Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA-Seq Data Processing
2.2. Survival Analysis of Samples with Overexpressed KLK6
2.3. Enrichment Analysis
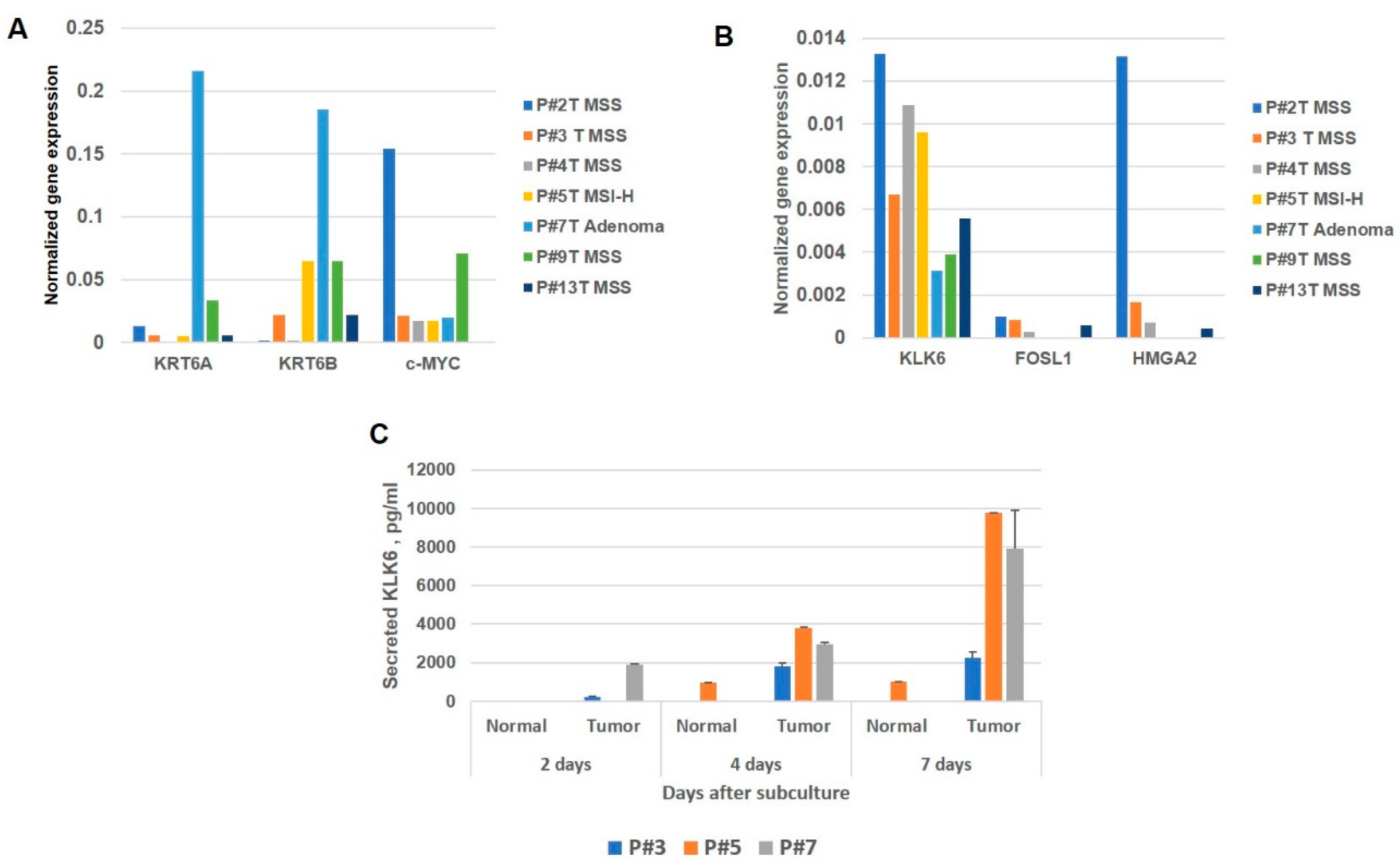
2.4. CRC Patient-Derived Organoid Cultures
2.5. Quantitative Reverse-Transcription Polymerase Chain Reaction
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) for KLK6
3. Results
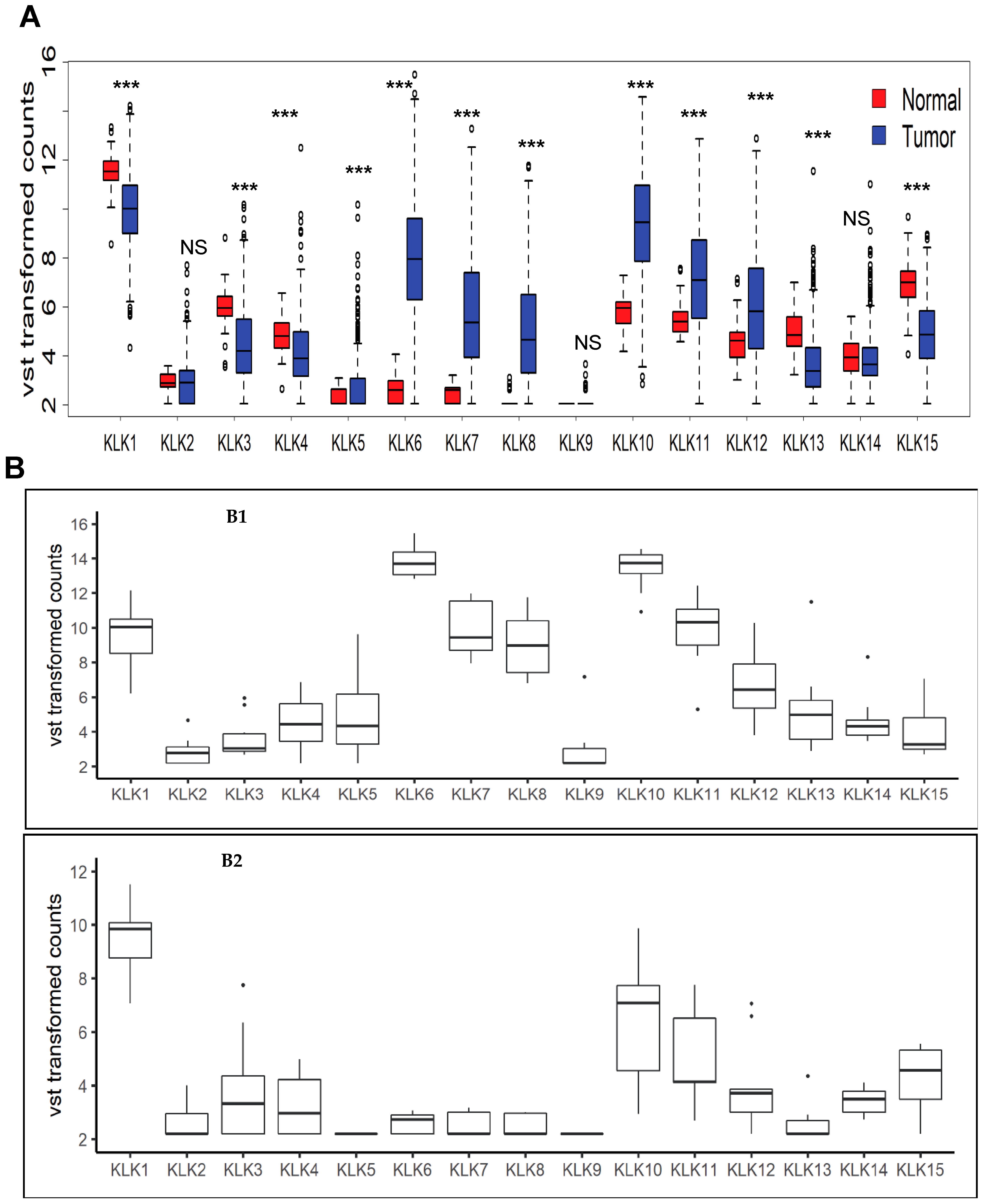
3.1. Pattern of Kallikrein Related Peptidases Family Expression in CRC Patients
3.2. Clinical and Molecular Characterization of KLK6-High Expressing Samples
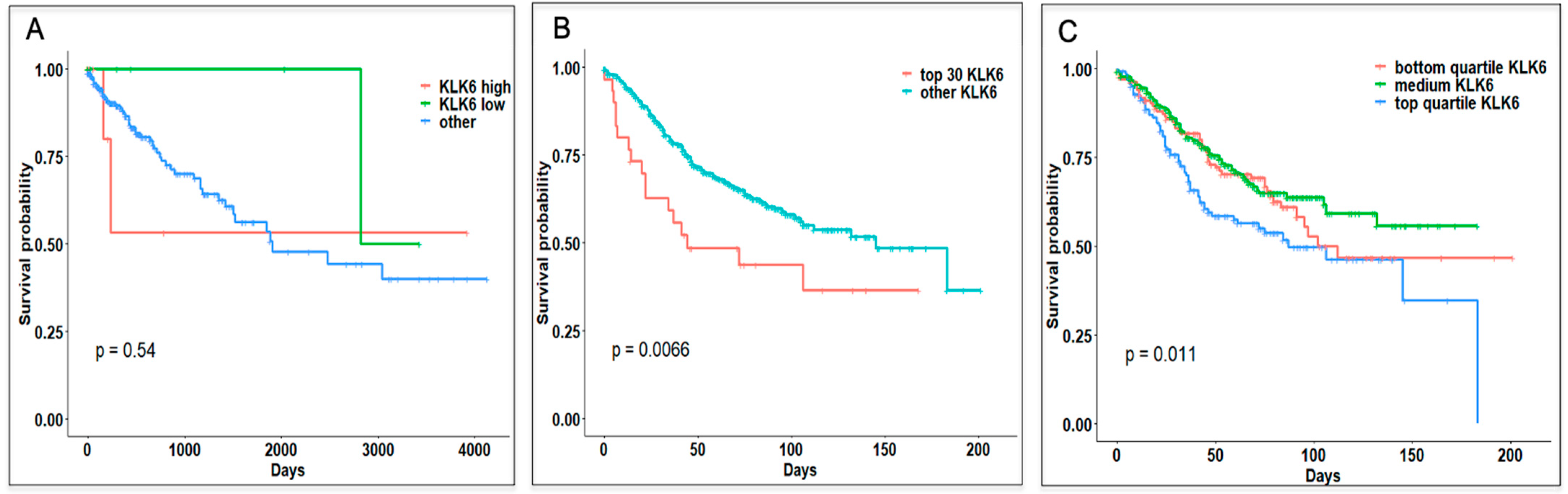
3.3. Survival Analysis
3.4. KLK6 Expression in Left and Right Sided CRC Cases
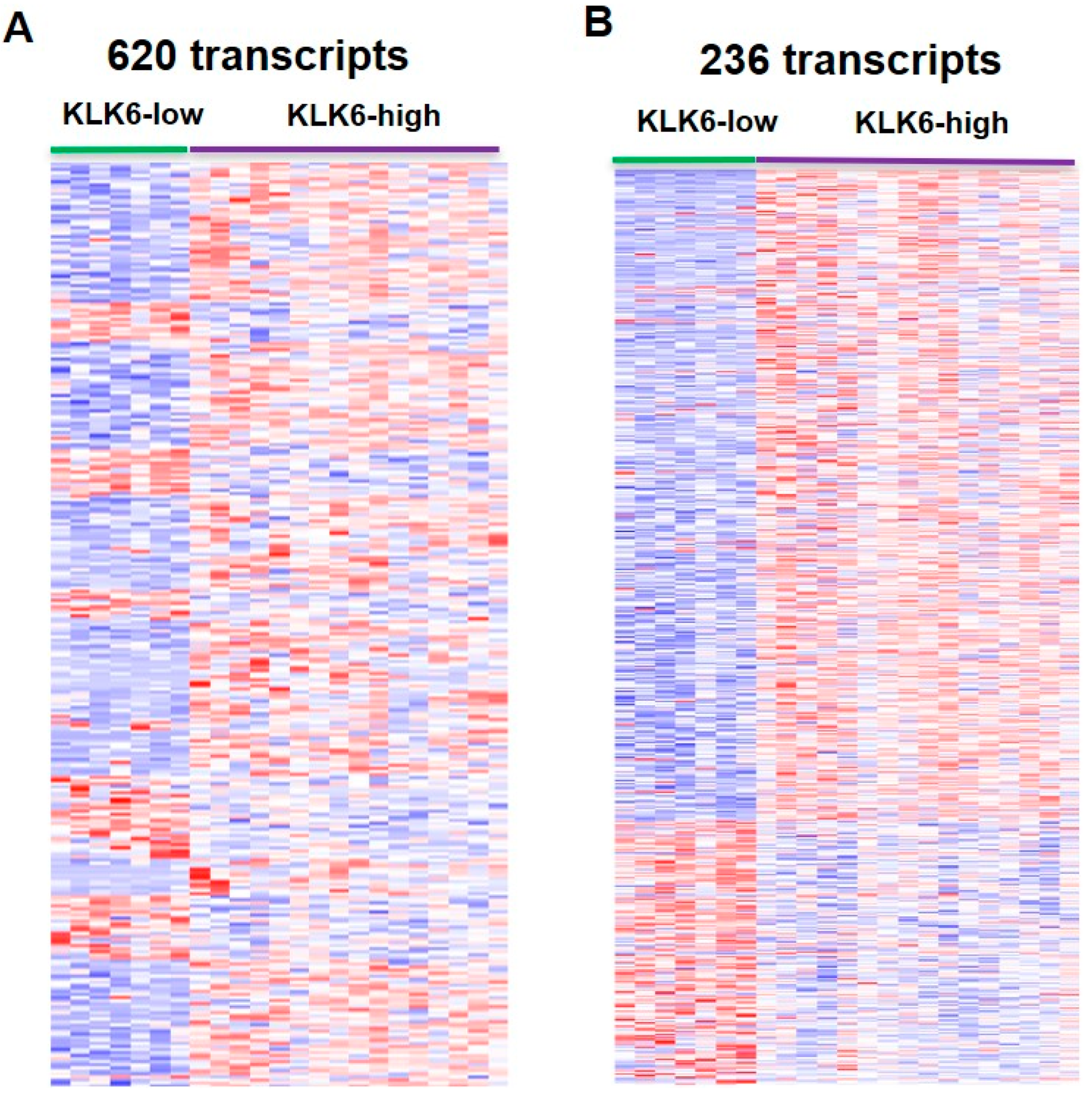
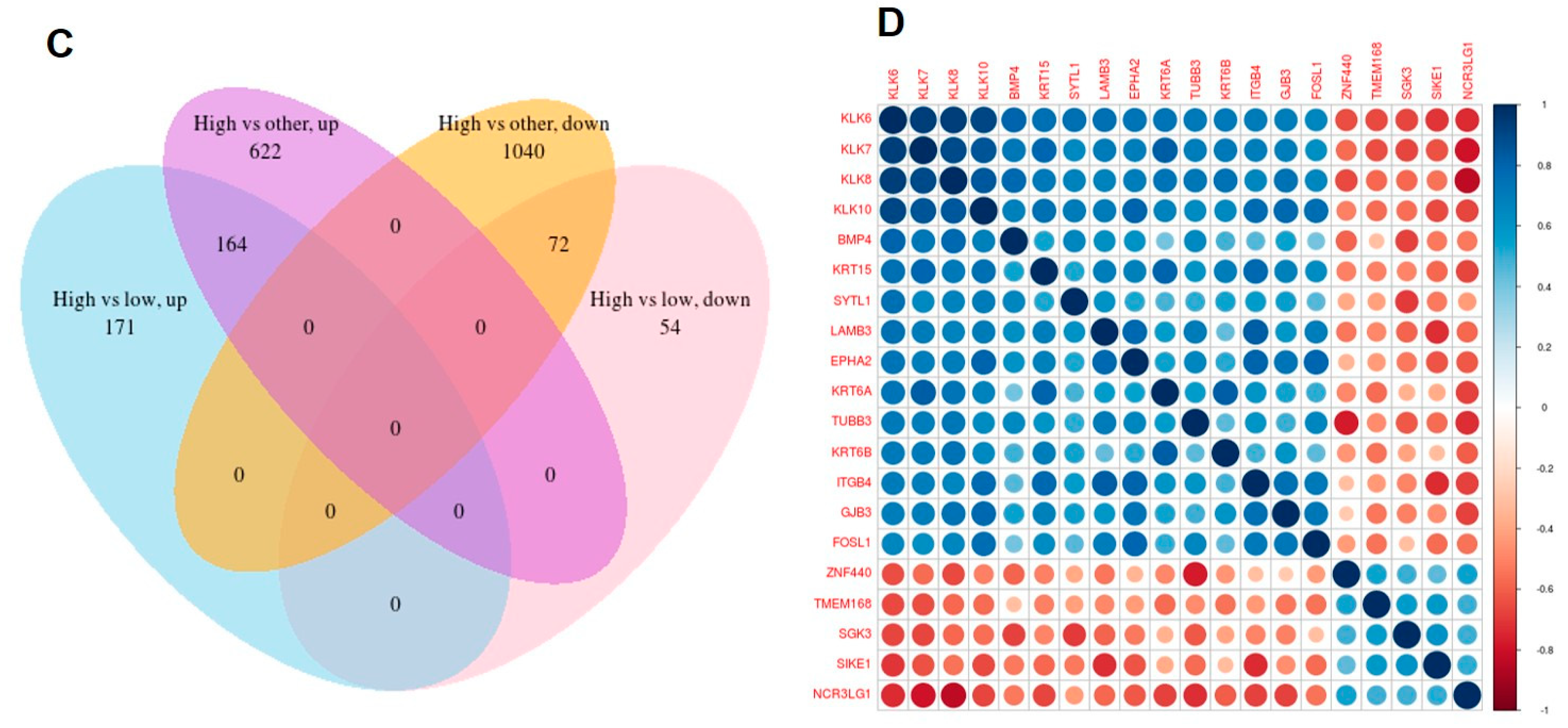
3.5. Differential Analysis of Samples with Overexpressed KLK6
3.6. Pathways and Significant Cellular Functions That Associated with KLK6 Overexpression
3.7. Analysis of Expression and Secretion of KLK6 in the CRC Patient-Derived Organoid Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallo, F.; De Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol Immunother. 2011, 60, 319–326. [Google Scholar] [CrossRef]
- Sotiropoulou, G.; Pampalakis, G.; Diamandis, E.P. Functional roles of human kallikrein-related peptidases. J. Biol. Chem. 2009, 284, 32989–32994. [Google Scholar] [CrossRef]
