Transcriptomic Changes in Mouse Bone Marrow-Derived Macrophages Exposed to Neuropeptide FF
Abstract
1. Introduction
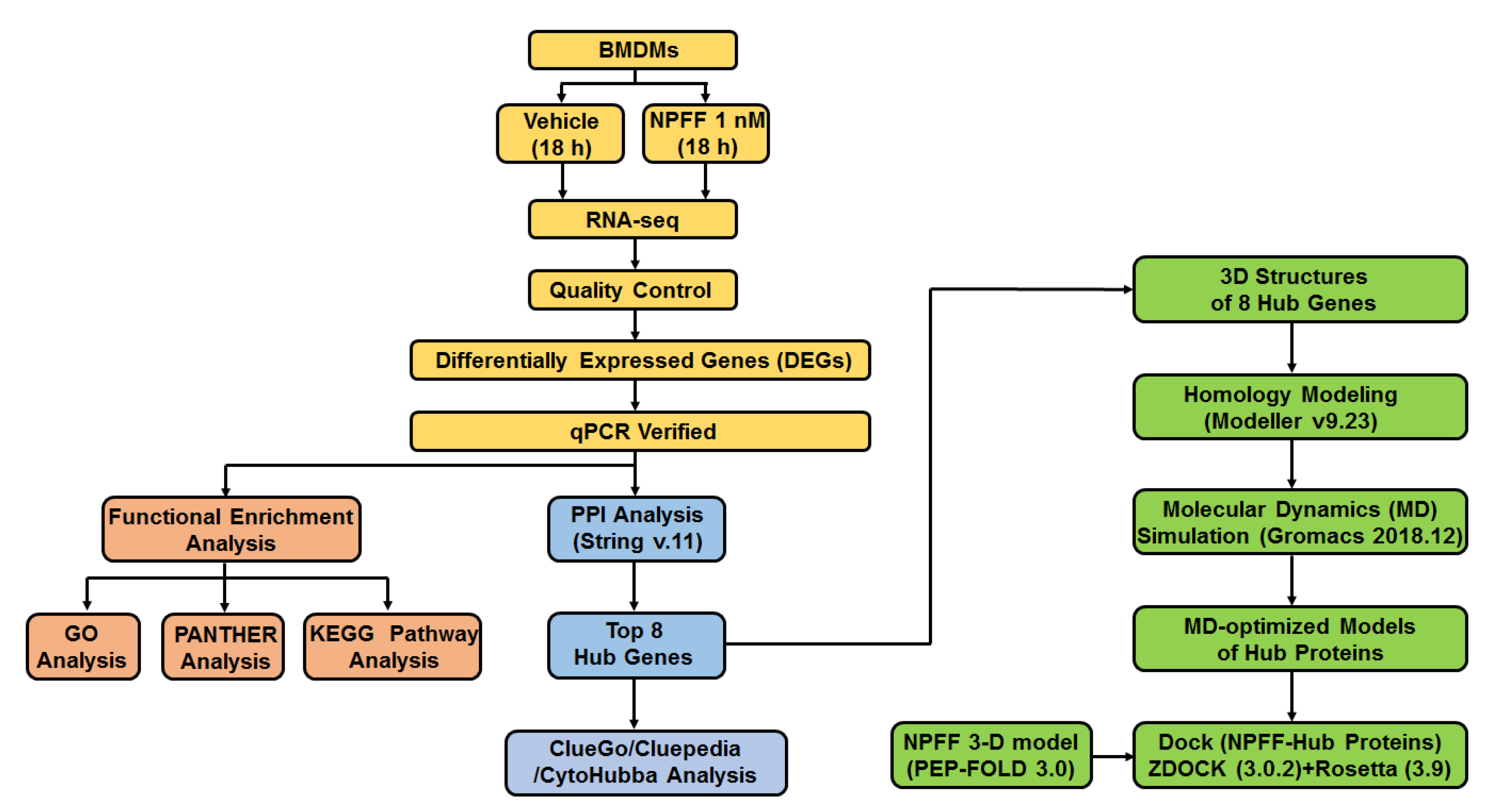
2. Materials and Methods
2.1. Ethical Statement
2.2. Mice
2.3. Materials
2.4. Isolation of BMDMs
2.5. Cell Sample Preparation and Microscope Detection
2.6. Cell Viability Assay
2.7. Flow Cytometry Experiment
2.8. RNA-Seq Sample Collection and Preparation
2.8.1. RNA Qualification and Quantification
2.8.2. Library Preparation for RNA Sequencing
2.8.3. Sequencing and Clustering
2.9. RNA-Seq Data Interpretation
2.9.1. Quality Control
2.9.2. Reads Mapping to the Musculus Reference Genome
2.9.3. Quantification of Gene Expression
2.9.4. Differential Expression Interpretation
2.9.5. GO and KEGG Enrichment Analysis
2.9.6. Protein–Protein Interaction (PPI) Network Analysis
2.10. Gene Expression Analysis
2.11. Western Blot
2.12. Immunofluorescence Stain Assay
2.13. Homology Modeling of Hub Proteins
2.14. Molecular Dynamics (MD) Simulation
2.15. Dock
2.16. Statistical Analysis
3. Results
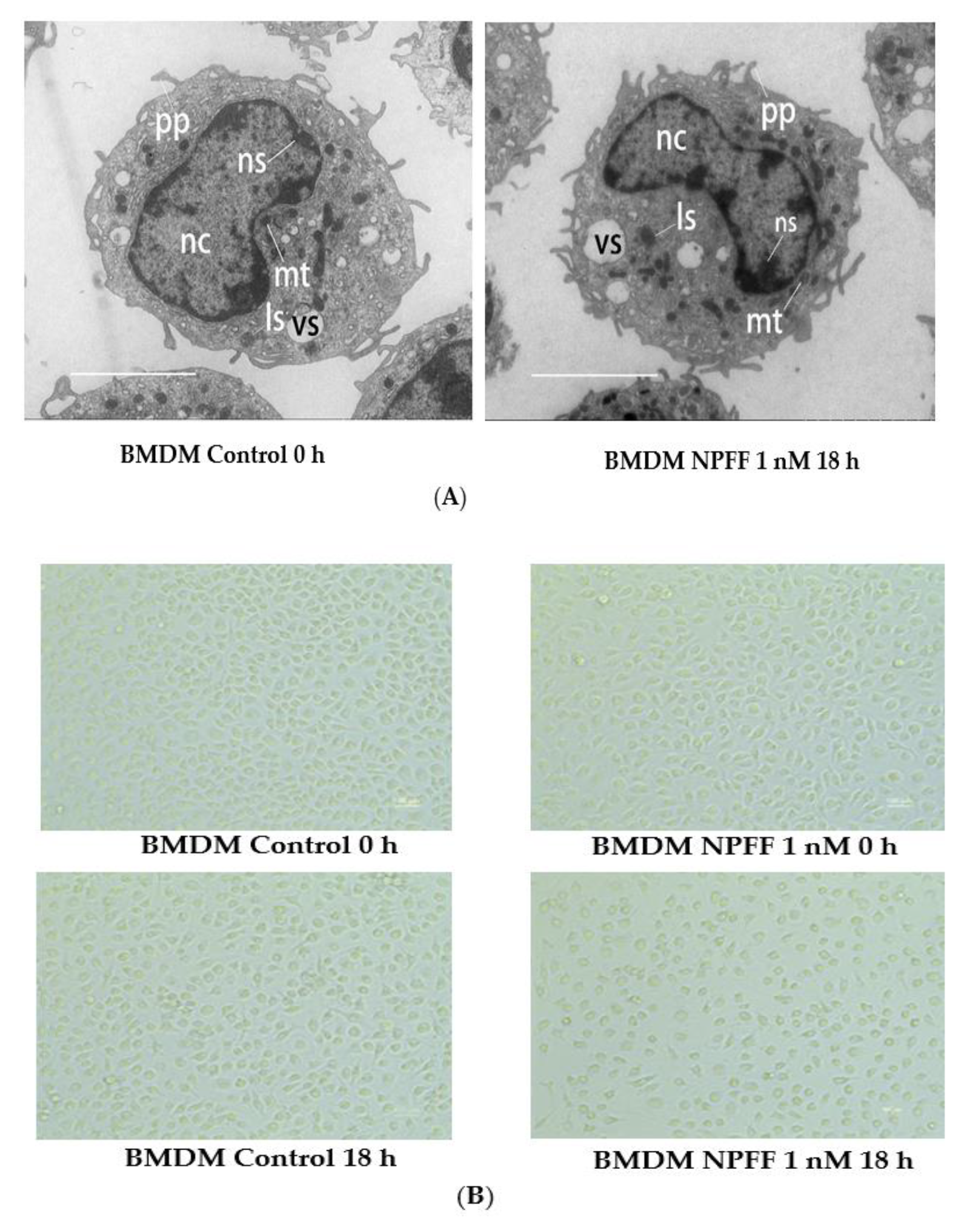
3.1. The Effect of NPFF on the Morphology and Viability of BMDMs
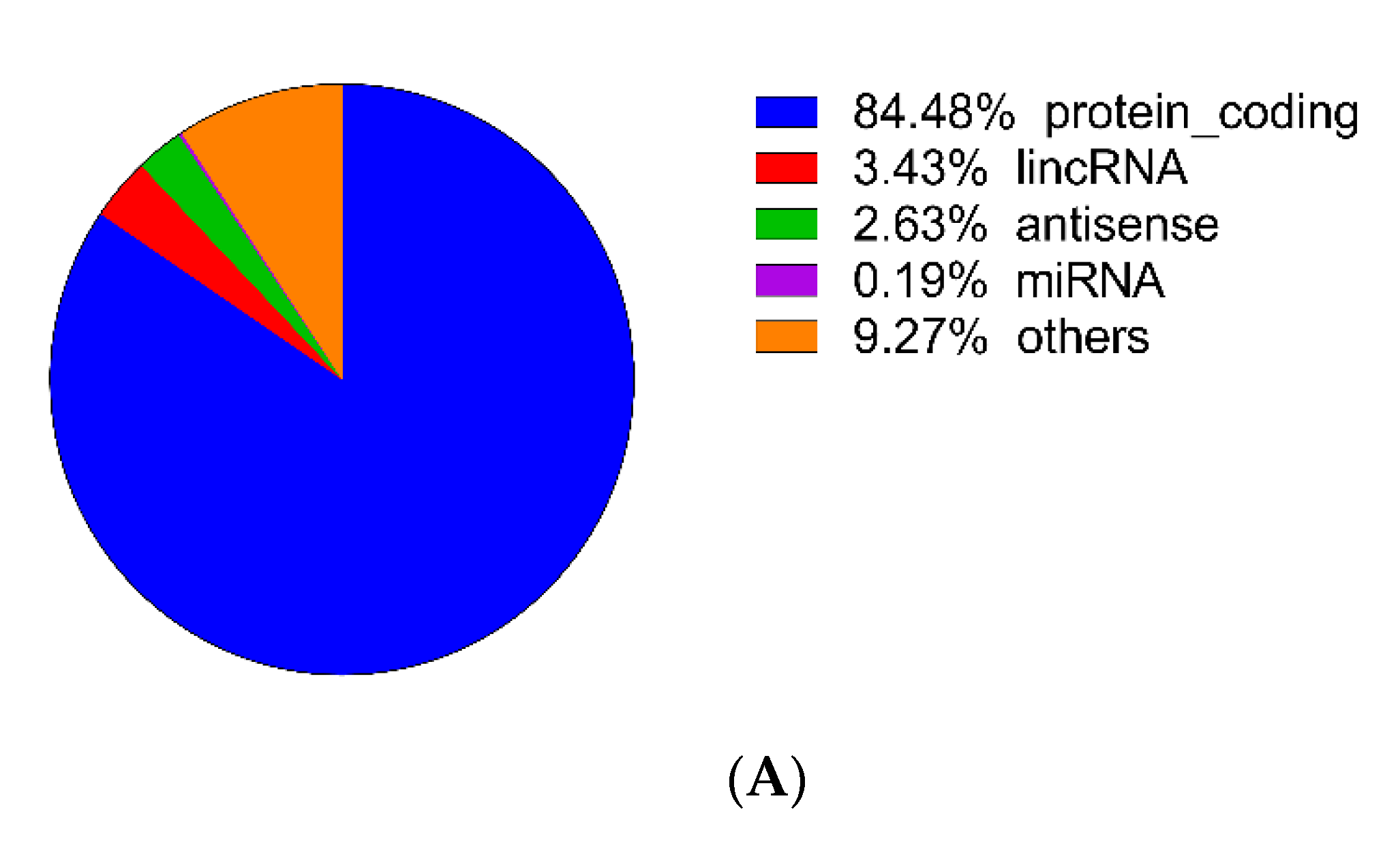
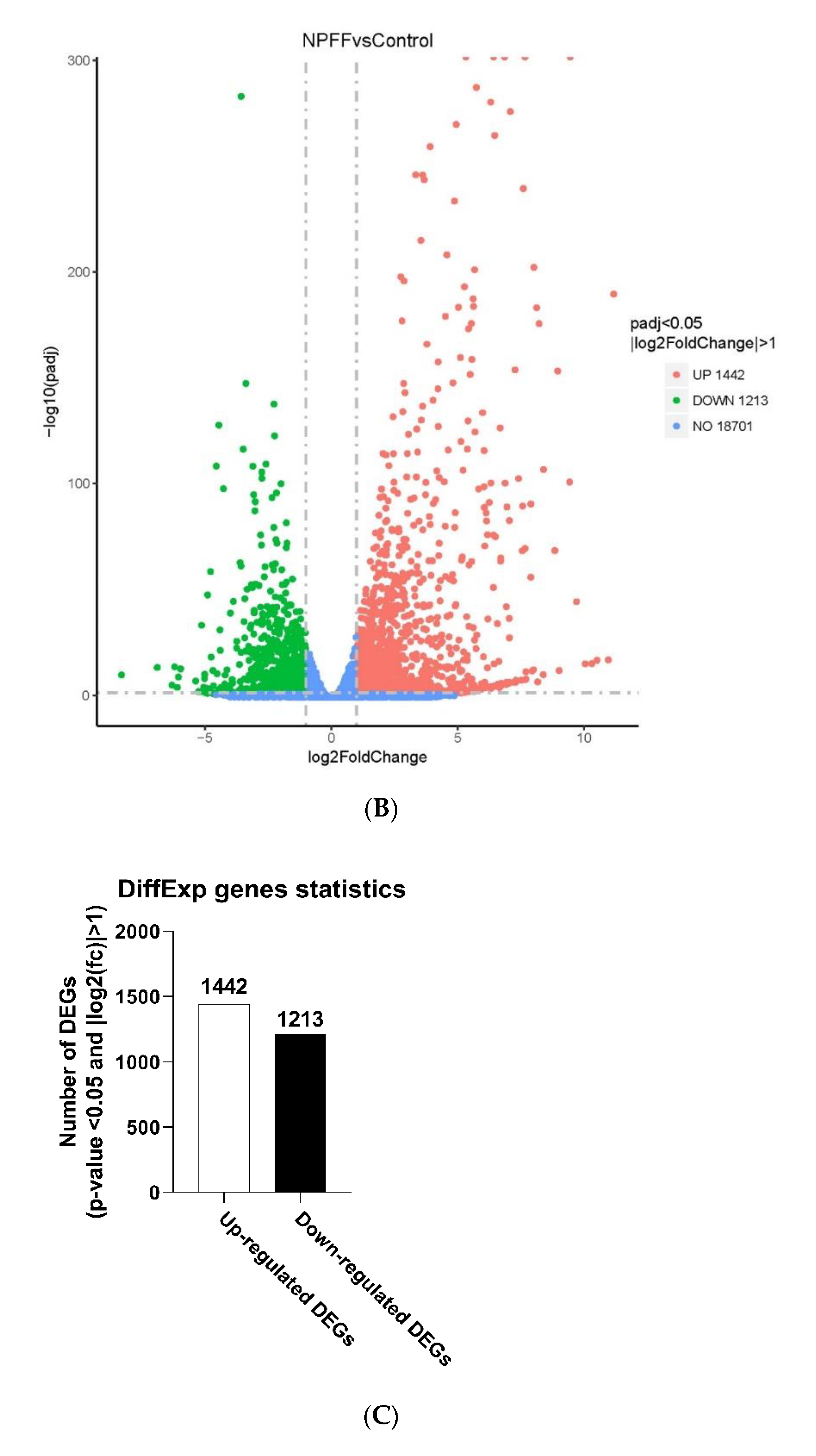
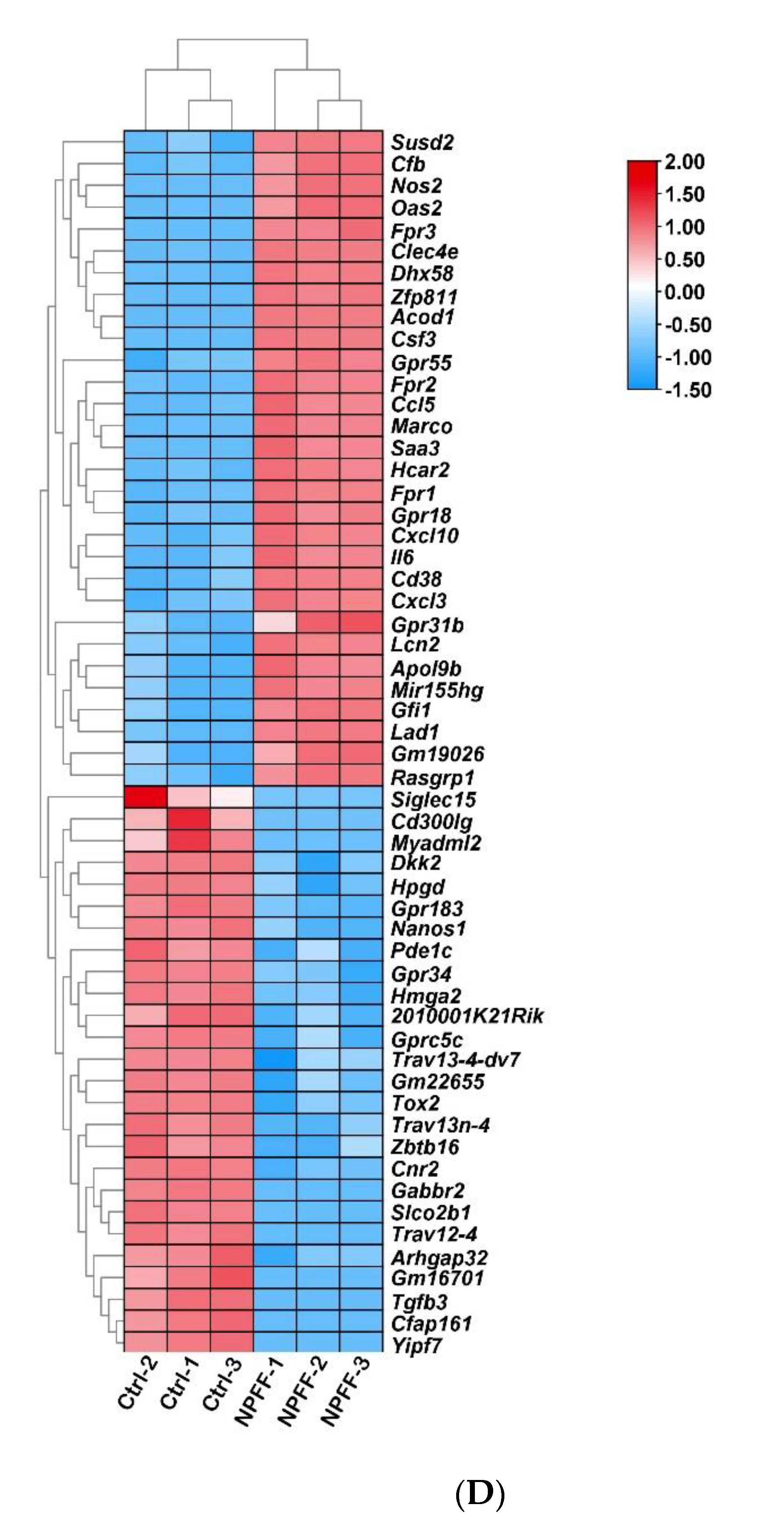
3.2. Identification of DEGs
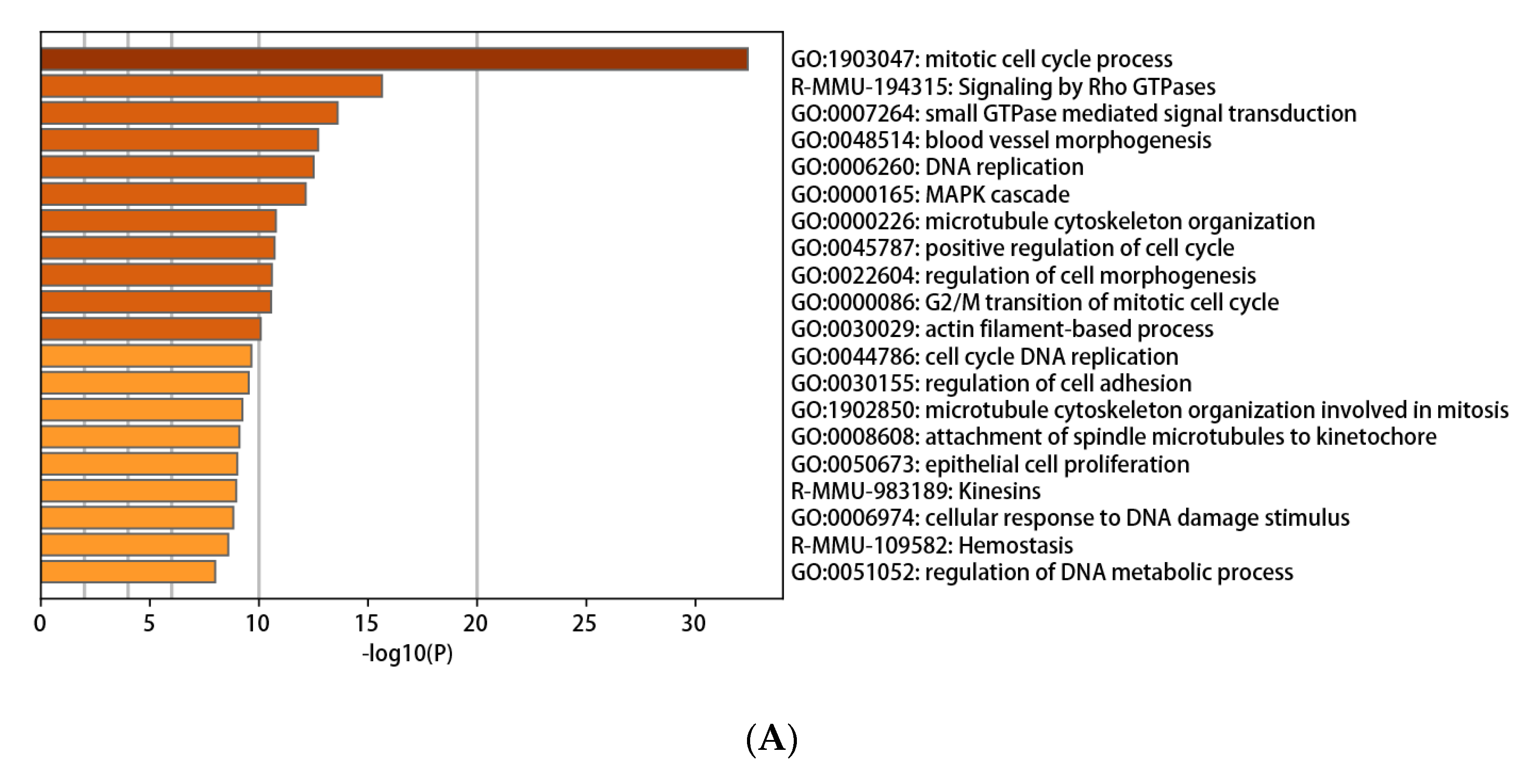
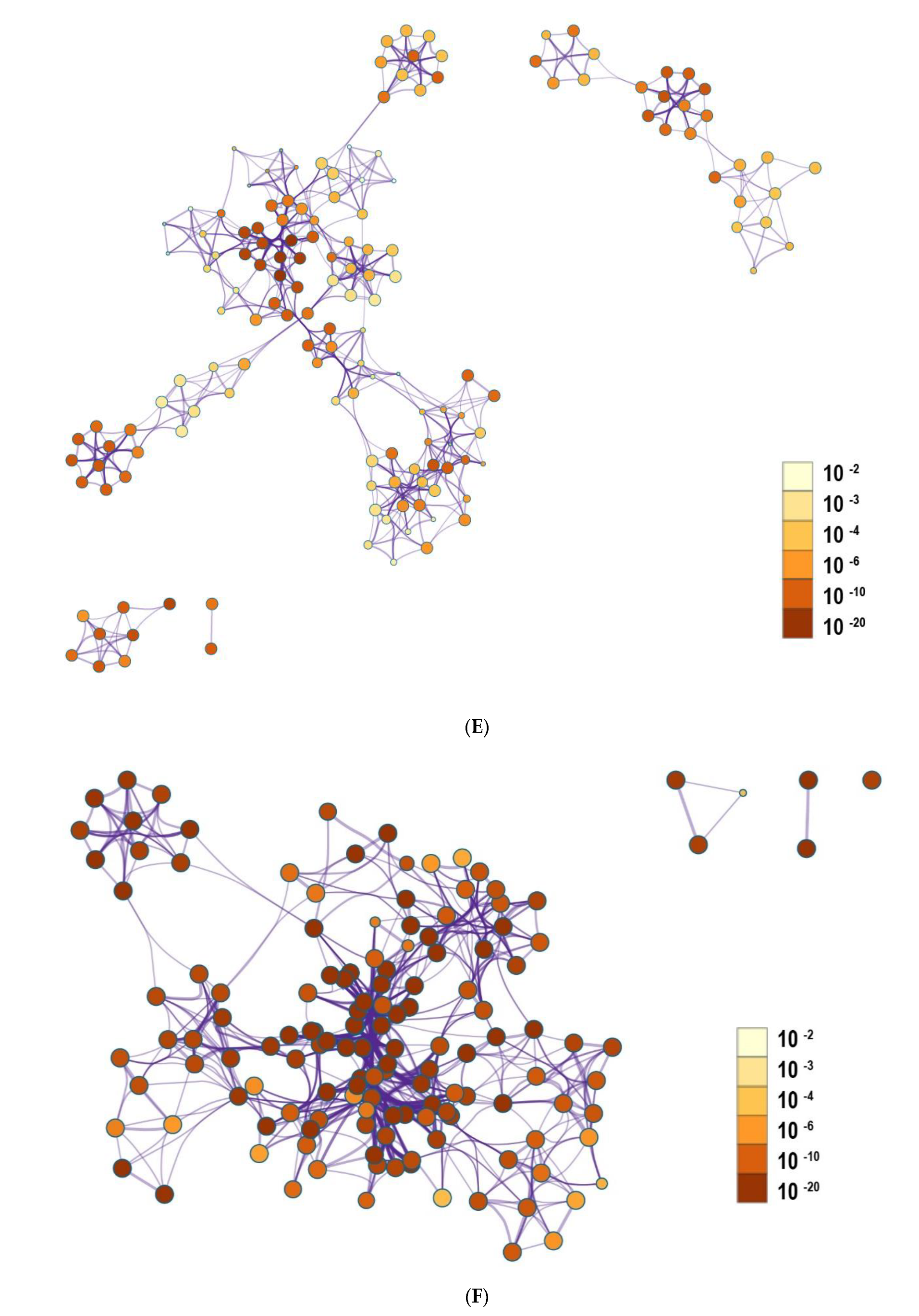
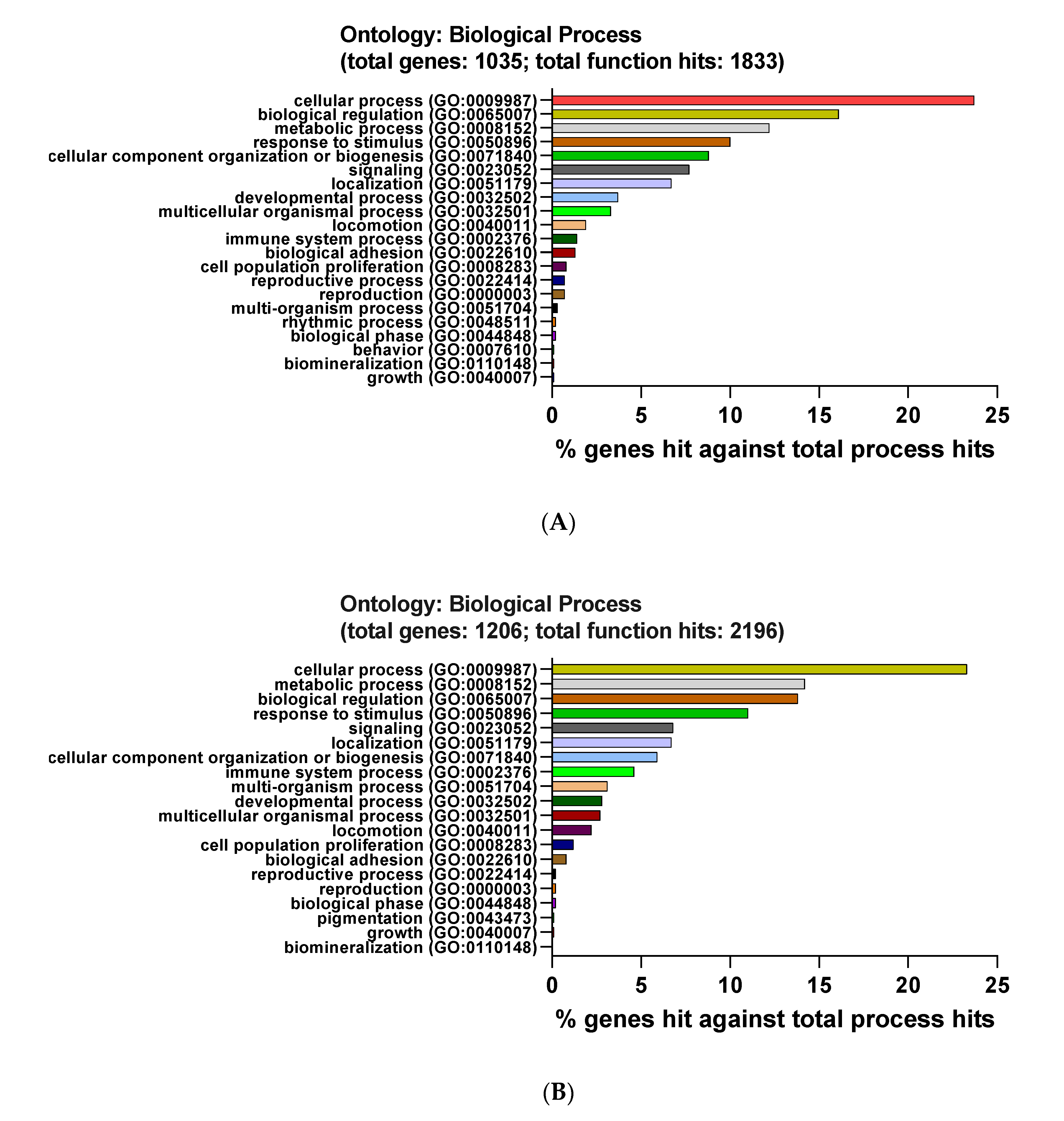
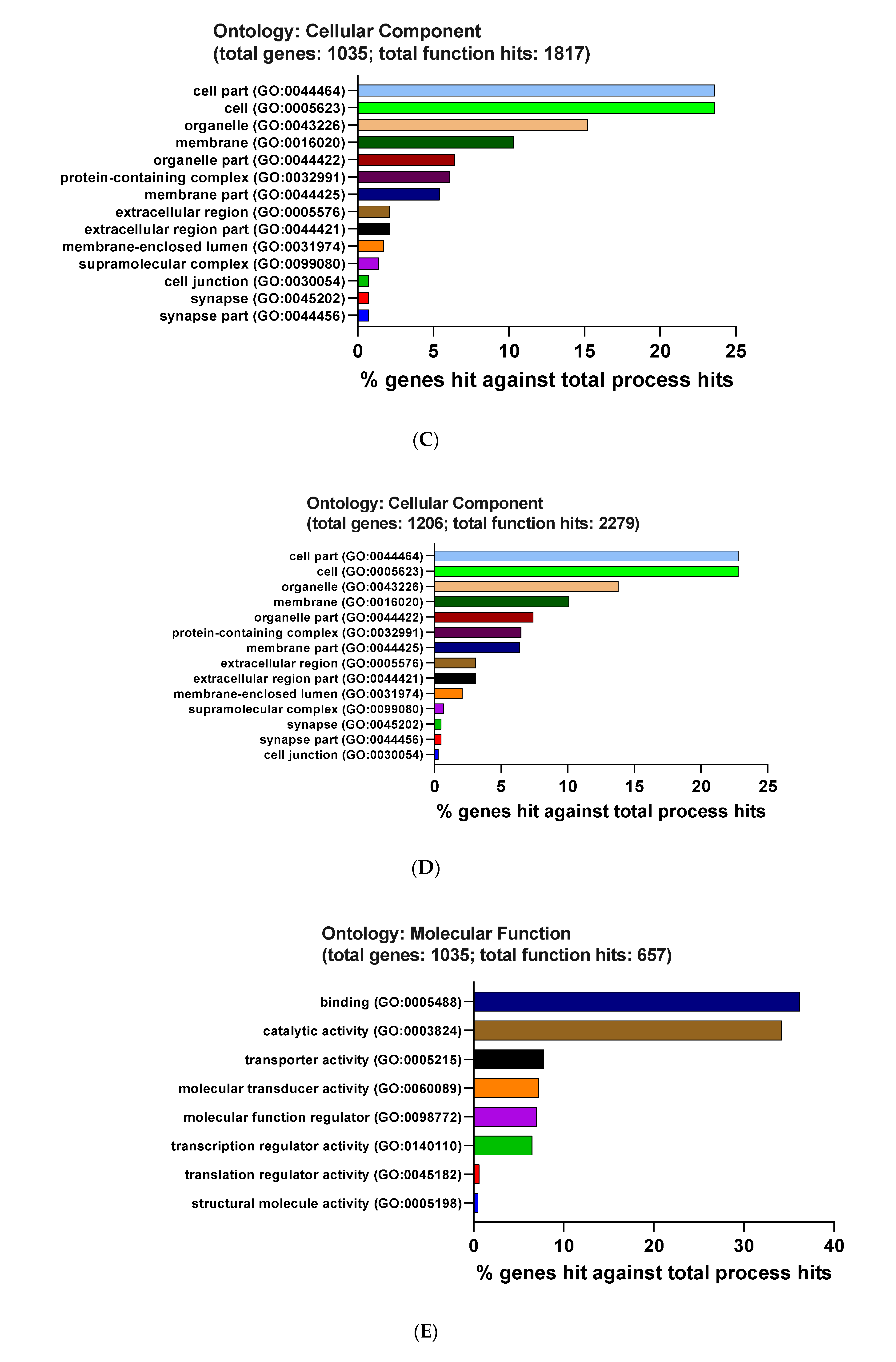
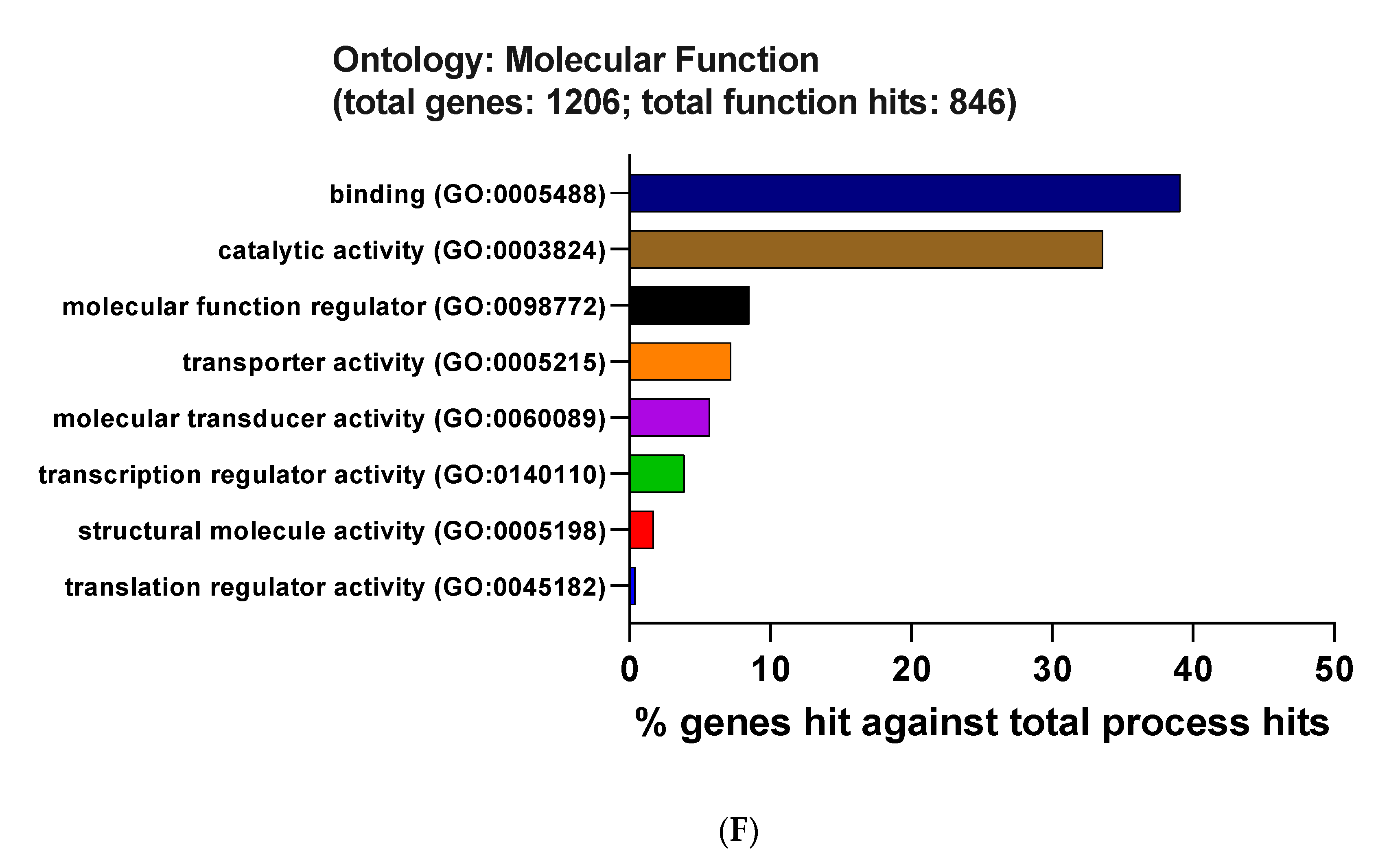
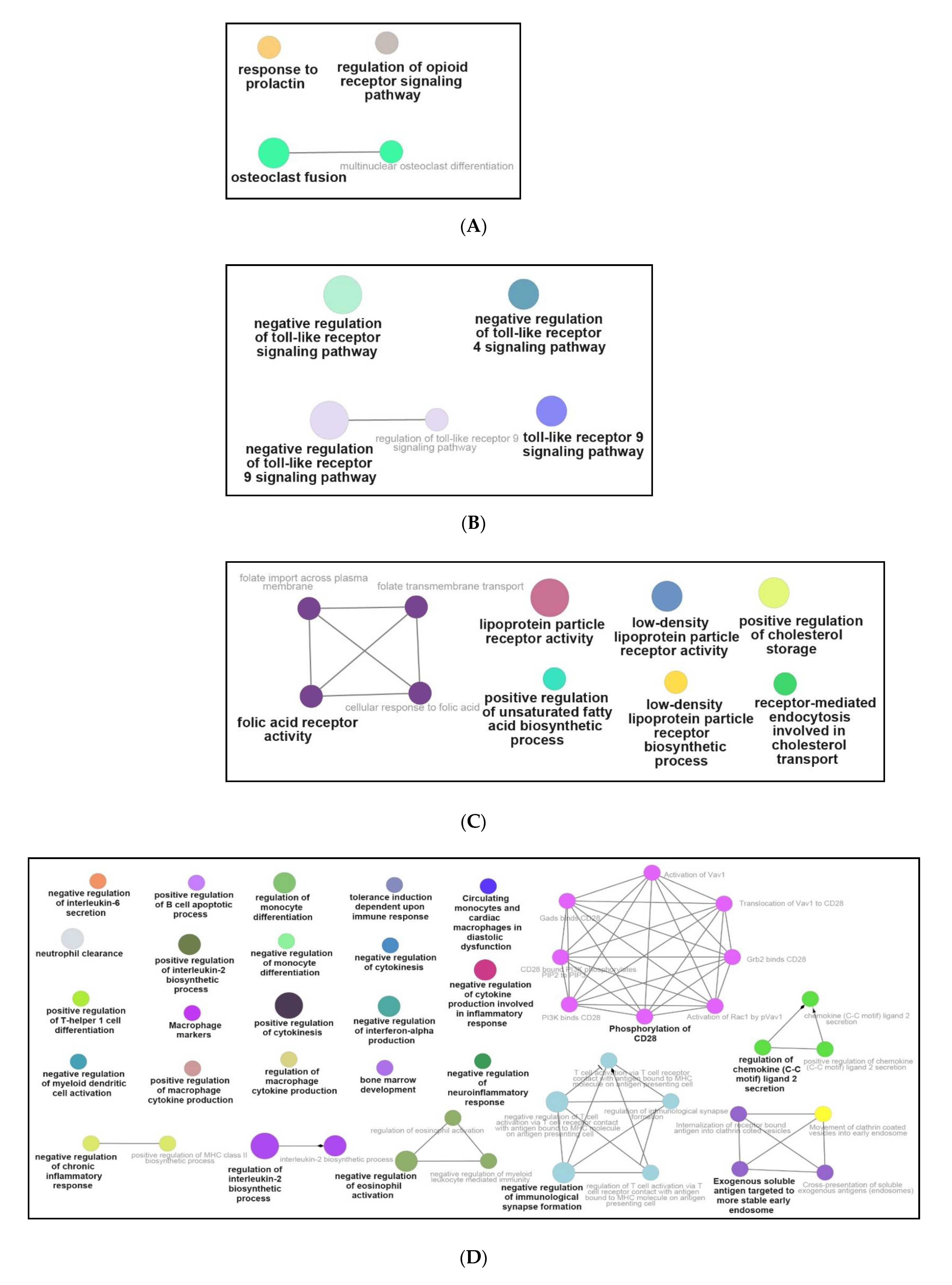
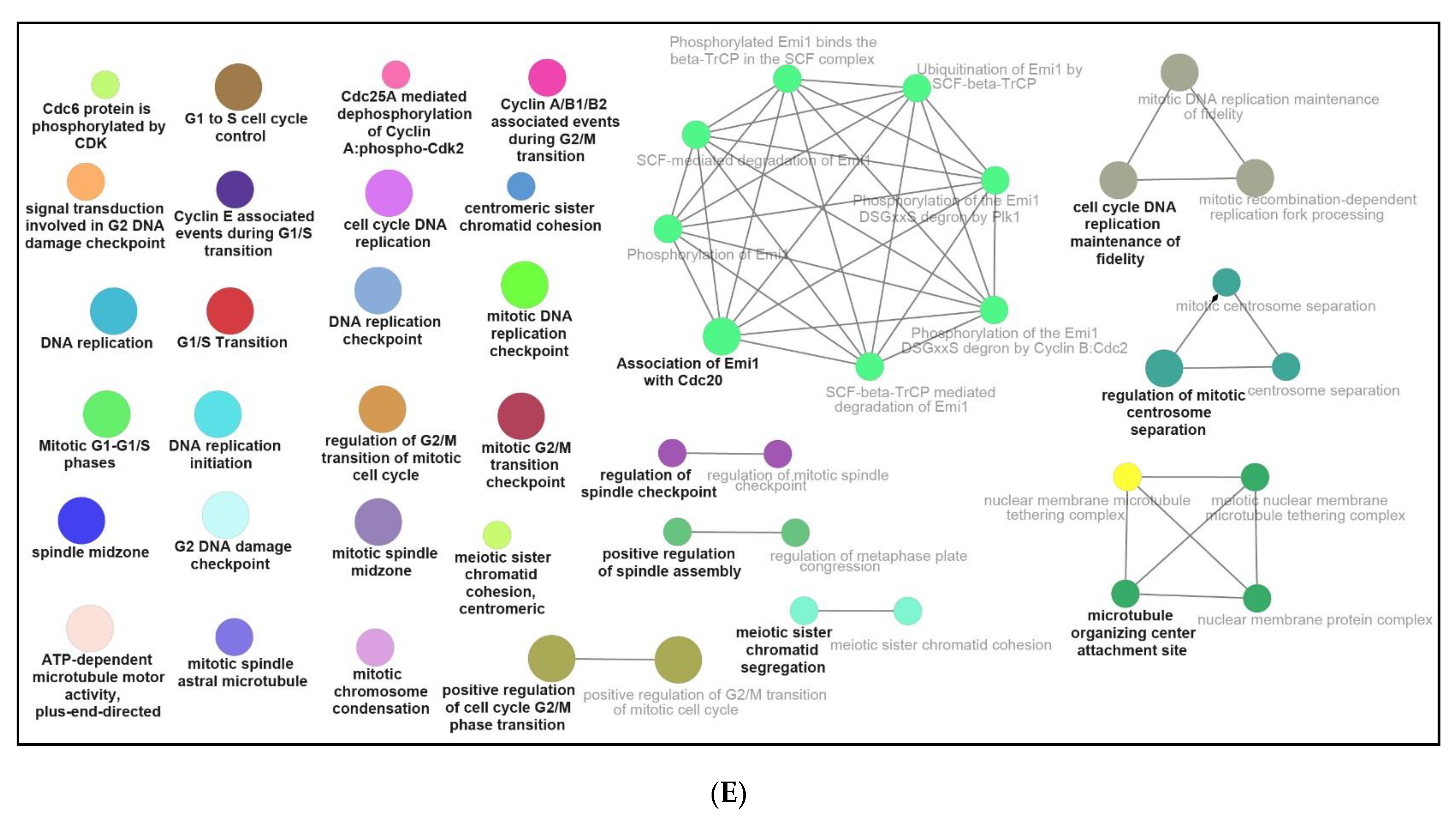
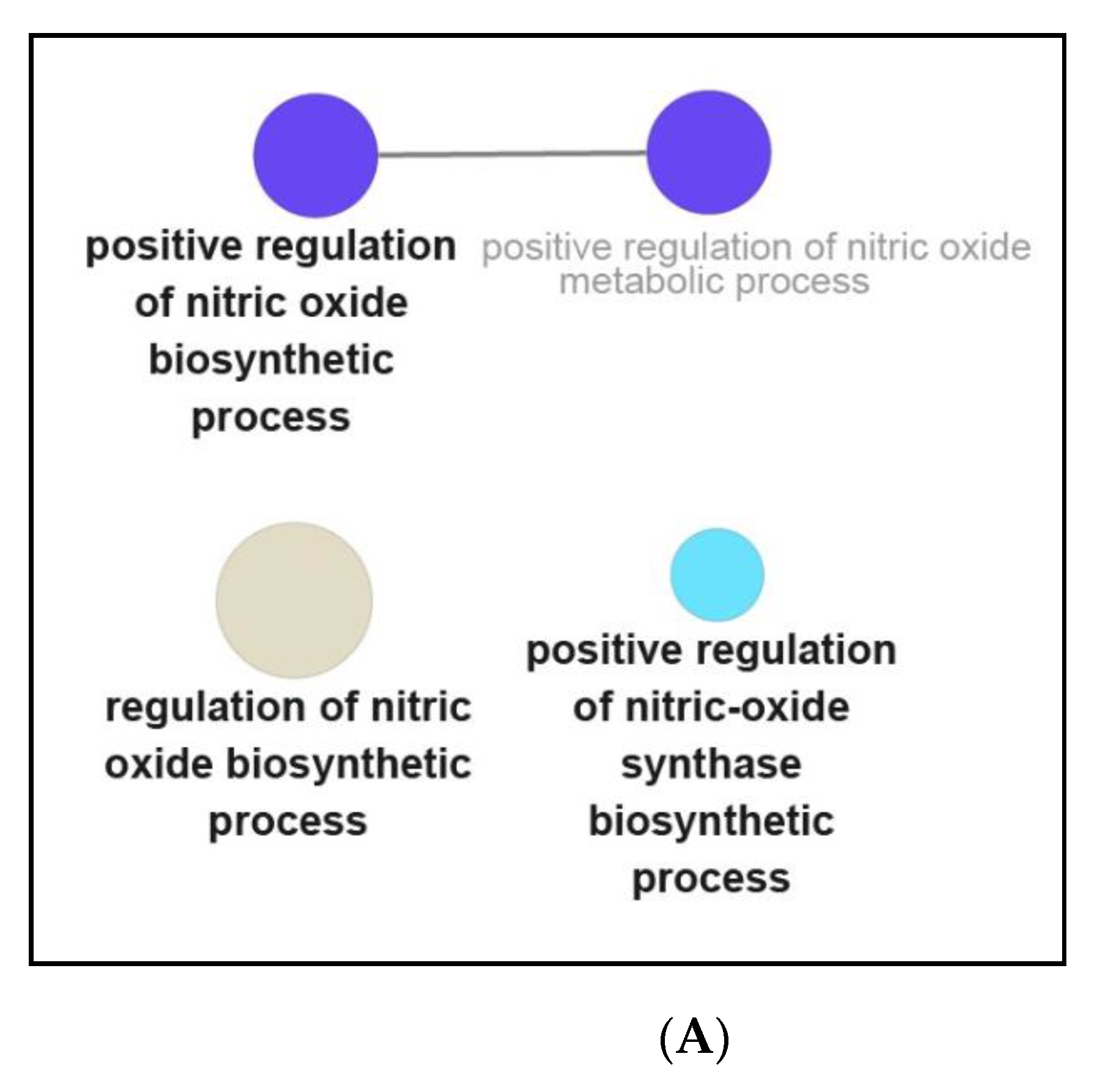
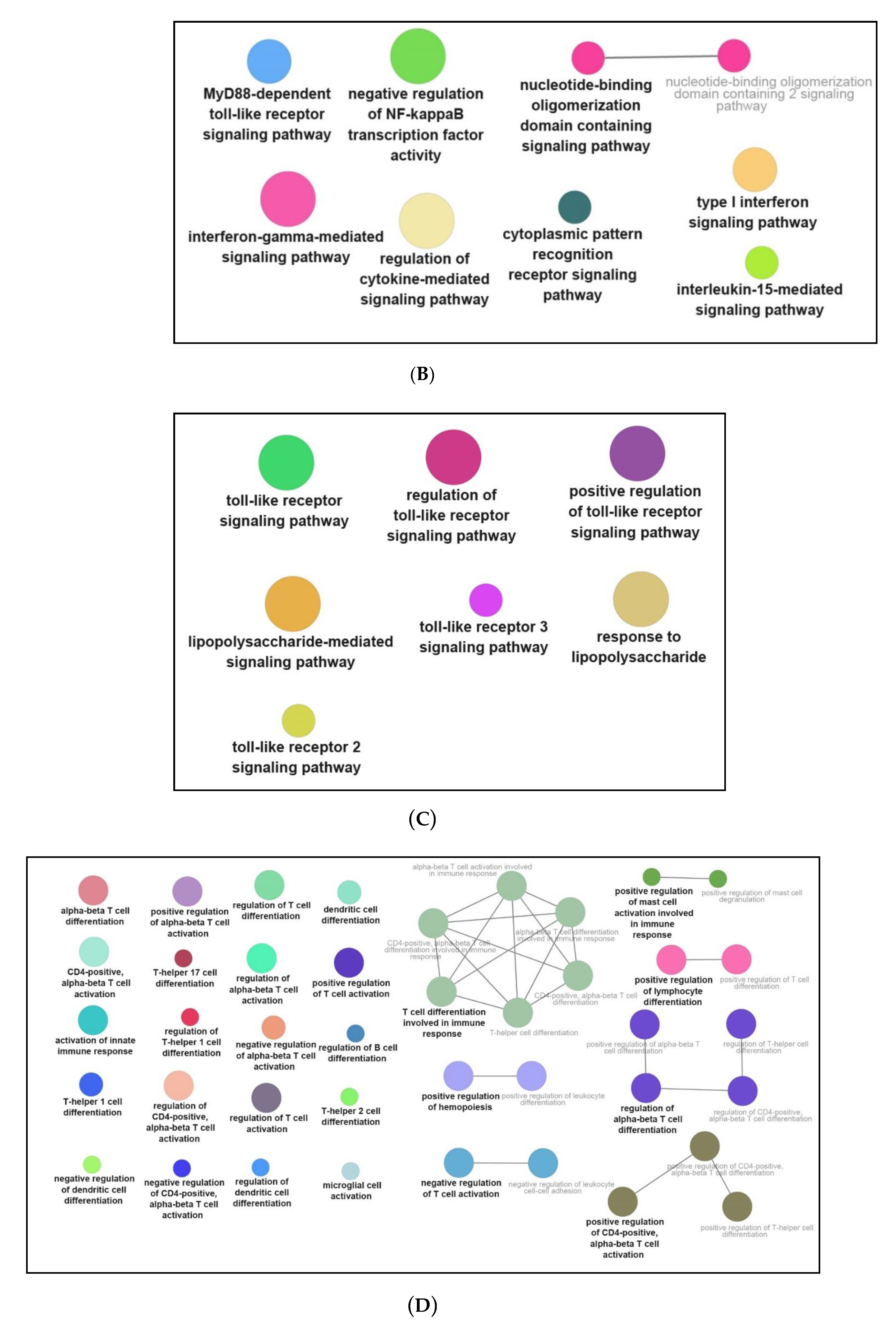
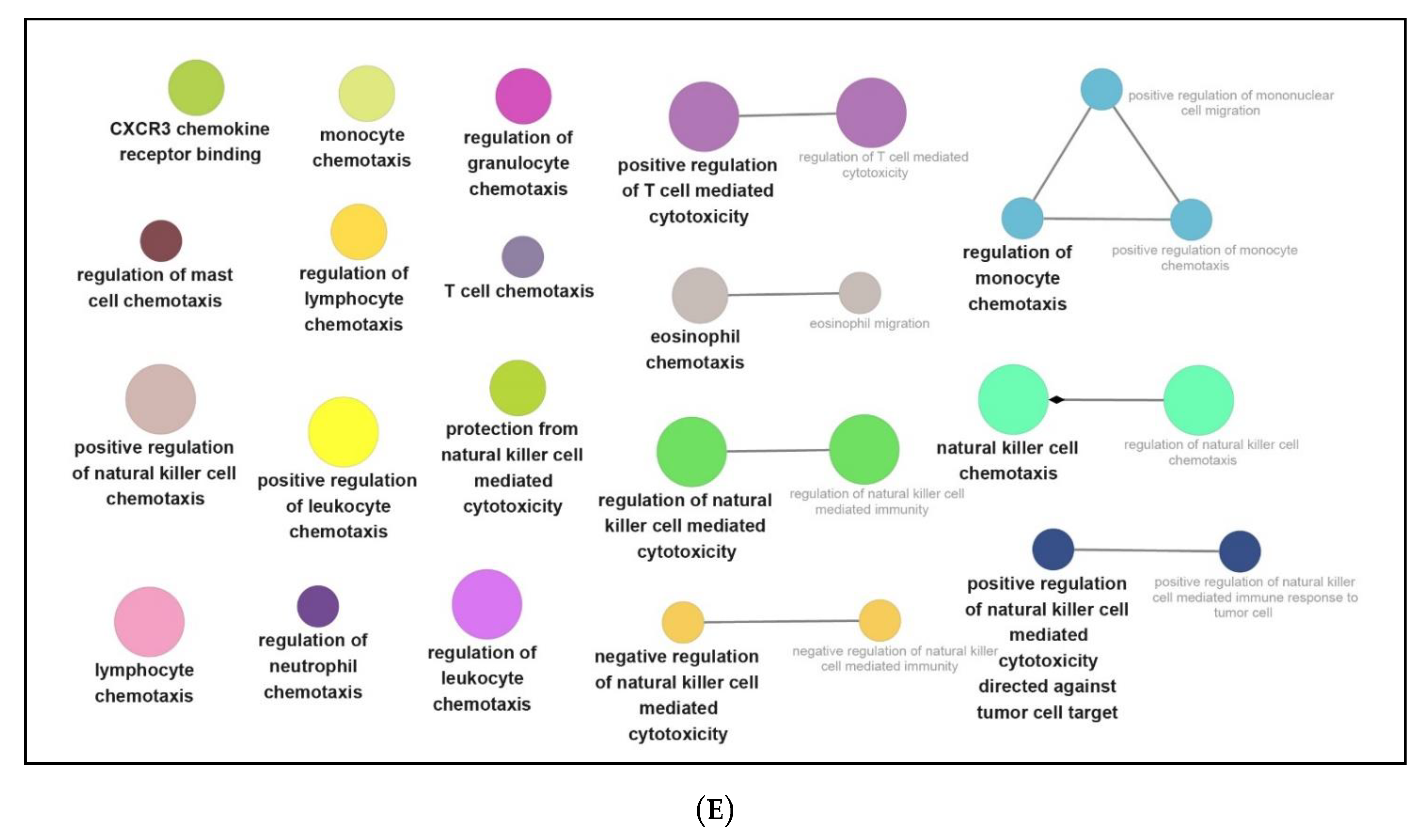
3.3. Functional and Pathway Enrichment Interpretation of DEGs
3.4. Identification of Hub Genes from Protein–Protein Interaction (PPI) Network
3.5. Common Transcription Factors Tied to Genes Down-Regulated by NPFF
3.6. Verification of Hub Genes with qPCR
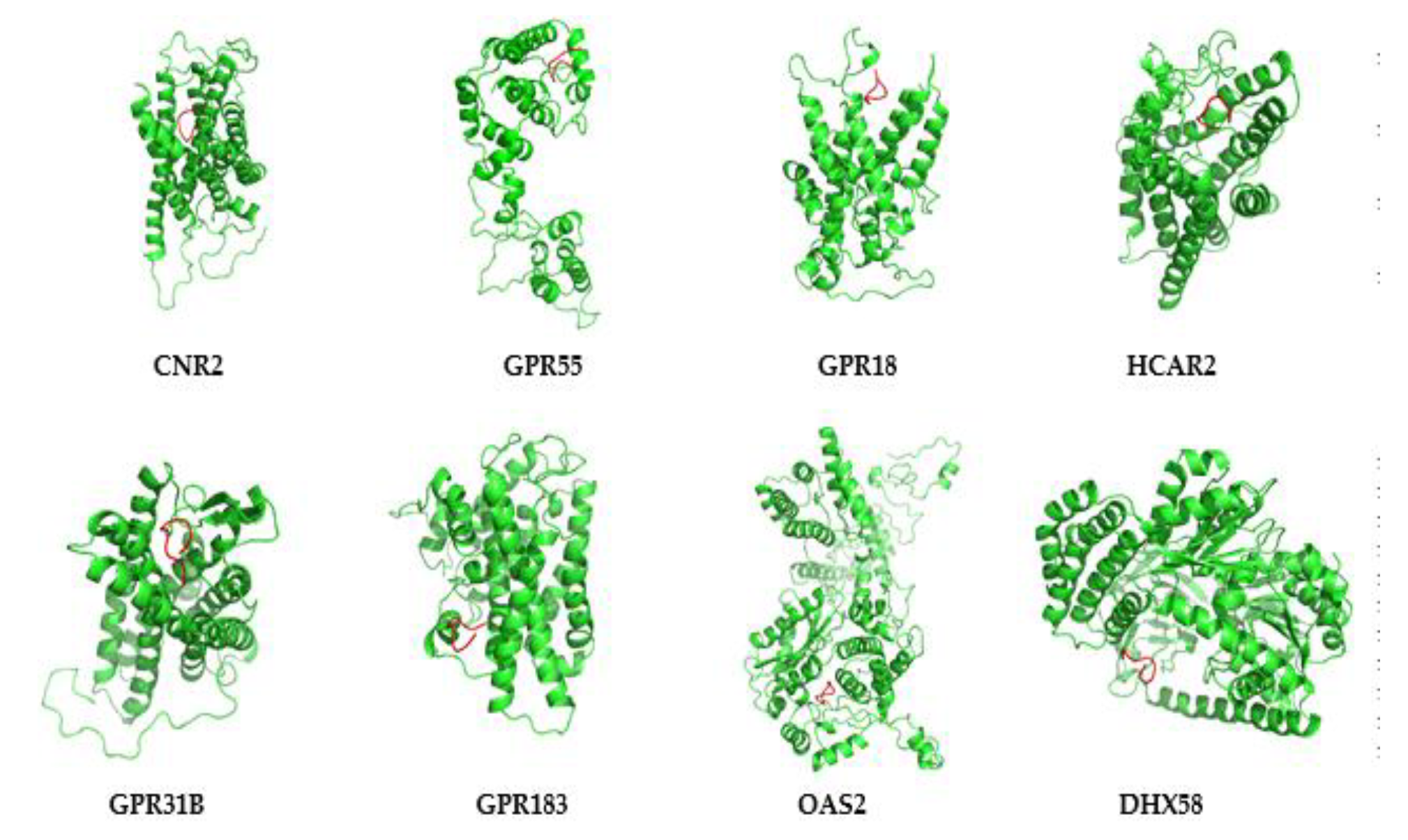
3.7. Protein Modeling of Hub Proteins
3.8. Molecular Dynamics Simulation of Hub Proteins
3.9. Peptide-Hub Protein Docking
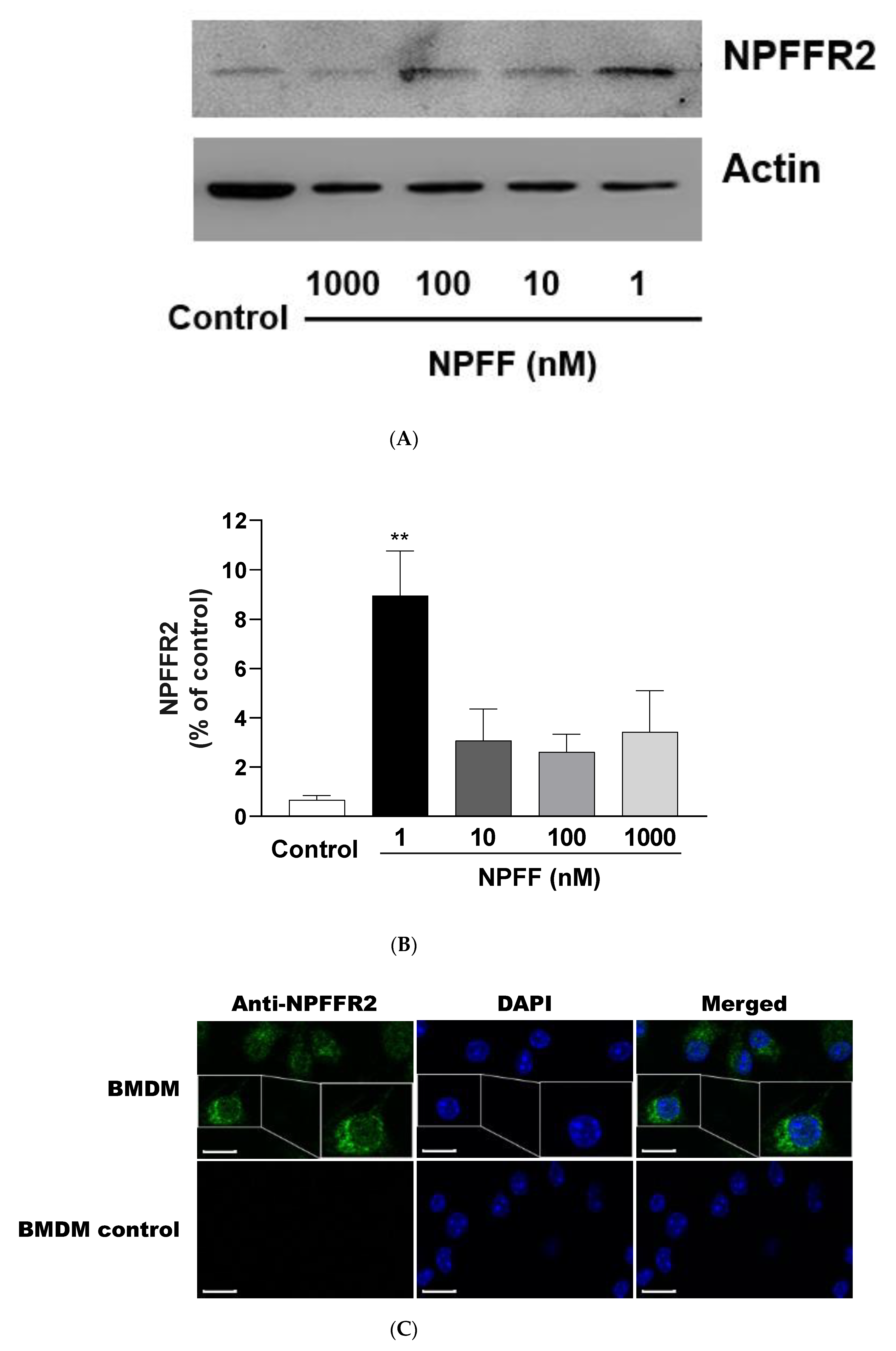
3.10. Expression of NPFFR2 on BMDMs
4. Discussion
