Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung
Abstract
1. Introduction
2. Emerging Pathogens and Colonization of the Lungs of Cystic Fibrosis Patients
3. Hypermutation and Genomic Within-Host Evolution
3.1. Hypermutation
3.2. Diversified Populations and Within-Host Genome Evolution
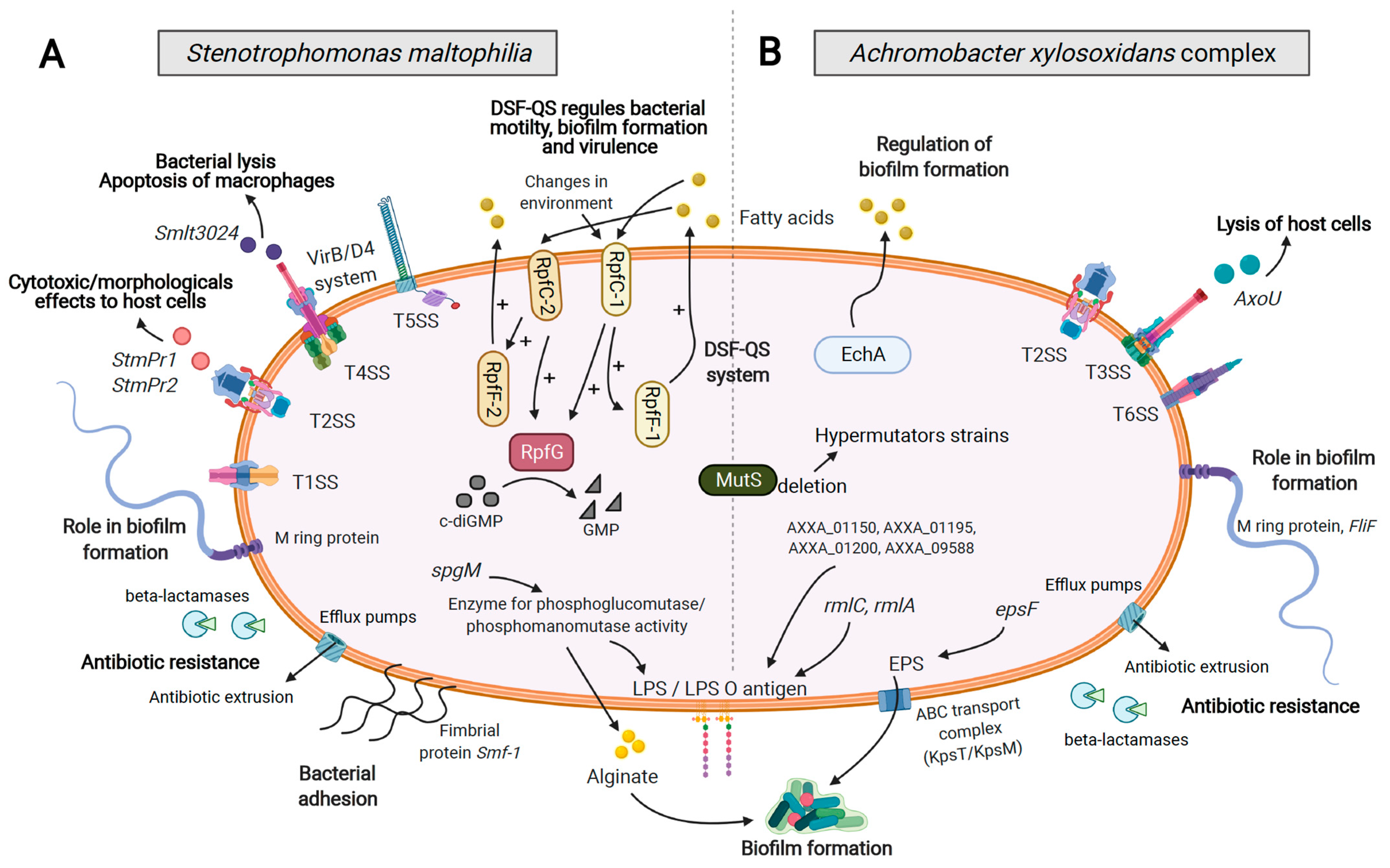
4. Virulence Factors and Secretion Systems
5. Pathoadaptive Traits
5.1. Virulence Attenuation
5.2. Adhesion, Motility, and Biofilm Formation
5.3. Antimicrobial Resistance
6. Competitive Interactions
7. Quorum Sensing Regulation
7.1. Diffusible Signal Factor (DSF) System
7.2. Other Quorum Sensing Factors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenza, G.; Tappe, D.; Turnwald, D.; Frosch, M.; König, C.; Hebestreit, H.; Abele-Horn, M. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 123–127. [Google Scholar] [CrossRef]
- Parkins, M.D.; Floto, R.A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Menetrey, Q.; Dupont, C.; Chiron, R.; Marchandin, H. Emerging bacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis from a microbiologist’s perspective. Rev. Mal. Respir. 2020, 37, 561–571. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report. Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.Pdf (accessed on 20 February 2021).
