UbiComb: A Hybrid Deep Learning Model for Predicting Plant-Specific Protein Ubiquitylation Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Benchmark Dataset
2.2. Sequence Encoding
2.3. Proposed Architecture
2.4. Model Evaluation and Performance Metrics
3. Results
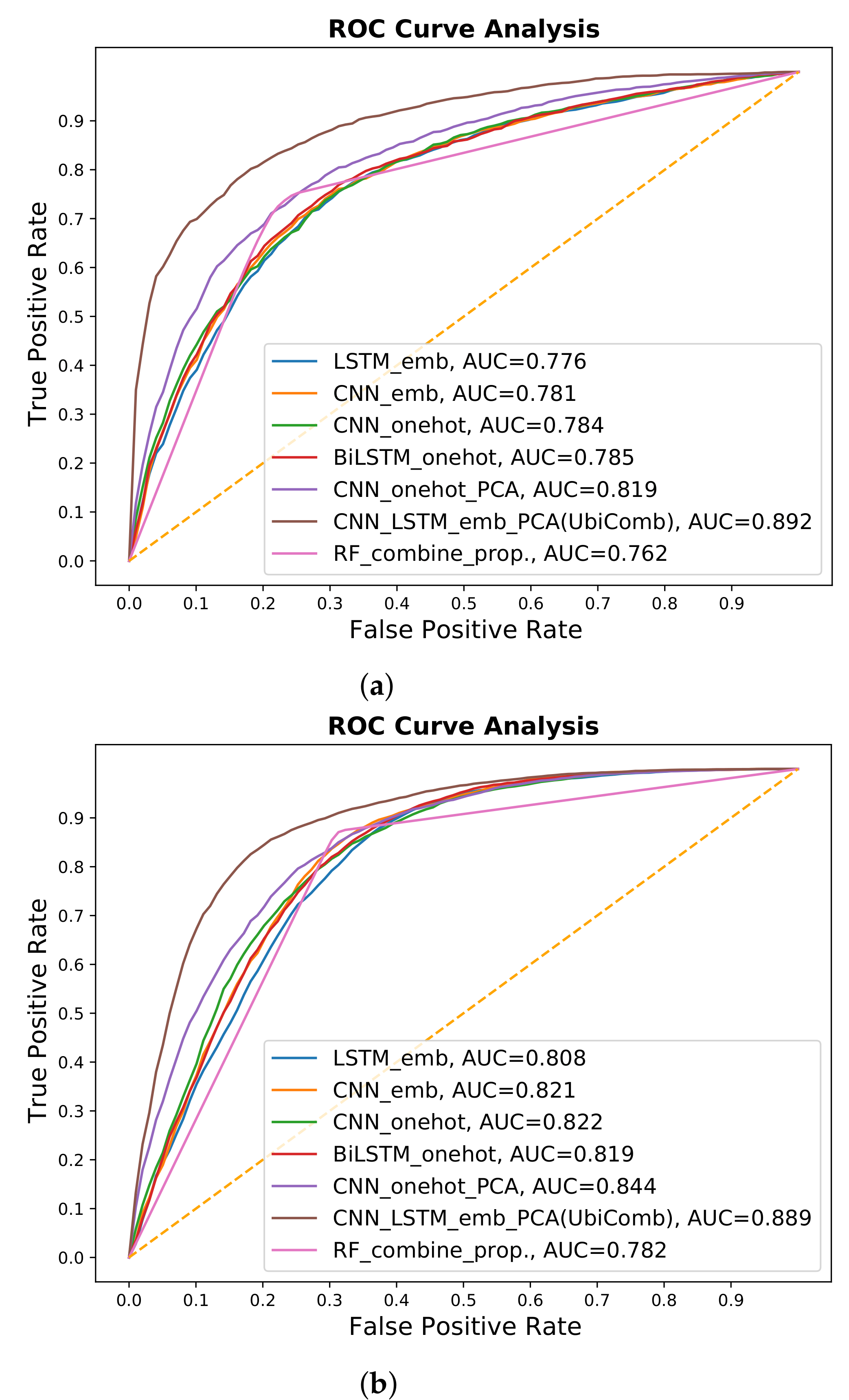
3.1. Experiment on Different Techniques
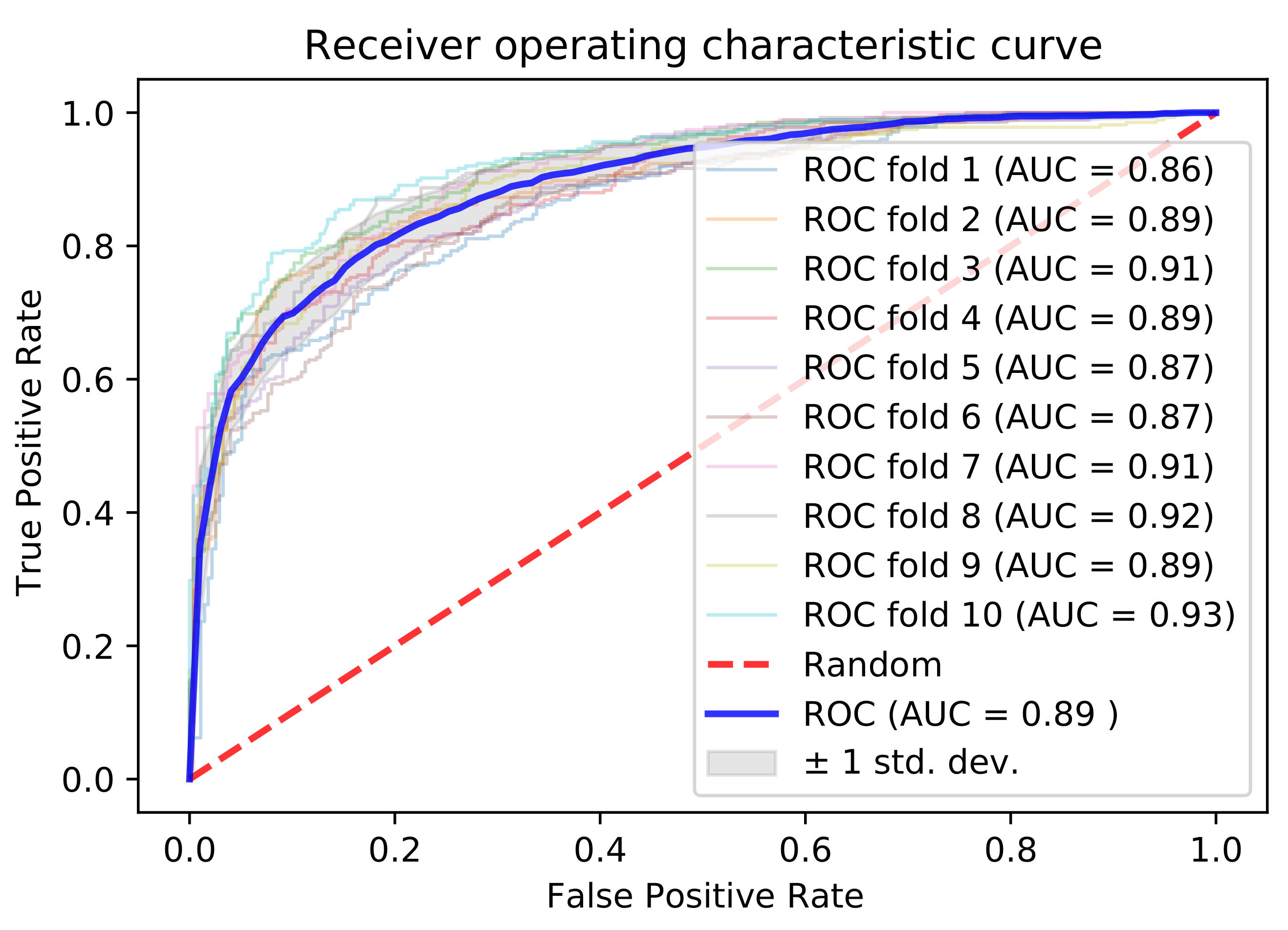
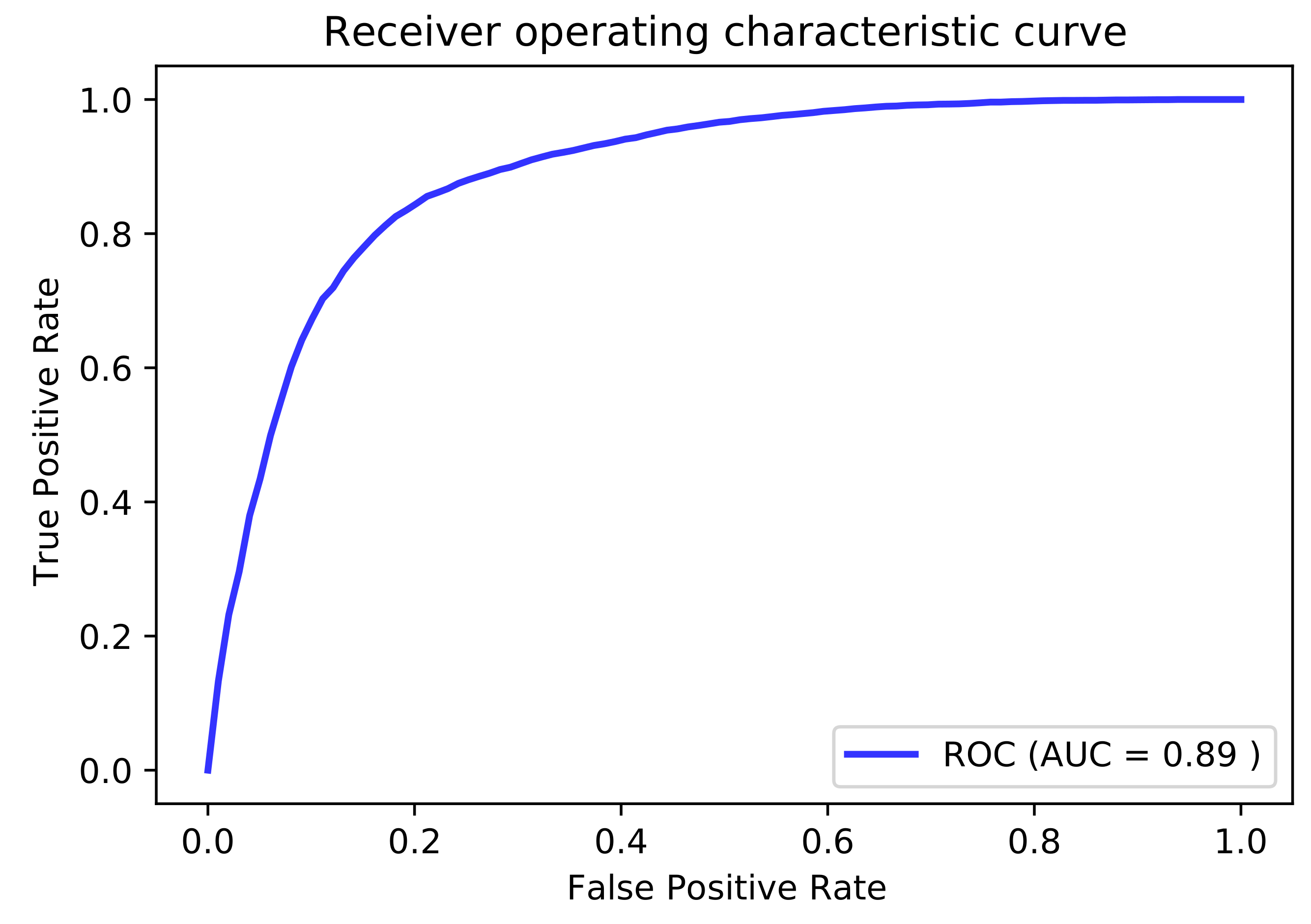
3.2. Cross-Validation Performance
3.3. Independent Dataset Comparison and Analysis of Published Tools
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishna, R.G.; Wold, F. Post-translational modifications of proteins. In Methods in Protein Sequence Analysis; Springer: Boston, MA, USA, 1993; pp. 167–172. [Google Scholar]
- Meng, Y.; Sandow, J.J.; Czabotar, P.E.; Murphy, J.M. The regulation of necroptosis by post-translational modifications. Cell Death Differ. 2021, 28, 861–883. [Google Scholar] [CrossRef]
- Seo, J.W.; Lee, K.J. Post-translational modifications and their biological functions: Proteomic analysis and systematic approaches. BMB Rep. 2004, 37, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Baslé, E.; Joubert, N.; Pucheault, M. Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol. 2010, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Vempati, R.K. Talin: A potential drug target for cancer therapy. Curr. Drug Metab. 2020, 21, 25–32. [Google Scholar] [CrossRef]
- Gao, C.; Higgins, P.J.; Zhang, W. AQP2: Mutations Associated with Congenital Nephrogenic Diabetes Insipidus and Regulation by Post-Translational Modifications and Protein-Protein Interactions. Cells 2020, 9, 2172. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zigo, M.; Zuidema, D.; Sutovsky, M.; Sutovsky, P. NEDD4-like ubiquitin ligase 2 protein (NEDL2) in porcine spermatozoa, oocytes, and preimplantation embryos and its role in oocyte fertilization†. Biol. Reprod. 2020, 104, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Aminu, B.; Roscow, O.; Zhang, W. Targeting the Ubiquitin Signaling Cascade in Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 791. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta BBA Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef]
- Song, L.; Luo, Z.Q. Post-translational regulation of ubiquitin signaling. J. Cell Biol. 2019, 218, 1776–1786. [Google Scholar] [CrossRef]
- Xu, G.; Jaffrey, S.R. The new landscape of protein ubiquitination. Nat. Biotechnol. 2011, 29, 1098–1100. [Google Scholar] [CrossRef]