- Devetzi, M.; Trangas, T.; Scorilas, A.; Xynopoulos, D.; Talieri, M. Parallel overexpression and clinical significance of kallikrein-related peptidases 7 and 14 (KLK7KLK14) in colon cancer. Thromb Haemost. 2013, 109, 716–725. [Google Scholar] [CrossRef]
- Vakrakou, A.; Devetzi, M.; Papachristopoulou, G.; Malachias, A.; Scorilas, A.; Xynopoulos, D.; Talieri, M. Kallikrein-related peptidase 6 (KLK6) expression in the progression of colon adenoma to carcinoma. Biol. Chem. 2014, 395, 1105–1117. [Google Scholar] [CrossRef]
- Alexopoulou, D.K.; Papadopoulos, I.N.; Scorilas, A. Clinical significance of kallikrein-related peptidase (KLK10) mRNA expression in colorectal cancer. Clin. Biochem. 2013, 46, 1453–1461. [Google Scholar] [CrossRef]
- Alexopoulou, D.K.; Kontos, C.K.; Christodoulou, S.; Papadopoulos, I.N.; Scorilas, A. KLK11 mRNA expression predicts poor disease-free and overall survival in colorectal adenocarcinoma patients. Biomark Med. 2014, 8, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Borgono, C.A.; Diamandis, E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 2004, 4, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Utsunomiya, T.; Mimori, K.; Tanaka, F.; Inoue, H.; Nagahara, H.; Murayama, S.; Mori, M. Clinical significance of human kallikrein gene 6 messenger RNA expression in colorectal cancer. Clin. Cancer Res. 2005, 11, 2889–2893. