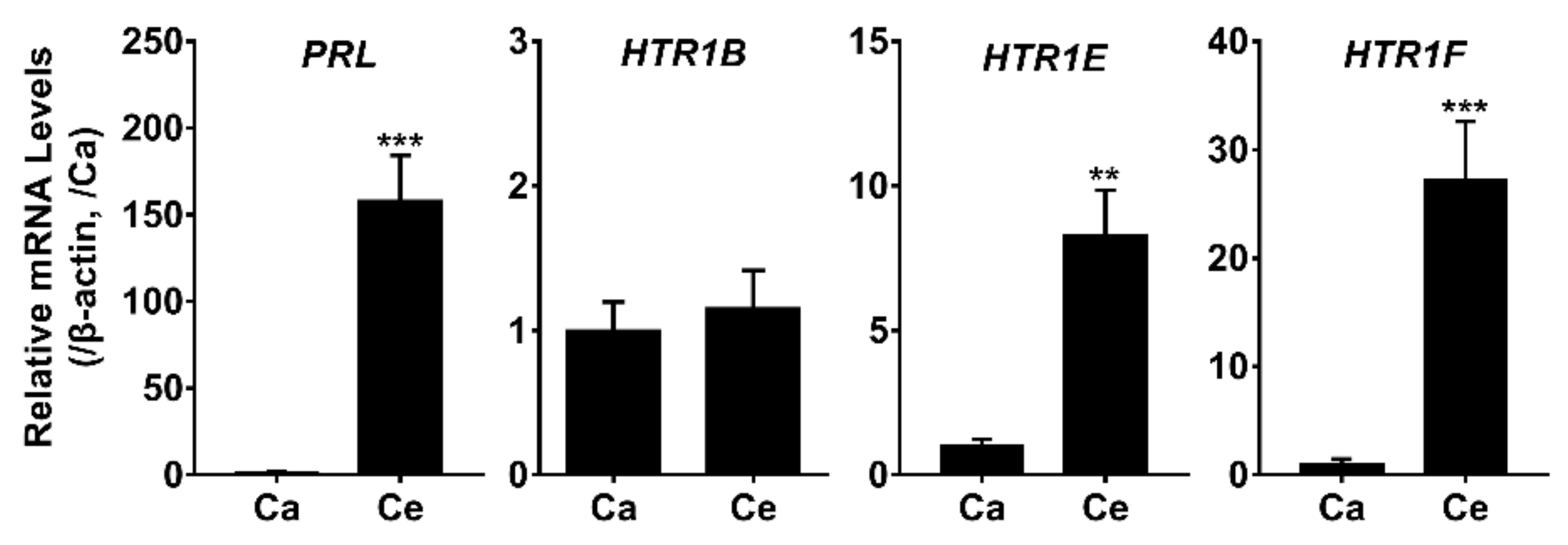
3.4. Tissue Distribution of HTR1B, HTR1E, and HTR1F in Chickens
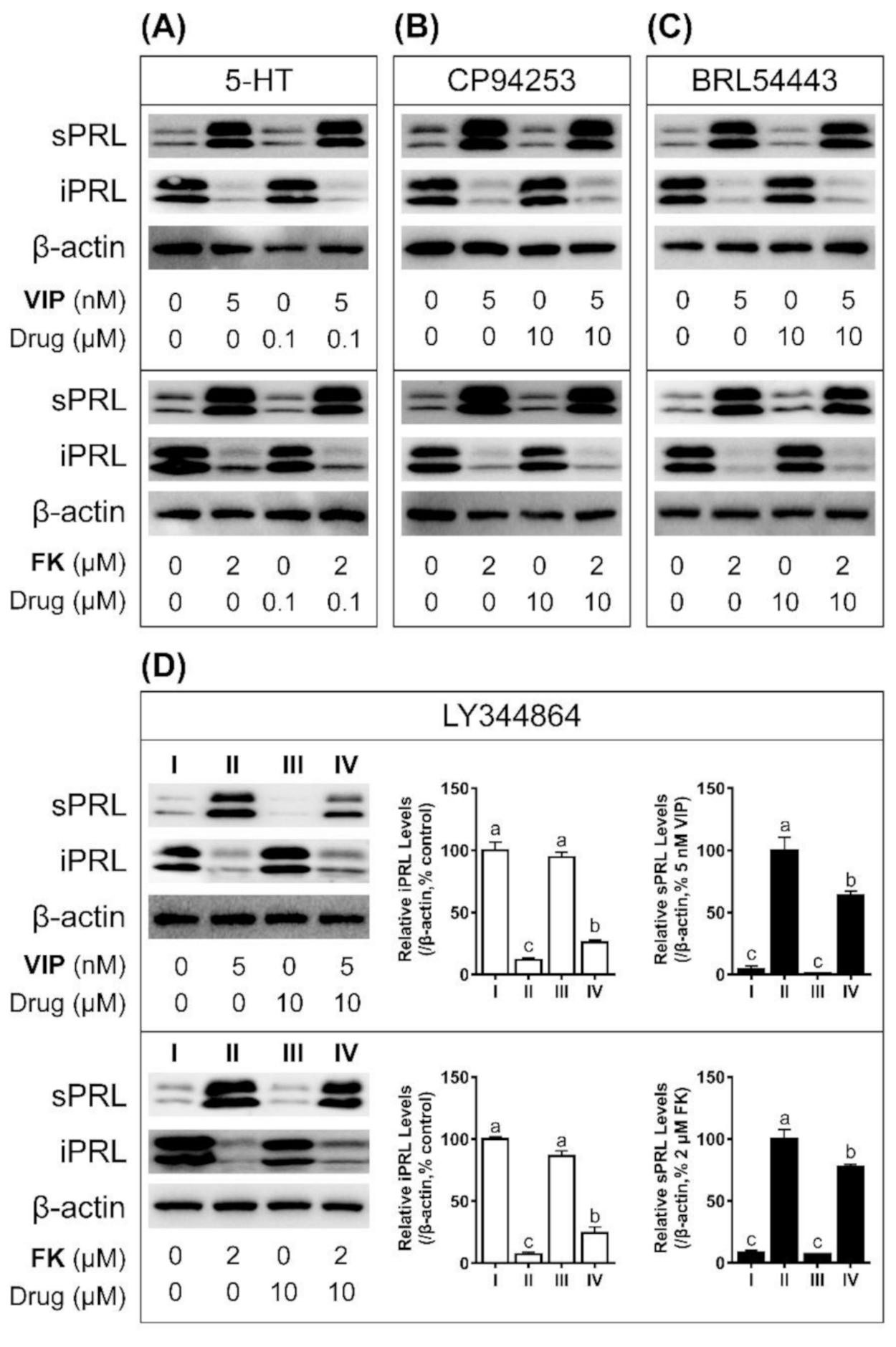
3.5. LY344864 Inhibits VIP-Induced PRL Secretion in Cultured Chicken Pituitary Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A. Structure and function of invertebrate 5-HT receptors: A review. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 791–804. [Google Scholar] [CrossRef]
- Thompson, A.J.; R Lummis, S. 5-HT3 receptors. Curr. Pharm. Des. 2006, 12, 3615–3630. [Google Scholar] [CrossRef]
- Derkach, V.; Surprenant, A.; North, R. 5-HT3 receptors are membrane ion channels. Nature 1989, 339, 706–709. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Voigt, M.M.; Laurie, D.J.; Seeburg, P.H.; Bach, A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991, 10, 4017–4023. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog. Brain Res. 2008, 172, 625–646. [Google Scholar]
- Fink, K.B.; Göthert, M.J.P.r. 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 2007, 59, 360–417. [Google Scholar] [CrossRef]
- Hållbus, M.; Magnusson, T.; Magnusson, O. Influence of 5-HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: A microdialysis study. Neurosci. Lett. 1997, 225, 57–60. [Google Scholar] [CrossRef]
- Shimron-Abarbanell, D.; Nöthen, M.M.; Erdmann, J.; Propping, P. Lack of genetically determined structural variants of the human serotonin-1E (5-HT1E) receptor protein points to its evolutionary conservation. Mol. Brain Res. 1995, 29, 387–390. [Google Scholar] [CrossRef]
- Amlaiky, N.; Ramboz, S.; Boschert, U.; Plassat, J.L.; Hen, R. Isolation of a mouse “5HT1E-like” serotonin receptor expressed predominantly in hippocampus. J. Biol. Chem. 1992, 267, 19761–19764. [Google Scholar] [CrossRef]
- Adham, N.; Kao, H.-T.; Schecter, L.; Bard, J.; Olsen, M.; Urquhart, D.; Durkin, M.; Hartig, P.R.; Weinshank, R.L.; Branchek, T.A. Cloning of another human serotonin receptor (5-HT1F): A fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 408–412. [Google Scholar] [CrossRef]
- Usman, H.O.; Balaban, C.D. Distribution of 5-HT1F receptors in monkey vestibular and trigeminal ganglion cells. Front. Neurol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Silberstei, S.D. Serotonin (5-HT) and migraine. Headache J. Head Face Pain 1994, 34, 408–417. [Google Scholar] [CrossRef]
- Reuter, U.; Israel, H.; Neeb, L. The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migraine. Ther. Adv. Neurol. Disord. 2015, 8, 46–54. [Google Scholar] [CrossRef]