- Gröschel, M.I.; Meehan, C.J.; Barilar, I.; Diricks, M.; Gonzaga, A.; Steglich, M.; Conchillo-Solé, O.; Scherer, I.-C.; Mamat, U.; Luz, C.F.; et al. The phylogenetic landscape and nosocomial spread of the multidrug-resistant opportunist Stenotrophomonas maltophilia. Nat. Commun. 2020, 11, 2044. [Google Scholar] [CrossRef]
- Green, H.; Jones, A.M. The microbiome and emerging pathogens in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tzanetou, K.; Triantaphillis, G.; Tsoutsos, D.; Petropoulou, D.; Ganteris, G.; Malamou-Lada, E.; Ziroyiannis, P. Stenotrophomonas maltophilia peritonitis in CAPD patients: Susceptibility to antibiotics and treatment outcome: A report of five cases. Perit. Dial. Int. 2004, 24, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Araoka, H.; Baba, M.; Yoneyama, A. Risk factors for mortality among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Japan, 1996. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 605–608. [Google Scholar] [CrossRef]
- Hauser, A.R.; Jain, M.; Bar-Meir, M.; McColley, S.A. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin. Microbiol. Rev. 2011, 24, 29–70. [Google Scholar] [CrossRef]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef]
- Liu, C.; Pan, F.; Guo, J.; Yan, W.; Jin, Y.; Liu, C.; Qin, L.; Fang, X. Hospital acquired pneumonia due to Achromobacter spp. in a geriatric ward in China: Clinical characteristic, genome variability, biofilm production, antibiotic resistance and integron in isolated strains. Front. Microbiol. 2016, 7, 621. [Google Scholar] [CrossRef] [PubMed]
- Barragán, E.P.; Pérez, J.S.; Corbella, L.; Orellana, M.Á.; Fernández-Ruiz, M. Achromobacter xylosoxidans bacteremia: Clinical and microbiological features in a 10-year case series. Rev. Esp. Quimioter. 2018, 31, 268–273. [Google Scholar]
- Hansen, C.R.; Pressler, T.; Nielsen, K.G.; Jensen, P.Ø.; Bjarnsholt, T.; Høiby, N. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J. Cyst. Fibros. 2010, 9, 51–58. [Google Scholar] [CrossRef]
- Lambiase, A.; Catania, M.R.; del Pezzo, M.; Rossano, F.; Terlizzi, V.; Sepe, A.; Raia, V. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.D.; Greysson-Wong, J.; Somayaji, R.; Waddell, B.; Whelan, F.J.; Storey, D.G.; Rabin, H.R.; Surette, M.G.; Parkins, M.D. Prevalence and outcomes of Achromobacter species infections in adults with cystic fibrosis: A North American Cohort Study. J. Clin. Microbiol. 2017, 55, 2074–2085. [Google Scholar] [CrossRef]
- Tetart, M.; Wallet, F.; Kyheng, M.; Leroy, S.; Perez, T.; Le Rouzic, O.; Wallaert, B.; Prevotat, A. Impact of Achromobacter xylosoxidans isolation on the respiratory function of adult patients with cystic fibrosis. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.; Yau, Y.; Prasad, S.; Lu, A.; Atenafu, E.; Crandall, I.; Tom, S.; Tullis, E.; Ratjen, F. Stenotrophomonas maltophilia in cystic fibrosis: Serologic response and effect on lung disease. Am. J. Respir. Crit. Care Med. 2011, 183, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Barsky, E.E.; Williams, K.A.; Priebe, G.P.; Sawicki, G.S. Incident Stenotrophomonas maltophilia infection and lung function decline in cystic fibrosis. Pediatr. Pulmonol. 2017, 52, 1276–1282. [Google Scholar] [CrossRef]