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Lindmark, G.; Israelsson, A.; Palmqvist, R.; Oberg, A.; Hammarstrom, M.L.; Hammarstrom, S. Lymph node tissue kallikrein-related peptidase 6 mRNA: A progression marker for colorectal cancer. Br. J. Cancer 2012, 107, 150–157. [Google Scholar] [CrossRef]
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 2011, 6, 479–507. [Google Scholar] [CrossRef]
- Henkhaus, R.S.; Gerner, E.W.; Ignatenko, N.A. Kallikrein 6 is a mediator of K-RAS-dependent migration of colon carcinoma cells. Biol. Chem. 2008, 389, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Sells, E.; Pandey, R.; Chen, H.; Skovan, B.A.; Cui, H.; Ignatenko, N.A. Specific microRNA-mRNA Regulatory Network of Colon Cancer Invasion Mediated by Tissue Kallikrein-Related Peptidase 6. Neoplasia 2017, 19, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef]
- Ignatenko, N.A.; Holubec, H.; Besselsen, D.G.; Blohm-Mangone, K.A.; Padilla-Torres, J.L.; Nagle, R.B.; de Alboranc, I.M.; Guillen, R.J.; Gerner, E.W. Role of c-Myc in intestinal tumorigenesis of the ApcMin/+ mouse. Technol. Cancer Res. Treat. 2006, 5, 1658–1664. [Google Scholar]
- Morishita, A.; Zaidi, M.R.; Mitoro, A.; Sankarasharma, D.; Szabolcs, M.; Okada, Y.; D’Armiento, J.; Chada, K. HMGA2 is a driver of tumor metastasis. Cancer Res. 2013, 73, 4289–4299. [Google Scholar] [CrossRef]