- Goldstein, D.; Roon, K.; Offen, W.; Ramadan, N.; Phebus, L.; Johnson, K.; Schaus, J.; Ferrari, M. Selective seratonin 1F (5-HT1F) receptor agonist LY334370 for acute migraine: A randomised controlled trial. Lancet 2001, 358, 1230–1234. [Google Scholar] [CrossRef]
- Amrutkar, D.V.; Ploug, K.B.; Hay-Schmidt, A.; Porreca, F.; Olesen, J.; Jansen-Olesen, I.J.P. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene–related peptide in the rat trigeminovascular system. Pain 2012, 153, 830–838. [Google Scholar] [CrossRef]
- Almaça, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.O.; Caicedo, A. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016, 17, 3281–3291. [Google Scholar] [CrossRef]
- Denbow, D.M.; Van Krey, H.P.; Cherry, J.A. Feeding and drinking response of young chicks to injections of serotonin into the lateral ventricle of the brain. Poult. Sci. 1982, 61, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.F.; Huston, T.M. Inhibitory effect of serotonin on ovulation in the domestic fowl, Gallus domesticus. Anim. Reprod. Sci. 1978, 1, 69–73. [Google Scholar] [CrossRef]
- Hall, T.R.; Harvey, S.; Chadwick, A. Serotonin and acetylcholine affect the release of prolactin and growth hormone from pituitary glands of domestic fowl in vitro in the presence of hypothalamic tissue. Eur. J. Endocrinol. 1984, 105, 455–462. [Google Scholar] [CrossRef]
- Dennis, R.L.; Chen, Z.Q.; Cheng, H.W. Serotonergic mediation of aggression in high and low aggressive chicken strains. Poult. Sci. 2008, 87, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Preuss, S.; Stratz, P.; Bennewitz, J. Investigating gene expression differences in two chicken groups with variable propensity to feather pecking. Anim. Genet. 2013, 44, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Stępińska, U.; Kuwana, T.; Olszańska, B. Serotonin receptors are selectively expressed in the avian germ cells and early embryos. Zygote 2015, 23, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Zhou, Y.; Cui, L.; Li, J.; Wu, C.; Wan, Y.; Li, J.; Wang, Y. The interaction of MC3R and MC4R with MRAP2, ACTH, α-MSH and AgRP in chickens. J. Endocrinol. 2017, 234, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Deng, Y.; Zhang, Z.; Cao, B.; Li, J.; Sun, C.; Hu, Z.; Zhang, J.; Li, J.; Wang, Y. Melanocortin Receptor 4 (MC4R) Signaling System in Nile Tilapia. Int. J. Mol. Sci. 2020, 21, 7036. [Google Scholar] [CrossRef]