- Wettlaufer, J.; Klingel, M.; Yau, Y.; Stanojevic, S.; Tullis, E.; Ratjen, F.; Waters, V. Longitudinal study of Stenotrophomonas maltophilia antibody levels and outcomes in cystic fibrosis patients. J. Cyst. Fibros. 2017, 16, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Mayer-Hamblett, N.; Aitken, M.L.; Rubenfeld, G.D.; Ramsey, B.W. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax 2004, 59, 955–959. [Google Scholar] [CrossRef]
- Waters, V.J.; Gómez, M.I.; Soong, G.; Amin, S.; Ernst, R.K.; Prince, A. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect. Immun. 2007, 75, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.S.; Schoeb, T.; Swords, W.E. Cooperativity between Stenotrophomonas maltophilia and Pseudomonas aeruginosa during polymicrobial airway infections. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.K.; Rau, M.H.; Johansen, H.K.; Ciofu, O.; Jelsbak, L.; Yang, L.; Folkesson, A.; Jarmer, H.Ø.; Aanæs, K.; von Buchwald, C.; et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J. 2012, 6, 31–45. [Google Scholar] [CrossRef]
- Gade, S.S.; Nørskov-Lauritsen, N.; Ridderberg, W. Prevalence and species distribution of Achromobacter sp. cultured from cystic fibrosis patients attending the Aarhus centre in Denmark. J. Med. Microbiol. 2017, 66, 686–689. [Google Scholar] [CrossRef]
- Registre Français de la Mucoviscidose –Bilan des Données 2018, Vaincre la Mucoviscidose Paris, décembre. Available online: https://www.vaincrelamuco.org/sites/default/files/registre_francais_de_la_mucoviscidose_bilan_donnees_2018.pdf (accessed on 20 February 2021).
- Chiron, R.; Marchandin, H.; Counil, F.; Jumas-Bilak, E.; Freydière, A.-M.; Bellon, G.; Husson, M.-O.; Turck, D.; Brémont, F.; Chabanon, G.; et al. Clinical and microbiological features of Inquilinus Sp. isolates from five patients with cystic fibrosis. J. Clin. Microbiol. 2005, 43, 3938–3943. [Google Scholar] [CrossRef]
- Coutinho, C.P.; Dos Santos, S.C.; Madeira, A.; Mira, N.P.; Moreira, A.S.; Sá-Correia, I. Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: Epidemiology, clonal variation, and genome-wide expression alterations. Front. Cell. Infect. Microbiol. 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Aujoulat, F.; Chiron, R.; Condom, P.; Jumas-Bilak, E.; Marchandin, H. Highly diversified Pandoraea pulmonicola population during chronic colonization in cystic fibrosis. Front. Microbiol. 2017, 8, 1892. [Google Scholar] [CrossRef]
- Cools, P.; Ho, E.; Vranckx, K.; Schelstraete, P.; Wurth, B.; Franckx, H.; Ieven, G.; Van Simaey, L.; Van Daele, S.; Verhulst, S.; et al. Epidemic Achromobacter xylosoxidans strain among Belgian cystic fibrosis patients and review of literature. BMC Microbiol. 2016, 16, 122. [Google Scholar] [CrossRef]
- Capaldo, C.; Beauruelle, C.; Saliou, P.; Rault, G.; Ramel, S.; Héry-Arnaud, G. Investigation of Stenotrophomonas maltophilia epidemiology in a French cystic fibrosis center. Respir. Med. Res. 2020, 78, 100757. [Google Scholar] [CrossRef]
- Cullen, L.; McClean, S. Bacterial adaptation during chronic respiratory infections. Pathogens 2015, 4, 66–89. [Google Scholar] [CrossRef]
- Hogardt, M.; Heesemann, J. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int. J. Med. Microbiol. 2010, 300, 557–562. [Google Scholar] [CrossRef]
- Aujoulat, F.; Roger, F.; Bourdier, A.; Lotthé, A.; Lamy, B.; Marchandin, H.; Jumas-Bilak, E. From environment to man: Genome evolution and adaptation of human opportunistic bacterial pathogens. Genes 2012, 3, 191–232. [Google Scholar] [CrossRef]