- Chen, H.; Sells, E.; Pandey, R.; Abril, E.R.; Hsu, C.H.; Krouse, R.S.; Nagle, R.B.; Pampalakis, G.; Sotiropoulou, G.; Ignatenko, N.A. Kallikrein 6 protease advances colon tumorigenesis via induction of the high mobility group A2 protein. Oncotarget 2019, 10, 6062–6078. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Eissa, A.; Poda, G.; Diamandis, E.P. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat. Rev. Drug Discov. 2015, 14, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Tailor, P.D.; Kodeboyina, S.K.; Bai, S.; Patel, N.; Sharma, S.; Ratnani, A.; Copland, J.A.; She, J.X.; Sharma, A. Diagnostic and prognostic biomarker potential of kallikrein family genes in different cancer types. Oncotarget 2018, 9, 17876–17888. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ding, Z.; Zhang, H.; Chen, Q. Identification of Prognostic Biomarkers and Drugs Targeting Them in Colon Adenocarcinoma: A Bioinformatic Analysis. Integr. Cancer Ther. 2019, 18, 1534735419864434. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, N.A.; Zhang, H.; Watts, G.S.; Skovan, B.A.; Stringer, D.E.; Gerner, E.W. The chemopreventive agent alpha-difluoromethylornithine blocks Ki-ras-dependent tumor formation and specific gene expression in Caco-2 cells. Mol. Carcinog. 