- Starita, L.M.; Parvin, J.D. The multiple nuclear functions of BRCA1: Transcription, ubiquitination and DNA repair. Curr. Opin. Cell Biol. 2003, 15, 345–350. [Google Scholar] [CrossRef]
- Park, H.B.; Kim, J.W.; Baek, K.H. Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int. J. Mol. Sci. 2020, 21, 3904. [Google Scholar] [CrossRef]
- Porro, A.; Berti, M.; Pizzolato, J.; Bologna, S.; Kaden, S.; Saxer, A.; Ma, Y.; Nagasawa, K.; Sartori, A.A.; Jiricny, J. FAN1 interaction with ubiquitylated PCNA alleviates replication stress and preserves genomic integrity independently of BRCA2. Nat. Commun. 2017, 8, 1073. [Google Scholar] [CrossRef]
- Stankovic-Valentin, N.; Melchior, F. Control of SUMO and ubiquitin by ROS: Signaling and disease implications. Mol. Asp. Med. 2018, 63, 3–17. [Google Scholar] [CrossRef]
- Corn, J.E.; Vucic, D. Ubiquitin in inflammation: The right linkage makes all the difference. Nat. Struct. Mol. Biol. 2014, 21, 297–300. [Google Scholar] [CrossRef]
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Ubiquitination in periodontal disease: A review. Int. J. Mol. Sci. 2017, 18, 1476. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Jo, U.; Kohrman, A.; Rezaeian, A.H.; Chou, P.C.; Logothetis, C.; Lin, H.K. Posttranslational regulation of Akt in human cancer. Cell Biosci. 2014, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef]
- Jahan, A.S.; Elbæk, C.R.; Damgaard, R.B. Met1-linked ubiquitin signalling in health and disease: Inflammation, immunity, cancer, and beyond. Cell Death Differ. 2021, 28, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Saracco, S.A.; Hansson, M.; Scalf, M.; Walker, J.M.; Smith, L.M.; Vierstra, R.D. Tandem affinity purification and mass spectrometric analysis of ubiquitylated proteins in Arabidopsis. Plant J. 2009, 59, 344–358. [Google Scholar] [CrossRef]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Dreher, K.; Callis, J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.R.; Lu, R.; Sadanandom, A.; Malcuit, I.; Moffett, P.; Brice, D.C.; Schauser, L.; Jaggard, D.A.; Xiao, S.; Coleman, M.J.; et al. Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10865–10869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, L. Crosstalk between ubiquitination and other post-translational protein modifications in plant immunity. Plant Commun. 2020, 1, 100041. [Google Scholar] [CrossRef]