- Wu, C.; Lv, C.; Wan, Y.; Li, X.; Zhang, J.; Li, J.; Wang, Y. Arginine vasotocin (AVT)/mesotocin (MT) receptors in chickens: Evidence for the possible involvement of AVT-AVPR1 signaling in the regulation of oviposition and pituitary prolactin expression. Gen. Comp. Endocrinol. 2019, 281, 91–104. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, Y.; Fang, C.; Chen, J.; Ouyang, W.; Li, J.; Wang, Y. The orphan G protein-coupled receptor 25 (GPR25) is activated by Apelin and Apela in non-mammalian vertebrates. Biochem. Biophys. Res. Commun. 2018, 501, 408–414. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Wu, C.; Wan, Y.; Fang, C.; Li, J.; Fang, W.; Yi, R.; Zhu, G.; Li, J. Characterization of the Apelin/Elabela Receptors (APLNR) in Chickens, Turtles, and Zebrafish: Identification of a Novel Apelin-Specific Receptor in Teleosts. Front. Endocrinol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, J.; He, C.; Meng, F.; Bu, G.; Zhu, G.; Li, J.; Wang, Y. Molecular characterization of neuropeptide Y (NPY) receptors (Y1, Y4 and Y6) and investigation of the tissue expression of their ligands (NPY, PYY and PP) in chickens. Gen. Comp. Endocrinol. 2017, 240, 46–60. [Google Scholar] [CrossRef]
- Bu, G.; Wang, C.Y.; Cai, G.; Leung, F.C.; Xu, M.; Wang, H.; Huang, G.; Li, J.; Wang, Y. Molecular characterization of prolactin receptor (cPRLR) gene in chickens: Gene structure, tissue expression, promoter analysis, and its interaction with chicken prolactin (cPRL) and prolactin-like protein (cPRL-L). Mol. Cell. Endocrinol. 2013, 370, 149–162. [Google Scholar] [CrossRef]
- Bu, G.; Lin, D.; Cui, L.; Huang, L.; Lv, C.; Huang, S.; Wan, Y.; Fang, C.; Li, J.; Wang, Y. Characterization of neuropeptide B (NPB), neuropeptide W (NPW), and their receptors in chickens: Evidence for NPW being a novel inhibitor of pituitary GH and prolactin secretion. Endocrinology 2016, 157, 3562–3576. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Vincens, P.; Roest Crollius, H.; Louis, A. Genomicus 2018: Karyotype evolutionary trees and on-the-fly synteny computing. Nucleic Acids Res. 2018, 46, D816–D822. [Google Scholar] [CrossRef]
- Lee, M.D.; Simansky, K.J. CP-94,253: A selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology 1997, 131, 264–270. [Google Scholar] [CrossRef]
- Tatarczyńska, E.; Antkiewicz-Michaluk, L.; Kłodzińska, A.; Stachowicz, K.; Chojnacka-Wójcik, E. Antidepressant-like effect of the selective 5-HT1B receptor agonist CP 94253: A possible mechanism of action. Eur. J. Pharmacol. 2005, 516, 46–50. [Google Scholar] [CrossRef]
- Garcia, R.; Cotter, A.; Leslie, K.; Olive, M. The selective 5-HT1B receptor agonist, CP 94,253, atten-uates methamphetamine self-administration in male rats. J. Int. Neuropsychopharmacol. 2017, 20, 644–653. [Google Scholar] [CrossRef]