2004, 39, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Ciferri, A.; Crumbliss, A.L. The Assembling and Contraction Mechanisms of Striated Muscles. Front. Chem. 2018, 6, 570. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, J.; He, N.; He, J.; Zhu, B. Titin mutation associated with responsiveness to checkpoint blockades in solid tumors. JCI Insight 2019, 4, e127901. [Google Scholar] [CrossRef]
- Ruckert, F.; Hennig, M.; Petraki, C.D.; Wehrum, D.; Distler, M.; Denz, A.; Schroder, M.; Dawelbait, G.; Kalthoff, H.; Saeger, H.D.; et al. Co-expression of KLK6 and KLK10 as prognostic factors for survival in pancreatic ductal adenocarcinoma. Br. J. Cancer 2008, 99, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Magdolen, V.; Seidl, C.; Dorn, J.; Drecoll, E.; Kotzsch, M.; Yang, F.; Schmitt, M.; Schilling, O.; Rockstroh, A.; et al. Kallikrein-related peptidases 4, 5, 6 and 7 regulate tumour-associated factors in serous ovarian cancer. Br. J. Cancer 2018, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.B.; Shen, X.M.; Chen, B.S.; Du, X.L.; Luo, M.L.; Cai, Y.; Han, Y.L.; Xu, X.; Zhan, Q.M.; et al. Exogenous expression of Esophagin/SPRR3 attenuates the tumorigenicity of esophageal squamous cell carcinoma cells via promoting apoptosis. Int. J. Cancer 2008, 122, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gu, Y.; Li, X.; Sun, H.; Zha, H.; Xie, J.; Zhao, J.; Huang, M.; Chen, L.; Peng, Q.; et