- Yang, A.; Cho, K.; Park, H.S. Chemical biology approaches for studying posttranslational modifications. RNA Biol. 2018, 15, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.R.; Trelle, M.B.; Thingholm, T.E.; Jensen, O.N. Analysis of posttranslational modifications of proteins by tandem mass spectrometry: Mass Spectrometry For Proteomics Analysis. Biotechniques 2006, 40, 790–798. [Google Scholar] [CrossRef]
- Shetty, P.; Ramprasad, R. Automated knowledge extraction from polymer literature using natural language processing. Iscience 2021, 24, 101922. [Google Scholar] [CrossRef]
- Oliwa, T.; Furner, B.; Schmitt, J.; Schneider, J.; Ridgway, J.P. Development of a predictive model for retention in HIV care using natural language processing of clinical notes. J. Am. Med. Inf. Assoc. 2021, 28, 104–112. [Google Scholar] [CrossRef]
- Mohammad, F.; Kim, Y.C. Energy load forecasting model based on deep neural networks for smart grids. Int. J. Syst. Assur. Eng. Manag. 2020, 11, 824–834. [Google Scholar] [CrossRef]
- Oneata, D.; Caranica, A.; Stan, A.; Cucu, H. An evaluation of word-level confidence estimation for end-to-end automatic speech recognition. arXiv 2021, arXiv:2101.05525. [Google Scholar]
- Ilyas, T.; Khan, A.; Umraiz, M.; Kim, H. Seek: A framework of superpixel learning with cnn features for unsupervised segmentation. Electronics 2020, 9, 383. [Google Scholar] [CrossRef]
- Islam, N.U.; Park, J. Face Attribute Modification Using Fine-Tuned Attribute-Modification Network. Electronics 2020, 9, 743. [Google Scholar] [CrossRef]
- Islam, N.U.; Park, J. Depth Estimation From a Single RGB Image Using Fine-Tuned Generative Adversarial Network. IEEE Access 2021, 9, 32781–32794. [Google Scholar] [CrossRef]
- Alam, W.; Ali, S.D.; Tayara, H.; To Chong, K. A CNN-based RNA n6-methyladenosine site predictor for multiple species using heterogeneous features representation. IEEE Access 2020, 8, 138203–138209. [Google Scholar] [CrossRef]
- Ali, S.D.; Alam, W.; Tayara, H.; Chong, K. Identification of functional piRNAs using a convolutional neural network. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020. [Google Scholar] [CrossRef]
- Shujaat, M.; Wahab, A.; Tayara, H.; Chong, K.T. pcPromoter-CNN: A CNN-Based Prediction and Classification of Promoters. Genes 2020, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Khanal, J.; Nazari, I.; Tayara, H.; Chong, K.T. 4mCCNN: Identification of N4-Methylcytosine Sites in Prokaryotes Using Convolutional Neural Network. IEEE Access 2019, 7, 145455–145461. [Google Scholar] [CrossRef]