- Lightowler, S.; Stean, T.; Upton, N.; Vimal, M.; Kennett, G.; Porter, R.; Brown, A. Effect of BRL 54443 (3-(-1-methylpiperidin-4-yl)-1H-indol-5-ol), a 5-ht1E/1F receptor agonist, on general behaviour and maximal electroshock seizure threshold in the rat. Br. J. Pharmacol. Proc. Suppl. 1998, 237P. [Google Scholar]
- Klein, M.T.; Dukat, M.; Glennon, R.A.; Teitler, M. Toward selective drug development for the human 5-hydroxytryptamine 1E receptor: A comparison of 5-hydroxytryptamine 1E and 1F receptor structure-affinity relationships. J. Pharmacol. Exp. Ther. 2011, 337, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Avenell, K.; Young, T.; Ho, M.; Porter, R.; Vimal, M.; Middlemiss, D. BRL 54443, a potent agonist with selectivity for human cloned 5-ht1E and 5-ht1F receptors. Br. J. Pharmacol. Proc. Suppl. 1998, 233P. [Google Scholar]
- Cohen, M.L.; Schenck, K. 5-Hydroxytryptamine1F receptors do not participate in vasoconstriction: Lack of vasoconstriction to LY344864, a selective serotonin1F receptor agonist in rabbit saphenous vein. J. Pharmacol. Exp. 1999, 290, 935–939. [Google Scholar]
- Shahidi, S.; Sadeghian, R.; Komaki, A.; Asl, S.S. Intracerebroventricular microinjection of the 5-HT1F receptor agonist LY 344864 inhibits methamphetamine conditioned place preference reinstatement in rats. Pharmacol. Biochem. Behav. 2018, 173, 27–35. [Google Scholar] [CrossRef]
- Vila-Pueyo, M. Targeted 5-HT 1F therapies for migraine. Neurotherapeutics 2018, 15, 291–303. [Google Scholar] [CrossRef]
- Boess, F.; Martin, I. Molecular biology of 5-HT receptors. Neuropharmacology 1994, 33, 275–317. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Seuwen, K.; Magnaldo, I.; Pouysségur, J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5–HT 1B receptors coupled to a G i-protein. Nature 1988, 335, 254–256. [Google Scholar] [CrossRef]
- Schoeffter, P.; Hoyer, D. 5-Hydroxytryptamine 5-HT 1B and 5-HT 1D receptors mediating inhibition of adenylate cyclase activity. Naunyn-Schmiedeberg′s Arch. Pharmacol. 1989, 340, 285–292. [Google Scholar] [CrossRef]
- Lione, A.M.; Errico, M.; Lin, S.L.; Cowen, D.S. Activation of Extracellular Signal-Regulated Kinase (ERK) and Akt by Human Serotonin 5-HT1B Receptors in Transfected BE (2)-C Neuroblastoma Cells Is Inhibited by RGS4. J. Neurochem. 2000, 75, 934–938. [Google Scholar] [CrossRef]
- Mendez, J.; Kadia, T.M.; Somayazula, R.K.; El-Badawi, K.I.; Cowen, D.S. Differential coupling of serotonin 5-HT1A and 5-HT1B receptors to activation of ERK2 and inhibition of adenylyl cyclase in transfected CHO cells. J. Neurochem. 1999, 73, 162–168. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef]