al. S100A6 promotes the proliferation and migration of cervical cancer cells via the PI3K/Akt signaling pathway. Oncol. Lett. 2018, 15, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lv, X.; Zhang, Z.; Xie, S. S100A6/miR193a regulates the proliferation, invasion, migration and angiogenesis of lung cancer cells through the P53 acetylation. Am. J. Transl. Res. 2019, 11, 4634–4649. [Google Scholar] [PubMed]
- Salyakina, D.; Tsinoremas, N.F. Non-coding RNAs profiling in head and neck cancers. NPJ Genom. Med. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Goettig, P.; Magdolen, V.; Brandstetter, H. Natural and synthetic inhibitors of kallikrein-related peptidases (KLKs). Biochimie 2010, 92, 1546–1567. [Google Scholar] [CrossRef]
- Liang, G.; Chen, X.; Aldous, S.; Pu, S.F.; Mehdi, S.; Powers, E.; Giovanni, A.; Kongsamut, S.; Xia, T.; Zhang, Y.; et al. Virtual Screening and X-ray Crystallography for Human Kallikrein 6 Inhibitors with an Amidinothiophene P1 Group. ACS Med. Chem. Lett. 2012, 3, 159–164. [Google Scholar] [CrossRef][Green Version]
- Sotiropoulou, G.; Pampalakis, G. Targeting the kallikrein-related peptidases for drug development. Trends Pharm. Sci. 2012, 33, 623–634. [Google Scholar] [CrossRef]
- Sananes, A.; Cohen, I.; Shahar, A.; Hockla, A.; De Vita, E.; Miller, A.K.; Radisky, E.S.; Papo, N. A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering. J. Biol. Chem. 2018, 293, 12663–12680. [Google Scholar] [CrossRef] [PubMed]