- Tung, C.W.; Ho, S.Y. Computational identification of ubiquitylation sites from protein sequences. BMC Bioinform. 2008, 9, 310. [Google Scholar] [CrossRef]
- Radivojac, P.; Vacic, V.; Haynes, C.; Cocklin, R.R.; Mohan, A.; Heyen, J.W.; Goebl, M.G.; Iakoucheva, L.M. Identification, analysis, and prediction of protein ubiquitination sites. Proteins Struct. Funct. Bioinform. 2010, 78, 365–380. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chen, S.A.; Hung, H.Y.; Ou, Y.Y. Incorporating distant sequence features and radial basis function networks to identify ubiquitin conjugation sites. PLoS ONE 2011, 6, e17331. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Su, M.G.; Kao, H.J.; Jhong, J.H.; Weng, S.L.; Lee, T.Y. UbiSite: Incorporating two-layered machine learning method with substrate motifs to predict ubiquitin-conjugation site on lysines. BMC Syst. Biol. 2016, 10, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.Z.; Wang, X.F.; Wang, C.; Yan, R.X.; Zhang, Z. Prediction of ubiquitination sites by using the composition of k-spaced amino acid pairs. PLoS ONE 2011, 6, e22930. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, J.D.; Shi, S.P.; Suo, S.B.; Huang, S.Y.; Liang, R.P. Incorporating key position and amino acid residue features to identify general and species-specific Ubiquitin conjugation sites. Bioinformatics 2013, 29, 1614–1622. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Y.; Song, J.; Zhang, Z. hCKSAAP_UbSite: Improved prediction of human ubiquitination sites by exploiting amino acid pattern and properties. Biochim. Biophys. Acta BBA Proteins Proteom. 2013, 1834, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.R.; Xiao, X.; Lin, W.Z.; Chou, K.C. iUbiq-Lys: Prediction of lysine ubiquitination sites in proteins by extracting sequence evolution information via a gray system model. J. Biomol. Struct. Dyn. 2015, 33, 1731–1742. [Google Scholar] [CrossRef]
- Wang, J.R.; Huang, W.L.; Tsai, M.J.; Hsu, K.T.; Huang, H.L.; Ho, S.Y. ESA-UbiSite: Accurate prediction of human ubiquitination sites by identifying a set of effective negatives. Bioinformatics 2017, 33, 661–668. [Google Scholar] [CrossRef]