4.1. The Effect of NPFF on the Morphology and Viability of BMDMs
4.2. NPFF Regulated Different Functional Enrichment Pathways of BMDMs
4.3. Common Transcription Factors Tied to NPFF-Regulated DEGs in BMDMs
4.4. The Concentration of NPFF in the Experimental System for High-Throughput Sequencing
4.5. Possible Modes of Interaction between NPFF and Hub Proteins
4.6. Expression of NPFFR2 on BMDMs
4.7. The Effects of Neuropeptides on Immune Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weischenfeldt, J.; Porse, B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008, 2008, pdb.prot5080. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Fratta, W.; Majane, E.A.; Costa, E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acad. Sci. USA 1985, 82, 7757–7761. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Marusich, J.; Li, J.X.; Zhang, Y. Neuropeptide FF and Its Receptors: Therapeutic Applications and Ligand Development. J. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Tao, T.; Iadarola, M.J. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides 2008, 42, 1–18. [Google Scholar] [CrossRef]
- Simonin, F.; Schmitt, M.; Laulin, J.P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA 2006, 103, 466–471. [Google Scholar] [CrossRef]
- Sunter, D.; Hewson, A.K.; Lynam, S.; Dickson, S.L. Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci. Lett. 2001, 313, 145–148. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, X.; Chen, P.; Yu, Y.; Wang, Y.; Xie, L. Neuropeptide FF Promotes Recovery of Corneal Nerve Injury Associated With Hyperglycemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7754–7765. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Yu, Z.; Tsai, S.C.; Hsu, P.H.; Chen, J.C. Neuropeptide FF receptor 2 inhibits capsaicin-induced CGRP Upregulation in mouse trigeminal ganglion. J. Headache Pain 2020, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Liu, H.-L.; Day, Y.-J.; Chang, C.-C.; Hsu, P.-H.; Chen, J.-C. Activation of NPFFR2 leads to hyperalgesia through the spinal inflammatory mediator CGRP in mice. Exp. Neurol. 2017, 291, 62–73. [Google Scholar] [CrossRef] [PubMed]