- De Vita, E.; Schuler, P.; Lovell, S.; Lohbeck, J.; Kullmann, S.; Rabinovich, E.; Sananes, A.; Hessling, B.; Hamon, V.; Papo, N.; et al. Depsipeptides Featuring a Neutral P1 Are Potent Inhibitors of Kallikrein-Related Peptidase 6 with On-Target Cellular Activity. J. Med. Chem. 2018, 61, 8859–8874. [Google Scholar] [CrossRef] [PubMed]
- De Veer, S.J.; Furio, L.; Swedberg, J.E.; Munro, C.A.; Brattsand, M.; Clements, J.A.; Hovnanian, A.; Harris, J.M. Selective Substrates and Inhibitors for Kallikrein-Related Peptidase 7 (KLK7) Shed Light on KLK Proteolytic Activity in the Stratum Corneum. J. Investig. Derm. 2017, 137, 430–439. [Google Scholar] [CrossRef] [PubMed]


| Clinical, Pathological and Molecular Characteristics | Groups | ||
|---|---|---|---|
| KLK6-High Group (n = 16), % (Number of Cases/Group) | KLK6-Low Group (n = 7), % (Number of Cases/Group) | ||
| Gender | female | 44 (7/16) | 71 (5/7) |
| male | 56 (9/16) | 29 (2/7) | |
| Tumor stage | Stage I | 19 (3/16) | 28.6 (2/7) |
| Stage II A | 37 (6/16) | 28.6 (2/7) | |
| Stage II B | 0 * | 14. 29 (1/7) | |
| Stage III | 6.25 (1/16) | 0 | |
| Stage III B | 6.25 (1/16) | 14.29 (1/7) | |
| Stage III C | 6.25 (1/16) | 14.29 (1/7) | |
| Stage IV | 19 (3/16) | 0 | |