- Li, Y.; Xie, P.; Lu, L.; Wang, J.; Diao, L.; Liu, Z.; Guo, F.; He, Y.; Liu, Y.; Huang, Q.; et al. An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase-substrate interaction network. Nat. Commun. 2017, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Walsh, I.; Di Domenico, T.; Tosatto, S.C. RUBI: rapid proteomic-scale prediction of lysine ubiquitination and factors influencing predictor performance. Amino Acids 2014, 46, 853–862. [Google Scholar] [CrossRef]
- Feng, K.Y.; Huang, T.; Feng, K.R.; Liu, X.J. Using WPNNA classifier in ubiquitination site prediction based on hybrid features. Protein Pept. Lett. 2013, 20, 318–323. [Google Scholar]
- Nguyen, V.N.; Huang, K.Y.; Huang, C.H.; Lai, K.R.; Lee, T.Y. A new scheme to characterize and identify protein ubiquitination sites. IEEE/ACM Trans. Comput. Biol. Bioinform. 2016, 14, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dass, J.F.P. Non-canonical pathway network modelling and ubiquitination site prediction through homology modelling of NF-κB. Gene 2016, 581, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Ma, Z.; Yin, M. Prediction of lysine ubiquitylation with ensemble classifier and feature selection. Int. J. Mol. Sci. 2011, 12, 8347–8361. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gupta, M.; Bist, A.S. Prediction of ubiquitination sites using UbiNets. Adv. Fuzzy Syst. 2018, 2018, 5125103. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Wang, X.; Wang, H.; Xu, Y. DeepUbi: A deep learning framework for prediction of ubiquitination sites in proteins. BMC Bioinform. 2019, 20, 86. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Q.; Jiang, J.; Li, W.; Wang, Y. Capsule network for protein ubiquitination site prediction. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, Z.; Lee, T.Y. Incorporating deep learning with word embedding to identify plant ubiquitylation sites. Front. Cell Dev. Biol. 2020, 8, 572195. [Google Scholar] [CrossRef] [PubMed]
- Mosharaf, M.P.; Hassan, M.M.; Ahmed, F.F.; Khatun, M.S.; Moni, M.A.; Mollah, M.N.H. Computational prediction of protein ubiquitination sites mapping on Arabidopsis thaliana. Comput. Biol. Chem. 2020, 85, 107238. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Y.; Wang, H.; Xu, Y. A deep learning method to more accurately recall known lysine acetylation sites. BMC Bioinform. 2019, 20, 49. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Thapa, N.; Chaudhari, M.; McManus, S.; Roy, K.; Newman, R.H.; Saigo, H.; Kc, D.B. DeepSuccinylSite: A deep learning based approach for protein succinylation site prediction. BMC Bioinform. 2020, 21, 63. [Google Scholar] [CrossRef]