- La Rochelle, A.D.; Guillemyn, K.; Dumitrascuta, M.; Martin, C.; Utard, V.; Quillet, R.; Schneider, S.; Daubeuf, F.; Willemse, T.; Mampuys, P.; et al. A bifunctional-biased mu-opioid agonist-neuropeptide FF receptor antagonist as analgesic with improved acute and chronic side effects. Pain 2018, 159, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Maletinska, L.; Ticha, A.; Nagelova, V.; Spolcova, A.; Blechova, M.; Elbert, T.; Zelezna, B. Neuropeptide FF analog RF9 is not an antagonist of NPFF receptor and decreases food intake in mice after its central and peripheral administration. Brain Res. 2013, 1498, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp. Physiol. 2004, 89, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, H.; Li, Y.; Zhang, Z.; Wang, L.; Mao, X.; Li, J.; Qi, X. FOXO1A promotes neuropeptide FF transcription subsequently regulating the expression of feeding-related genes in spotted sea bass (Lateolabrax maculatus). Mol. Cell. Endocrinol. 2020, 110871. [Google Scholar] [CrossRef]
- Li, Q.; Wen, H.; Li, Y.; Zhang, Z.; Zhou, Y.; Qi, X. Evidence for the Direct Effect of the NPFF Peptide on the Expression of Feeding-Related Factors in Spotted Sea Bass (Lateolabrax maculatus). Front. Endocrinol. (Lausanne) 2019, 10, 545. [Google Scholar] [CrossRef]
- Zhang, L.; Ip, C.K.; Lee, I.J.; Qi, Y.; Reed, F.; Karl, T.; Low, J.K.; Enriquez, R.F.; Lee, N.J.; Baldock, P.A.; et al. Diet-induced adaptive thermogenesis requires neuropeptide FF receptor-2 signalling. Nat. Commun. 2018, 9, 4722. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, B.; Klausen, C.; Yi, Y.; Li, Y.; Xiong, S.; von Dadelszen, P.; Leung, P.C.K. NPFF increases fusogenic proteins syncytin 1 and syncytin 2 via GCM1 in first trimester primary human cytotrophoblast cells. FASEB J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Goncharuk, V. Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front. Endocrinol. (Lausanne) 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Waqas, S.F.H.; Hoang, A.C.; Lin, Y.-T.; Ampem, G.; Azegrouz, H.; Balogh, L.; Thuróczy, J.; Chen, J.-C.; Gerling, I.C.; Nam, S.; et al. Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J. Clin. Investig. 2017, 127, 2842–2854. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Yu, Y.-L.; Hong, W.-C.; Yeh, T.-S.; Chen, T.