| Stage IV A | 6.25 (1/16) | 0 | |
| Metastasis ≥M1 | 25 (4/16) | 0 | |
| Lymph node positive | 44 (7/16) | 28.6 (2/5) | |
| Molecular subtype ** | MSS | 43.75 (7/16) | 42.85 (3/7) |
| MSI-L | 31.25 (5/16) | 14.2 (1/7) | |
| MSI-H | 25 (4/16) | 42.85 (3/7) | |
| Mutations *** | APC | 75 (12/16) | 100 (6/6) |
| Titin (TTN) | 75 (12/16) | 0 | |
| K-RAS | 68.75(11/16) | 0 | |
| MUC16 | 56.25 (9/16) | 0 | |
| TP53 | 50 (8/16) | 66.67 (4/6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.; Zhou, M.; Chen, Y.; Darmoul, D.; Kisiel, C.C.; Nfonsam, V.N.; Ignatenko, N.A. Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer. Genes 2021, 12, 749. https://doi.org/10.3390/genes12050749
Pandey R, Zhou M, Chen Y, Darmoul D, Kisiel CC, Nfonsam VN, Ignatenko NA. Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer. Genes. 2021; 12(5):749. https://doi.org/10.3390/genes12050749
Chicago/Turabian StylePandey, Ritu, Muhan Zhou, Yuliang Chen, Dalila Darmoul, Conner C. Kisiel, Valentine N. Nfonsam, and Natalia A. Ignatenko. 2021. "Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer" Genes 12, no. 5: 749. https://doi.org/10.3390/genes12050749
APA StylePandey, R., Zhou, M., Chen, Y., Darmoul, D., Kisiel, C. C., Nfonsam, V. N., & Ignatenko, N. A. (2021). Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer. Genes, 12(5), 749. https://doi.org/10.3390/genes12050749