- Veschetti, L.; Sandri, A.; Patuzzo, C.; Melotti, P.; Malerba, G.; Lleò, M.M. Mobilome analysis of Achromobacter spp. isolates from chronic and occasional lung infection in cystic fibrosis patients. Microorganisms 2021, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Ridderberg, W.; Jensen Handberg, K.; Nørskov-Lauritsen, N. Prevalence of hypermutator isolates of Achromobacter spp. from cystic fibrosis patients. Int. J. Med. Microbiol. 2020, 310, 151393. [Google Scholar] [CrossRef]
- Gabrielaite, M.; Bartell, J.A.; Nørskov-Lauritsen, N.; Pressler, T.; Nielsen, F.C.; Johansen, H.K.; Marvig, R.L. Transmission and antibiotic resistance of Achromobacter in cystic fibrosis. J. Clin. Microbiol. 2021, 59, e02911-20. [Google Scholar] [CrossRef] [PubMed]
- Turrientes, M.C.; Baquero, M.R.; Sánchez, M.B.; Valdezate, S.; Escudero, E.; Berg, G.; Cantón, R.; Baquero, F.; Galán, J.C.; Martínez, J.L. Polymorphic mutation frequencies of clinical and environmental Stenotrophomonas maltophilia populations. Appl. Environ. Microbiol. 2010, 76, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, P.G.; Dittmer, S.; Steinmann, E.; Buer, J.; Rath, P.-M.; Steinmann, J. Adaptation of Stenotrophomonas maltophilia in cystic fibrosis: Molecular diversity, mutation frequency and antibiotic resistance. Int. J. Med. Microbiol. 2014, 304, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Pompilio, A.; Bettua, C.; Crocetta, V.; Giacobazzi, E.; Fiscarelli, E.; Jousson, O.; Di Bonaventura, G. Evolution of Stenotrophomonas maltophilia in cystic fibrosis lung over chronic infection: A genomic and phenotypic population study. Front. Microbiol. 2017, 8, 1590. [Google Scholar] [CrossRef]
- Alcaraz, E.; Centrón, D.; Camicia, G.; Quiroga, M.P.; Di Conza, J.; Passerini de Rossi, B. Stenotrophomonas maltophilia phenotypic and genotypic features through 4-year cystic fibrosis lung colonization. J. Med. Microbiol. 2021, 70, 001281. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; Ghosh, D.; Chakrabarti, M.; Gherardi, G.; Vitali, L.A.; Fiscarelli, E.; Di Bonaventura, G. Stenotrophomonas maltophilia phenotypic and genotypic diversity during a 10-year colonization in the lungs of a cystic fibrosis patient. Front. Microbiol. 2016, 7, 1551. [Google Scholar] [CrossRef]
- Hogardt, M.; Heesemann, J. Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung. Curr. Top. Microbiol. Immunol. 2013, 358, 91–118. [Google Scholar] [CrossRef]
- Winstanley, C.; O’Brien, S.; Brockhurst, M.A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Michon, A.-L.; Jumas-Bilak, E.; Nørskov-Lauritsen, N.; Chiron, R.; Marchandin, H. Intrapatient diversity of Achromobacter spp. involved in chronic colonization of cystic fibrosis airways. Infect. Genet. Evol. 2015, 32, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ridderberg, W.; Nielsen, S.M.; Nørskov-Lauritsen, N. Genetic adaptation of Achromobacter sp. during persistence in the lungs of cystic fibrosis patients. PLoS ONE 2015, 10, e0136790. [Google Scholar] [CrossRef]
- Ormerod, K.L.; George, N.M.; Fraser, J.A.; Wainwright, C.; Hugenholtz, P. Comparative genomics of non-pseudomonal bacterial species colonising paediatric cystic fibrosis patients. PeerJ 2015, 3, e1223. [Google Scholar] [CrossRef]
- Jeukens, J.; Freschi, L.; Vincent, A.T.; Emond-Rheault, J.-G.; Kukavica-Ibrulj, I.; Charette, S.J.; Levesque, R.C. A pan-genomic approach to understand the basis of host adaptation in Achromobacter. Genome Biol. Evol. 2017, 9, 1030–1046. [Google Scholar] [CrossRef]