-C.; Chen, J.-C. NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents. Int. J. Mol. Sci. 2017, 18, 1810. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Liu, T.-Y.; Yang, C.-Y.; Yu, Y.-L.; Chen, T.-C.; Day, Y.-J.; Chang, C.-C.; Huang, G.-J.; Chen, J.-C. Chronic activation of NPFFR2 stimulates the stress-related depressive behaviors through HPA axis modulation. Psychoneuroendocrinology 2016, 71, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Huang, Y.L.; Tsai, S.C.; Chen, J.C. Ablation of NPFFR2 in Mice Reduces Response to Single Prolonged Stress Model. Cells 2020, 9, 2479. [Google Scholar] [CrossRef]
- Portelli, J.; Meurs, A.; Bihel, F.; Hammoud, H.; Schmitt, M.; De Kock, J.; Utard, V.; Humbert, J.P.; Bertin, I.; Buffel, I.; et al. Neuropeptide FF receptors as novel targets for limbic seizure attenuation. Neuropharmacology 2015, 95, 415–423. [Google Scholar] [CrossRef]
- Mouledous, L.; Barthas, F.; Zajac, J.M. Opposite control of body temperature by NPFF1 and NPFF2 receptors in mice. Neuropeptides 2010, 44, 453–456. [Google Scholar] [CrossRef]
- Yang, H.Y.; Iadarola, M.J. Activation of spinal neuropeptide FF and the neuropeptide FF receptor 2 during inflammatory hyperalgesia in rats. Neuroscience 2003, 118, 179–187. [Google Scholar] [CrossRef]
- Vilim, F.S.; Aarnisalo, A.A.; Nieminen, M.L.; Lintunen, M.; Karlstedt, K.; Kontinen, V.K.; Kalso, E.; States, B.; Panula, P.; Ziff, E. Gene for pain modulatory neuropeptide NPFF: Induction in spinal cord by noxious stimuli. Mol. Pharmacol. 1999, 55, 804–811. [Google Scholar]
- Pertovaara, A.; Hämäläinen, M.M.; Kauppila, T.; Panula, P. Carrageenan-induced changes in spinal nociception and its modulation by the brain stem. Neuroreport 1998, 9, 351–355. [Google Scholar] [CrossRef]
- Sun, Y.L.; Sun, T.; Zhang, X.Y.; He, N.; Zhuang, Y.; Li, J.Y.; Fang, Q.; Wang, K.R.; Wang, R. NPFF2 Receptor is Involved in the Modulatory Effects of Neuropeptide FF for Macrophage Cell Line. Protein Pept. Lett. 2014, 21, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhang, X.Y.; Sun, T.; He, N.; Li, J.Y.; Zhuang, Y.; Zeng, Q.; Yu, J.; Fang, Q.; Wang, R. The anti-inflammatory potential of neuropeptide FF in vitro and in vivo. Peptides 2013, 47, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kuang, Y.; Zuo, Z.; Zhang, J.; Ma, X.; Xing, X.; Liu, L.; Miao, Y.; Ren, T.; Li, H.; et al. Cellular processes involved in RAW 264.7 macrophages exposed to NPFF: A transcriptional study. Peptides 2021, 136, 170469. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Matteucci, C.; Argaw-Denboba, A.; Balestrieri, E.; Giovinazzo, A.; Miele, M.; D’Agostini, C.; Pica, F.; Grelli, S.; Paci, M.; Mastino, A.; et al. Deciphering cellular biological processes to clinical application: A new perspective for Tα1 treatment targeting multiple diseases. Expert Opin. Biol. Ther. 2018, 18, 23–31. [Google Scholar] [CrossRef]
- Ahn, J.H.; Hwang, S.H.; Cho, H.S.; Lee, M. Differential Gene Expression Common to Acquired and Intrinsic Resistance to BRAF Inhibitor Revealed by RNA-Seq Analysis. Biomol. Ther. 2019, 27, 302–310. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Lin, Q.; Wang, S.; Chen, T.; Jiang, H. Key genes and integrated modules in hematopoietic differentiation of human embryonic stem cells: A comprehensive bioinformatic analysis. Stem Cell Res. Ther. 2018, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Sun, Y.L.; Chen, Z.H.; Chen, X.H.; Yin, C.; Li, D.J.; Ma, X.L.; Zhao, F.; Zhang, G.; Shang, P.; Qian, A.R. Diamagnetic Levitation Promotes Osteoclast Differentiation from RAW264.7 Cells. IEEE Trans. Biomed. Eng. 2015, 62, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Hourai, Y.; Weng, Z. Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS ONE 2011, 6, e24657. [Google Scholar] [CrossRef] [PubMed]
- Mintseris, J.; Pierce, B.; Wiehe, K.; Anderson, R.; Chen, R.; Weng, Z. Integrating statistical pair potentials into protein complex prediction. Proteins 2007, 69, 511–520. [Google Scholar] [CrossRef]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Kim, Y.; Ghil, S. Modulation of G-protein-coupled receptor 55-mediated signaling by regulator of G-protein signaling 2. Biochem. Biophys. Res. Commun. 2020, 533, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H.; Cyster, J.G. G-Protein Coupled Receptor 18 Contributes to Establishment of the CD8 Effector T Cell Compartment. Front. Immunol. 