- Cao, M.; Chen, G.; Wang, L.; Wen, P.; Shi, S. Computational prediction and analysis for tyrosine post-translational modifications via elastic net. J. Chem. Inf. Model. 2018, 58, 1272–1281. [Google Scholar] [CrossRef]
- Yu, B.; Yu, Z.; Chen, C.; Ma, A.; Liu, B.; Tian, B.; Ma, Q. DNNAce: Prediction of prokaryote lysine acetylation sites through deep neural networks with multi-information fusion. Chemom. Intell. Lab. Syst. 2020, 200, 103999. [Google Scholar] [CrossRef]
- Quan, C.; Hua, L.; Sun, X.; Bai, W. Multichannel convolutional neural network for biological relation extraction. BioMed Res. Int. 2016, 2016, 1850404. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.; Chantsalnyam, T.; Tayara, H.; Chong, K.T. RecSNO: Prediction of Protein S-Nitrosylation Sites Using a Recurrent Neural Network. IEEE Access 2021, 9, 6674–6682. [Google Scholar] [CrossRef]
- Kulmanov, M.; Khan, M.A.; Hoehndorf, R. DeepGO: Predicting protein functions from sequence and interactions using a deep ontology-aware classifier. Bioinformatics 2018, 34, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Venkatarajan, M.S.; Braun, W. New quantitative descriptors of amino acids based on multidimensional scaling of a large number of physical–chemical properties. Mol. Model. Annu. 2001, 7, 445–453. [Google Scholar]
- Naseer, S.; Hussain, W.; Khan, Y.D.; Rasool, N. iPhosS (Deep)-PseAAC: Identify Phosphoserine Sites in Proteins using Deep Learning on General Pseudo Amino Acid Compositions via Modified 5-Steps Rule. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, Y.; Xu, D. Capsule network for protein post-translational modification site prediction. Bioinformatics 2019, 35, 2386–2394. [Google Scholar] [CrossRef]
- Hua, Y.; Zhao, Z.; Li, R.; Chen, X.; Liu, Z.; Zhang, H. Deep learning with long short-term memory for time series prediction. IEEE Commun. Mag. 2019, 57, 114–119. [Google Scholar] [CrossRef]
- Graham, B. Fractional max-pooling. arXiv 2014, arXiv:1412.6071. [Google Scholar]
- Ilyas, T.; Umraiz, M.; Khan, A.; Kim, H. DAM: Hierarchical Adaptive Feature Selection using Convolution Encoder Decoder Network for Strawberry Segmentation. Front. Plant Sci. 2021, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Hecht-Nielsen, R. Theory of the backpropagation neural network. In Neural Networks for Perception; Elsevier: Amsterdam, The Netherlands, 1992; pp. 65–93. [Google Scholar]