- Chung, H.; Lieberman, T.D.; Vargas, S.O.; Flett, K.B.; McAdam, A.J.; Priebe, G.P.; Kishony, R. Global and local selection acting on the pathogen Stenotrophomonas maltophilia in the human lung. Nat. Commun. 2017, 8, 14078. [Google Scholar] [CrossRef]
- Kostakioti, M.; Newman, C.L.; Thanassi, D.G.; Stathopoulos, C. Mechanisms of protein export across the bacterial outer membrane. J. Bacteriol. 2005, 187, 4306–4314. [Google Scholar] [CrossRef]
- Douzi, B.; Filloux, A.; Voulhoux, R. On the path to uncover the bacterial type II secretion system. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012, 367, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Brunet, Y.R.; Espinosa, L.; Harchouni, S.; Mignot, T.; Cascales, E. Imaging type VI secretion-mediated bacterial killing. Cell Rep. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Abby, S.S.; Cury, J.; Guglielmini, J.; Néron, B.; Touchon, M.; Rocha, E.P.C. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016, 6, 23080. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Liu, W.-B.; Tan, Y.-F.; Yang, H.-Y.; Zhang, T.-T.; Wang, T.; Wang, X.-Y.; Song, Y.-J.; Yang, R.-F.; Du, Z.-M. An interaction between the inner rod protein Ysci and the needle protein Yscf is required to assemble the needle structure of the Yersinia type three secretion system. J. Biol. Chem. 2017, 292, 5488–5498. [Google Scholar] [CrossRef] [PubMed]
- Lasica, A.M.; Ksiazek, M.; Madej, M.; Potempa, J. The Type IX Secretion System (T9SS): Highlights and recent insights into its structure and function. Front. Cell. Infect. Microbiol. 2017, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, S.U.; Carmody, L.A.; Gonzalez, C.F.; LiPuma, J.J. A type IV secretion system contributes to intracellular survival and replication of Burkholderia cenocepacia. Infect. Immun. 2008, 76, 5447–5455. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F.; Ruiz-Perez, F.; Cataldi, Á.; Larzábal, M. Type VI secretion system in pathogenic Escherichia coli: Structure, role in virulence, and acquisition. Front. Microbiol. 2019, 10, 1965. [Google Scholar] [CrossRef] [PubMed]
- Cianciotto, N.P. Type II secretion: A protein secretion system for all seasons. Trends Microbiol. 2005, 13, 581–588. [Google Scholar] [CrossRef]
- McCoy-Simandle, K.; Stewart, C.R.; Dao, J.; DebRoy, S.; Rossier, O.; Bryce, P.J.; Cianciotto, N.P. Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect. Immun. 2011, 79, 1984–1997. [Google Scholar] [CrossRef]
- Baldi, D.L.; Higginson, E.E.; Hocking, D.M.; Praszkier, J.; Cavaliere, R.; James, C.E.; Bennett-Wood, V.; Azzopardi, K.I.; Turnbull, L.; Lithgow, T.; et al. The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect. Immun. 2012, 80, 2042–2052. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Hansen, M.A.; Jensen, P.Ø.; Hansen, L.; Riber, L.; Cockburn, A.; Kolpen, M.; Rønne Hansen, C.; Ridderberg, W.; Eickhardt, S.; et al. Complete genome sequence of the cystic fibrosis pathogen Achromobacter xylosoxidans NH44784-1996 complies with important pathogenic phenotypes. PLoS ONE 2013, 8, e68484. [Google Scholar] [CrossRef]
- Karaba, S.M.; White, R.C.; Cianciotto, N.P. Stenotrophomonas maltophilia encodes a type II protein secretion system that promotes detrimental effects on lung epithelial cells. Infect. Immun. 2013, 81, 3210–3219. [Google Scholar] [CrossRef]
- DuMont, A.L.; Karaba, S.M.; Cianciotto, N.P. Type II secretion-dependent degradative and cytotoxic activities mediated by Stenotrophomonas maltophilia serine proteases Stmpr1 and Stmpr. Infect. Immun. 2015, 83, 3825–3837. [Google Scholar] [CrossRef]