2018, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Barría, Y.; Borquez, K.; Urra, B.; Rivera, A.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A. β-hydroxybutyrate and hydroxycarboxylic acid receptor 2 agonists activate the AKT, ERK and AMPK pathways, which are involved in bovine neutrophil chemotaxis. Sci. Rep. 2020, 10, 12491. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, L.; Nguyen, N.; Garzia, C.; Fletcher, E.; Stevenson, R.; Jaramillo, D.; Kuliopulos, A.; Covic, L. Lipid Receptor GPR31 (G-Protein-Coupled Receptor 31) Regulates Platelet Reactivity and Thrombosis Without Affecting Hemostasis. Arterioscler. Thromb. Vasc. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Moriyama, S.; Li, Z.; Zhou, L.; Flamar, A.L.; Klose, C.S.N.; Moeller, J.B.; Putzel, G.G.; Withers, D.R.; Sonnenberg, G.F.; et al. Anti-microbial Functions of Group 3 Innate Lymphoid Cells in Gut-Associated Lymphoid Tissues Are Regulated by G-Protein-Coupled Receptor 183. Cell Rep. 2018, 23, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xie, H.; Li, S.; Ye, H.; Li, S.; Ren, K.; Li, Y.; Xu, M.; Lin, W.; Duan, X.; et al. 2′, 5′-Oligoadenylate Synthetase 2 (OAS2) Inhibits Zika Virus Replication through Activation of Type Ι IFN Signaling Pathway. Viruses 2020, 12, 418. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, S.; Xu, L.; Yu, Y.; Zhang, T.; Wang, M. DExH-Box helicase 58 enhances osteoblast differentiation of osteoblastic cells via Wnt/β-Catenin signaling. Biochem. Biophys. Res. Commun. 2019, 511, 307–311. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Gibula-Tarlowska, E.; Kotlinska, J.H. Crosstalk between Opioid and Anti-Opioid Systems: An Overview and Its Possible Therapeutic Significance. Biomolecules 2020, 10, 1376. [Google Scholar] [CrossRef]
- Mouledous, L.; Mollereau, C.; Zajac, J.M. Opioid-modulating properties of the neuropeptide FF system. Biofactors 2010, 36, 423–429. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Chen, Z.; Ma, X.; Li, D.; Shang, P.; Qian, A. Neuropeptide FF attenuates RANKL-induced differentiation of macrophage-like cells into osteoclast-like cells. Arch. Oral Biol. 2015, 60, 282–292. [Google Scholar] [CrossRef]