- Bai, F.; Yin, T.; Johnstone, E.M.; Su, C.; Varga, G.; Little, S.P.; Nelson, D.L. Molecular cloning and pharmacological characterization of the guinea pig 5-HT1E receptor. Eur. J. Pharmacol. 2004, 484, 127–139. [Google Scholar] [CrossRef]
- Janssen, P.; Tack, J.; Sifrim, D.; Meulemans, A.L.; Lefebvre, R.A. Influence of 5-HT1 receptor agonists on feline stomach relaxation. Eur. J. Pharmacol. 2004, 492, 259–267. [Google Scholar] [CrossRef]
- Garrett, S.M.; Whitaker, R.M.; Beeson, C.C.; Schnellmann, R.G. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 2014, 350, 257–264. [Google Scholar] [CrossRef]
- Xu, J.; Yao, B.; Fan, X.; Langworthy, M.M.; Zhang, M.-Z.; Harris, R.C. Characterization of a putative intrarenal serotonergic system. Am. J. Physiol. Ren. Physiol. 2007, 293, F1468–F1475. [Google Scholar] [CrossRef]
- Albert, P.R.; Tiberi, M. Receptor signaling and structure: Insights from serotonin-1 receptors. Trends Endocrinol. Metab. 2001, 12, 453–460. [Google Scholar] [CrossRef]
- Bhalla, P.; Sharma, H.S.; Ma, X.; Wurch, T.; Pauwels, P.J.; Saxena, P.R. Molecular cloning, pharmacological properties and tissue distribution of the porcine 5-HT1B receptor. Br. J. Pharmacol. 2001, 133, 891–901. [Google Scholar] [CrossRef]
- Bonaventure, P.; Voorn, P.; Luyten, W.; Jurzak, M.; Schotte, A.; Leysen, J. Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: Clues for function. Neuroscience 1997, 82, 469–484. [Google Scholar] [CrossRef]
- Varnäs, K.; Hurd, Y.L.; Hall, H. Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse 2005, 56, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.H.; Folchert, A.; Bally-Cuif, L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp. Neurol. 2008, 511, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Salichon, N.; Gaspar, P.; Upton, A.L.; Picaud, S.; Hanoun, N.; Hamon, M.; De Maeyer, E.; Murphy, D.L.; Mössner, R.; Lesch, K.P. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J. Neurosci. 2001, 21, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Aoki, N.; Mori, C.; Fujita, E.; Matsushima, T.; Homma, K.J.; Yamaguchi, S. The dorsal arcopallium of chicks displays the expression of orthologs of mammalian fear related serotonin receptor subfamily genes. Sci. Rep. 2020, 10, 21183. [Google Scholar] [CrossRef]
- Klein, M.; Teitler, M. Distribution of 5-ht1E receptors in the mammalian brain and cerebral vasculature: An immunohistochemical and pharmacological study. Br. J. Pharmacol. 2012, 166, 1290–1302. [Google Scholar] [CrossRef]