- Kiranyaz, S.; Avci, O.; Abdeljaber, O.; Ince, T.; Gabbouj, M.; Inman, D.J. 1D convolutional neural networks and applications: A survey. Mech. Syst. Signal Process. 2021, 151, 107398. [Google Scholar] [CrossRef]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the International Joint Conference on Artificial Intelligence (IJCAI), Montreal, QC, Canada, 20–25 August 1995; Volume 14, pp. 1137–1145. [Google Scholar]
- Chen, Z.; Zhao, P.; Li, F.; Marquez-Lago, T.T.; Leier, A.; Revote, J.; Zhu, Y.; Powell, D.R.; Akutsu, T.; Webb, G.I.; et al. iLearn: An integrated platform and meta-learner for feature engineering, machine-learning analysis and modeling of DNA, RNA and protein sequence data. Brief. Bioinform. 2020, 21, 1047–1057. [Google Scholar] [CrossRef]
- Zhan, H.; Song, L.; Kamran, A.; Han, F.; Li, B.; Zhou, Z.; Liu, T.; Shen, L.; Li, Y.; Wang, F.; et al. Comprehensive Proteomic Analysis of Lysine Ubiquitination in Seedling Leaves of Nicotiana tabacum. ACS Omega 2020, 5, 20122–20133. [Google Scholar] [CrossRef]
- Kucheryavskiy, S.; Zhilin, S.; Rodionova, O.; Pomerantsev, A. Procrustes Cross-Validation—A Bridge between Cross-Validation and Independent Validation Sets. Anal. Chem. 2020, 92, 11842–11850. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, R.; Li, J.; Bao, L.; Xu, D.; Zhao, X. Large-scale prediction of protein ubiquitination sites using a multimodal deep architecture. BMC Syst. Biol. 2018, 12, 81–90. [Google Scholar] [CrossRef]
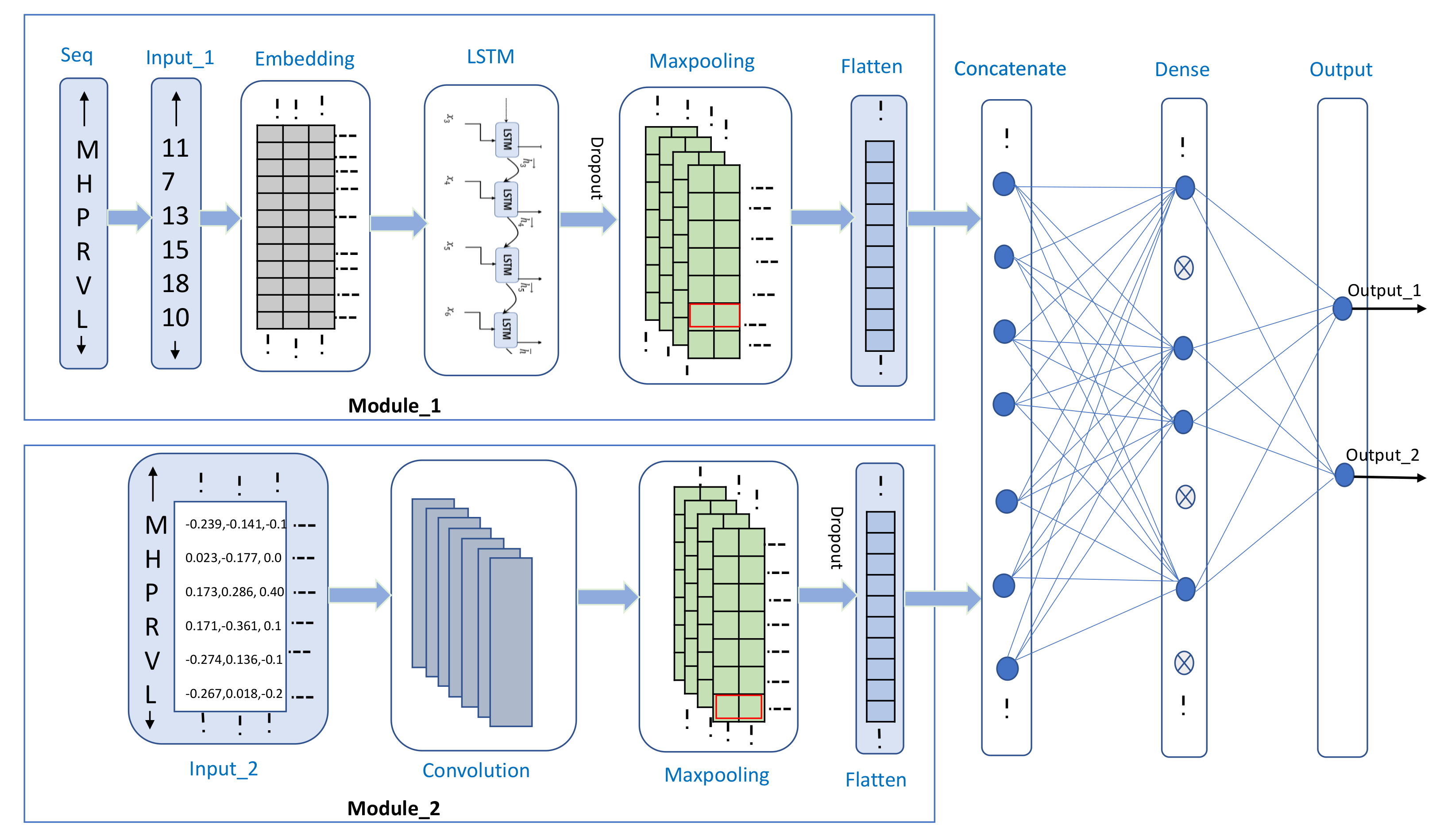

| Layers | Hyperparameter Settings | Output Shape |
|---|---|---|
| Input_1 | shape = (31) | (31) |
| Embedding | Input dim = 22 | |
| Output dim = 32 | (31, 32) | |
| Input shape = (31 ) | ||
| LSTM | units = 32 | |
| Kernal reg = L2 (1 × 10) | (31, 32) | |
| Recurrent reg = L2 (1 × 10) | ||
| Bias reg = L2 (1 × 10) | ||
| Dropout | Rate = 0.2 | (31, 32) |
| MaxPooling1D | Pool size = 2 | (15, 32) |
| Flatten_1 | Just flatten the matrix | (480) |
| Input_2 | shape = (31, 5) | (31, 5) |
| Conv1D | filters = 16 | |
| kernal_size = 3 | (29, 16) | |
| Activation = relu | ||
| MaxPooling1D | Pool size = 2 | (14, 16) |
| Dropout | Rate = 0.2 | (14, 16) |
| Flatten_2 | Just flatten the matrix | (224) |
| Concatenate | concatenate the Flatten_1 and Flatten_2 | (704) |
| Dense | Activation = relu | (16) |
| Units = 16 | ||
| Dropout | Rate = 0.4 | (16) |
| Dense | Activation = softmax | (2) |
| Units = 2 |
| Models | 10-Fold Cross Validation | Independent | ||