- Sun, Y.L.; Chen, Z.H.; Li, D.J.; Zhao, F.; Ma, X.L.; Shang, P.; Yang, T.; Qian, A. Neuropeptide FF inhibits LPS-mediated osteoclast differentiation of RAW264.7 cells. Protein Pept. Lett. 2014, 22, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Locker, F.; Lang, A.A.; Koller, A.; Lang, R.; Bianchini, R.; Kofler, B. Galanin modulates human and murine neutrophil activation in vitro. Acta Physiol. 2015, 213, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci. Rep. 2019, 9, 7237. [Google Scholar] [CrossRef] [PubMed]



| Genes | Primers | Sequences (5′ to 3′) | Products (bp) |
|---|---|---|---|
| Gapdh | Forward | TGTGTCCGTCGTGGATCTGA | 150 |
| Reverse | TTGCTGTTGAAGTCGCAGGAG | ||
| Cnr2 | Forward | GCTCTGTTCATATCAAATC | 84 |
| Reverse | ATCCTTTAGTATCATCTCTG | ||
| Gpr55 | Forward | CTCTGTTCTTTATACTCCTAC | 133 |
| Reverse | GGTTCTTCTGCTTCATAC | ||
| Gpr18 | Forward | ATTACCTTCGCAGTGTTC | 90 |
| Reverse | CTCTGACTCAAAGCATCTC | ||
| Hcar2 | Forward | CTGTCCACCTCCTCTATAC | 90 |
| Reverse | GCCACCTGAAGTTGTAAC | ||
| Gpr31b | Forward | CTGTCTACCTGTTCAACC | 76 |
| Reverse | AGATAGAAGGCAGCAAAG | ||
| Gpr183 | Forward | TGCTGCGATTCTCTGTAATG | 84 |
| Reverse | GTGCTTAGGAACTTAGGAAGAC | ||
| Oas2 | Forward | CATTGTTGTGTTCCTCTC | 96 |
| Reverse | AATTCTTCTAACTGCTTCTG | ||
| Dhx58 | Forward | AACCAAATCCACCAACAAC | 75 |
| Reverse | CACTTGCTGCTCATACATC |
| Gene Name Ensembl ID | Species Gene Type | Location Length | Expression Changes (NPFF vs. Control) | Function | Refs |
|---|---|---|---|---|---|
| Cnr2 (Cannabinoid Receptor 2) (ENSEMBL: ENSG00000188822) | Mus musculus Protein coding | Chr 4 (4084 bp) | Down-regulated | Is the receptor for cannabinoids, and is involved in the diseases such as polyarticular juvenile idiopathic arthritis, and cannabis abuse. Besides, it regulates the GPCR pathway and peptide ligand-binding receptors. | [49] |
| Gpr55 (G protein-coupled receptor 55) (ENSG00000135898) | Mus musculus Protein coding | Chr 1 (2550 bp) | Up-regulated | Involved in the cannabinoid receptor pathway, GPCR signaling pathway, and diseases include cannabis abuse and lysinuric protein intolerance. | [50] |
| Gpr18 (G protein-coupled receptor 18) (ENSG00000125245) | Mus musculus Protein coding | Chr 14 (1424 bp) | Up-regulated | Involved in the GPCR signaling pathways and GPCRs. | [51] |