- Bruinvels, A.; Landwehrmeyer, B.; Gustafson, E.; Durkin, M.; Mengod, G.; Branchek, T.; Hoyer, D.; Palacios, J.J.N. Localization of 5-HT1B, 5-HT1Dα, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 1994, 33, 367–386. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamada, J.; Kimura, I.; Watanabe, Y.; Horisaka, K. The effects of the serotonin1A receptor agonist buspirone on the blood glucose and pancreatic hormones in rats. Jpn. J. Pharmacol. 1992, 60, 145–148. [Google Scholar] [CrossRef]
- Björkstrand, E.; Ahlénius, S.; Smedh, U.; Uvnäs-Moberg, K. The oxytocin receptor antagonist 1-deamino-2-D-Tyr-(OEt)-4-Thr-8-Orn-oxytocin inhibits effects of the 5-HT1A receptor agonist 8-OH-DPAT on plasma levels of insulin, cholecystokinin and somatostatin. Regul. Pept. 1996, 63, 47–52. [Google Scholar] [CrossRef]
- veettil Mohanan, V.; Khan, R.; Paulose, C. Hypothalamic 5-HT functional regulation through 5-HT1A and 5-HT2C receptors during pancreatic regeneration. Life Sci. 2006, 78, 1603–1609. [Google Scholar] [CrossRef]
- Bhalla, P.; Sharma, H.S.; Wurch, T.; Pauwels, P.J.; Saxena, P.R. Molecular cloning and expression of the porcine trigeminal ganglion cDNA encoding a 5-ht1F receptor. Eur. J. Pharmacol. 2002, 436, 23–33. [Google Scholar] [CrossRef]
- Pascual, J.; Del Arco, C.; Romón, T.; Del Olmo, E.; Pazos, A. [3H] Sumatriptan binding sites in human brain: Regional-dependent labelling of 5-HT1D and 5-HT1F receptors. Eur. J. Pharmacol. 1996, 295, 271–274. [Google Scholar] [CrossRef]
- Neeb, L.; Meents, J.; Reuter, U. 5-HT 1F receptor agonists: A new treatment option for migraine attacks? Neurotherapeutics 2010, 7, 176–182. [Google Scholar] [CrossRef]
- Ouyang, Q.; Hu, S.; Wang, G.; Hu, J.; Zhang, J.; Li, L.; Hu, B.; He, H.; Liu, H.; Xia, L. Comparative Transcriptome Analysis Suggests Key Roles for 5-Hydroxytryptamlne Receptors in Control of Goose Egg Production. Genes 2020, 11, 455. [Google Scholar] [CrossRef]
- Sturkie, P.D. Avian Physiology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Mishra, S.K.; Chen, B.; Zhu, Q.; Xu, Z.; Ning, C.; Yin, H.; Wang, Y.; Zhao, X.; Fan, X.; Yang, M. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Lv, C.; Mo, C.; Liu, H.; Wu, C.; Li, Z.; Li, J.; Wang, Y. Dopamine D2-like receptors (DRD2 and DRD4) in chickens: Tissue distribution, functional analysis, and their involvement in dopamine inhibition of pituitary prolactin expression. Gene 2018, 651, 33–43. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Denef, C. Estradiol induces expression of 5-hydroxytryptamine (5-HT) 4, 5-HT5, and 5-HT6 receptor messenger ribonucleic acid in rat anterior pituitary cell aggregates and allows prolactin release via the 5-HT4 receptor. Endocrinology 2007, 148, 1384–1395. [Google Scholar] [CrossRef]