|---|---|---|---|---|
| Predictor | ACC | F-Score | ACC | F-Score |
| LSTM-emb | 0.700 | 0.735 | 0.734 | 0.779 |
| CNN-emb | 0.704 | 0.739 | 0.733 | 0.776 |
| BiLSTM-onehot | 0.725 | 0.729 | 0.757 | 0.777 |
| CNN-onehot | 0.719 | 0.731 | 0.748 | 0.778 |
| CNN-onehot-PCA | 0.748 | 0.750 | 0.768 | 0.786 |
| Comb-emb-PCA (UbiComb) | 0.804 | 0.795 | 0.818 | 0.825 |
| RF-Comb | 0.762 | 0.757 | 0.781 | 0.800 |
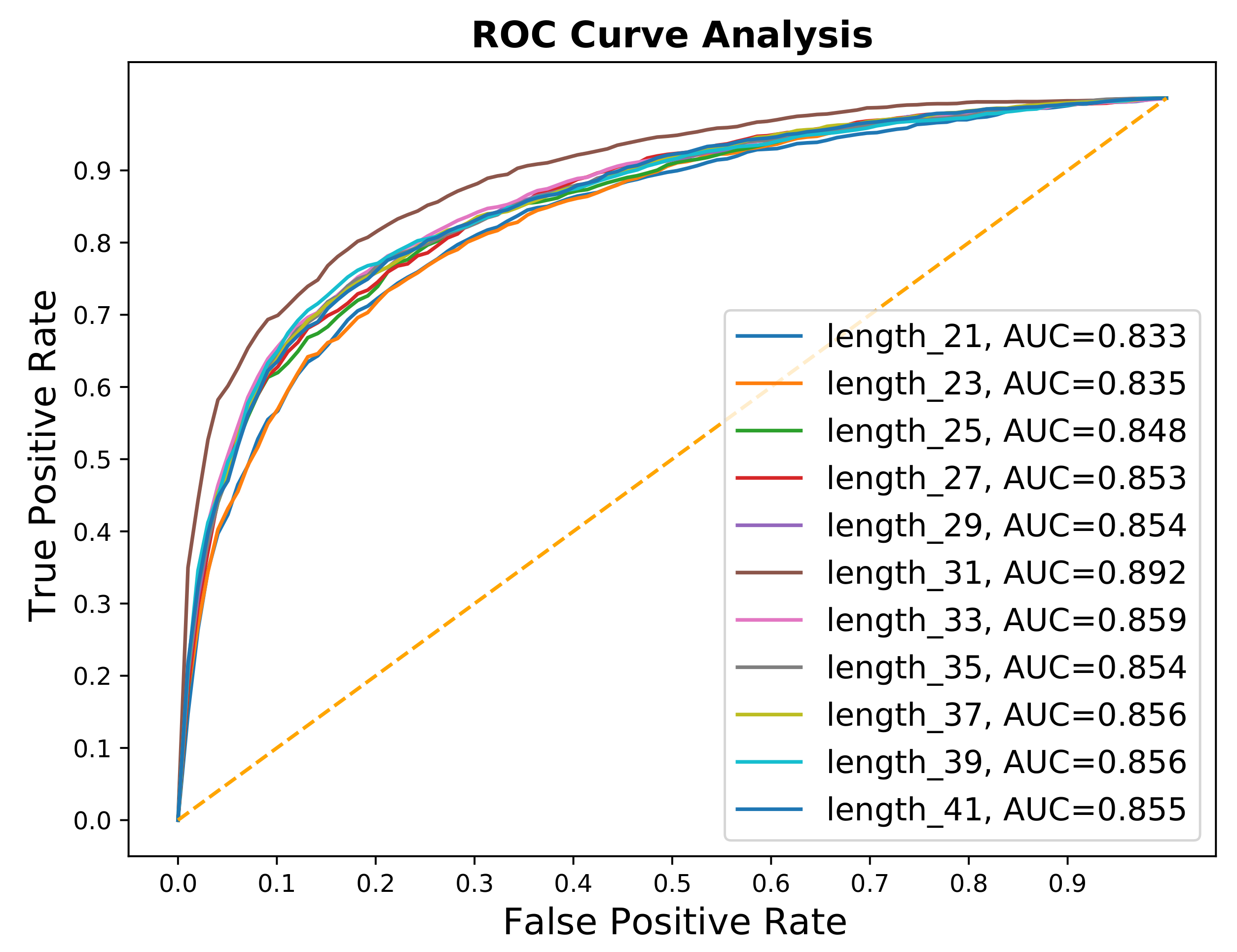
| Fragment | ACC | F-Score | AUC |
|---|---|---|---|
| 21 | 0.762 | 0.753 | 0.833 |
| 23 | 0.754 | 0.744 | 0.835 |
| 25 | 0.767 | 0.759 | 0.848 |
| 27 | 0.774 | 0.760 | 0.853 |
| 29 | 0.779 | 0.763 | 0.854 |
| 31 | 0.805 | 0.795 | 0.892 |
| 33 | 0.780 | 0.769 | 0.859 |
| 35 | 0.782 | 0.773 | 0.854 |
| 37 | 0.771 | 0.770 | 0.856 |
| 39 | 0.788 | 0.777 | 0.856 |
| 41 | 0.777 | 0.763 | 0.855 |
| Models | 10-Fold Cross Validation | Independent | ||
|---|---|---|---|---|
| Predictor | ACC | F-Score | ACC | F-Score |
| Wang et al., | 0.782 | 0.785 | 0.791 | 0.782 |
| UbiComb | 0.805 | 0.795 | 0.818 | 0.825 |
| Models | 10-Fold Cross Validation | Independent | ||
|---|---|---|---|---|
| Predictor | ACC | F-Score | ACC | F-Score |
| UbPred | 0.719 | 0.738 | 0.626 | 0.678 |
| iUbiq-Lys | 0.799 | 0.837 | 0.563 | 0.671 |
| Ubisite | 0.752 | 0.794 | 0.596 | 0.681 |
| Deep Ub | 0.683 | 0.703 | 0.674 | 0.687 |
| DeepUbi | 0.739 | 0.741 | 0.733 | 0.734 |
| Wang et al., | 0.756 | 0.767 | 0.733 | 0.749 |
| UbiComb | 0.805 | 0.795 | 0.818 | 0.825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siraj, A.; Lim, D.Y.; Tayara, H.; Chong, K.T. UbiComb: A Hybrid Deep Learning Model for Predicting Plant-Specific Protein Ubiquitylation Sites. Genes 2021, 12, 717. https://doi.org/10.3390/genes12050717
Siraj A, Lim DY, Tayara H, Chong KT. UbiComb: A Hybrid Deep Learning Model for Predicting Plant-Specific Protein Ubiquitylation Sites. Genes. 2021; 12(5):717. https://doi.org/10.3390/genes12050717
Chicago/Turabian StyleSiraj, Arslan, Dae Yeong Lim, Hilal Tayara, and Kil To Chong. 2021. "UbiComb: A Hybrid Deep Learning Model for Predicting Plant-Specific Protein Ubiquitylation Sites" Genes 12, no. 5: 717. https://doi.org/10.3390/genes12050717
APA StyleSiraj, A., Lim, D. Y., Tayara, H., & Chong, K. T. (2021). UbiComb: A Hybrid Deep Learning Model for Predicting Plant-Specific Protein Ubiquitylation Sites. Genes, 12(5), 717. https://doi.org/10.3390/genes12050717