| Hcar2 (Hydroxycarboxylic Acid Receptor 2) (ENSG00000182782) | Mus musculus Protein coding | Chr 5 (1947 bp) | Up-regulated | Involved in the pathways such as peptide ligand-binding receptors and GPCR signaling pathway, and is closely associated with diseases include diversion colitis and pellagra. | [52] |
| Gpr31b (G Protein-Coupled Receptor 31) (ENSG00000120436) | Mus musculus Protein coding | Chr 17 (960 bp) | Up-regulated | Anticipates in the process of ischemia, and regulates the pathways of free fatty acid receptors and GPCR. | [53] |
| Gpr183 (G Protein-Coupled Receptor 183) (ENSG00000169508) | Mus musculus Protein coding | Chr 14 (2942 bp) | Down-regulated | Involved in the immune response upon Epstein–Barr virus infection of primary B lymphocytes and regulates the function of thrombin receptor. | [54] |
| Oas2 (2′-5′-Oligoadenylate Synthetase 2) (ENSG00000111335) | Mus musculus Protein coding | Chr 5 (3957 bp) | Up-regulated | Modulates the innate immune system and interferon γ signaling and is involved in disorders such as microphthalmia with limb anomalies and tick-borne encephalitis. | [55] |
| Dhx58 (DExH-Box Helicase 58) (ENSG00000108771) | Mus musculus Protein coding | Chr 11 (2427 bp) | Up-regulated | Involved in the diseases such as rabies and measles and regulates several pathways include IFN-α/β pathways and innate immune system. | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Kuang, Y.; Zuo, Z. Transcriptomic Changes in Mouse Bone Marrow-Derived Macrophages Exposed to Neuropeptide FF. Genes 2021, 12, 705. https://doi.org/10.3390/genes12050705
Sun Y, Kuang Y, Zuo Z. Transcriptomic Changes in Mouse Bone Marrow-Derived Macrophages Exposed to Neuropeptide FF. Genes. 2021; 12(5):705. https://doi.org/10.3390/genes12050705
Chicago/Turabian StyleSun, Yulong, Yuanyuan Kuang, and Zhuo Zuo. 2021. "Transcriptomic Changes in Mouse Bone Marrow-Derived Macrophages Exposed to Neuropeptide FF" Genes 12, no. 5: 705. https://doi.org/10.3390/genes12050705
APA StyleSun, Y., Kuang, Y., & Zuo, Z. (2021). Transcriptomic Changes in Mouse Bone Marrow-Derived Macrophages Exposed to Neuropeptide FF. Genes, 12(5), 705. https://doi.org/10.3390/genes12050705