- Pajot, M.T.B.; Mounier, F.; di Sciullo, A.; Schmidt, B.; Kordon, C. Differential Sites of Action of 8OH-DPAT, a 5HT1A Agonist, on ACTH and PRL Secretion in the Rat. Neuroendocrinology 1995, 61, 159–166. [Google Scholar] [CrossRef]
- Apfelbaum, M.E. Role of vasoactive intestinal peptide and 5-ht2 receptor subtype in serotonin stimulation of basal and thyrotropin-releasing-hormone-induced prolactin release in vitro from rat pituitary cells. Neuroendocrinology 1998, 67, 45–50. [Google Scholar] [CrossRef]
- Jørgensen, H.; Knigge, U.; Warberg, J. Involvement of 5-HT1, 5-HT2, and 5-HT3 receptors in the mediation of the prolactin response to serotonin and 5-hydroxytryptophan. Neuroendocrinology 1992, 55, 336–343. [Google Scholar] [CrossRef]

| EC50 Values (nM) | ||||
|---|---|---|---|---|
| Drug | 5-HT | CP94253 | BRL54443 | LY344864 |
| cAMP/PKA signaling pathway | ||||
| HTR1B | 8.0 | 4.0 | / | / |
| HTR1E | 9.3 | / | 2.4 | / |
| HTR1F | 5.7 | / | / | 1.8 |
| MAPK/ERK signaling pathway | ||||
| HTR1B | >800 a | >200 a | / | / |
| HTR1E | >100 a | / | 28.8 | / |
| HTR1F | >500 a | / | / | >200 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Qiu, Y.; Ren, Q.; Zhang, X.; Cao, B.; Zou, Y.; Li, J.; Zhang, J.; Wang, Y. Molecular Cloning and Functional Characterization of Three 5-HT Receptor Genes (HTR1B, HTR1E, and HTR1F) in Chickens. Genes 2021, 12, 891. https://doi.org/10.3390/genes12060891
Sun C, Qiu Y, Ren Q, Zhang X, Cao B, Zou Y, Li J, Zhang J, Wang Y. Molecular Cloning and Functional Characterization of Three 5-HT Receptor Genes (HTR1B, HTR1E, and HTR1F) in Chickens. Genes. 2021; 12(6):891. https://doi.org/10.3390/genes12060891
Chicago/Turabian StyleSun, Caiyun, Yang Qiu, Qin Ren, Xiao Zhang, Baolong Cao, Yi Zou, Juan Li, Jiannan Zhang, and Yajun Wang. 2021. "Molecular Cloning and Functional Characterization of Three 5-HT Receptor Genes (HTR1B, HTR1E, and HTR1F) in Chickens" Genes 12, no. 6: 891. https://doi.org/10.3390/genes12060891
APA StyleSun, C., Qiu, Y., Ren, Q., Zhang, X., Cao, B., Zou, Y., Li, J., Zhang, J., & Wang, Y. (2021). Molecular Cloning and Functional Characterization of Three 5-HT Receptor Genes (HTR1B, HTR1E, and HTR1F) in Chickens. Genes, 12(6), 891. https://doi.org/10.3390/genes12060891