- Hayes, C.S.; Aoki, S.K.; Low, D.A. Bacterial contact-dependent delivery systems. Annu. Rev. Genet. 2010, 44, 71–90. [Google Scholar] [CrossRef]
- Souza, D.P.; Oka, G.U.; Alvarez-Martinez, C.E.; Bisson-Filho, A.W.; Dunger, G.; Hobeika, L.; Cavalcante, N.S.; Alegria, M.C.; Barbosa, L.R.S.; Salinas, R.K.; et al. Bacterial killing via a type IV secretion system. Nat. Commun. 2015, 6, 6453. [Google Scholar] [CrossRef]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.S.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef]
- Pickrum, A.M.; DeLeon, O.; Dirck, A.; Tessmer, M.H.; Riegert, M.O.; Biller, J.A.; Ledeboer, N.A.; Kirby, J.R.; Frank, D.W. Achromobacter xylosoxidans cellular pathology is correlated with activation of a type III secretion system. Infect. Immun. 2020, 88, e00136-20. [Google Scholar] [CrossRef]
- Tessmer, M.H.; Anderson, D.M.; Pickrum, A.M.; Riegert, M.O.; Frank, D.W. Identification and verification of ubiquitin-activated bacterial phospholipases. J. Bacteriol. 2019, 201, e00623-18. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Cenens, W.; Matsuyama, B.Y.; Oka, G.U.; Di Sessa, G.; Mininel, I.D.V.; Alves, T.L.; Farah, C.S. The opportunistic pathogen Stenotrophomonas maltophilia utilizes a type IV secretion system for interbacterial killing. PLoS Pathog. 2019, 15, e1007651. [Google Scholar] [CrossRef] [PubMed]
- Nas, M.Y.; White, R.C.; DuMont, A.L.; Lopez, A.E.; Cianciotto, N.P. Stenotrophomonas maltophilia encodes a VirB/VirD4 type IV secretion system that modulates apoptosis in human cells and promotes competition against heterologous bacteria, including Pseudomonas aeruginosa. Infect. Immun. 2019, 87, e00457-19. [Google Scholar] [CrossRef] [PubMed]
- Crossman, L.C.; Gould, V.C.; Dow, J.M.; Vernikos, G.S.; Okazaki, A.; Sebaihia, M.; Saunders, D.; Arrowsmith, C.; Carver, T.; Peters, N.; et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008, 9, R74. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, R.P.; Levy, C.E.; Yano, T. A heat-stable cytotoxic factor produced by Achromobacter xylosoxidans isolated from Brazilian patients with CF is associated with in vitro increased proinflammatory cytokines. J. Cyst. Fibros. 2012, 11, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, P.M.S.; Furumura, M.T.; Santos, A.M.; Sousa, A.C.T.; Kota, D.J.; Levy, C.E.; Yano, T. Cytotoxic activity of clinical Stenotrophomonas maltophilia. Lett. Appl. Microbiol. 2006, 43, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.E.; Sadikot, R.T. Achromobacter respiratory infections. Ann. Am. Thorac. Soc. 2015, 12, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, A.; Strateva, T. Stenotrophomonas maltophilia—A low-grade pathogen with numerous virulence factors. Infect. Dis. 2019, 51, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.; Gong, J.; Zhang, L.; Wang, G. Comparative genome characterization of Achromobacter members reveals potential genetic determinants facilitating the adaptation to a pathogenic lifestyle. Appl. Microbiol. Biotechnol. 2013, 97, 6413–6425. [Google Scholar] [CrossRef]
- Sousa, A.M.; Pereira, M.O. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—A review. Pathogens 2014, 3, 680–703. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Meyer, R.L.; Nørskov-Lauritsen, N. Differences in gene expression profiles between early and late isolates in monospecies Achromobacter biofilm. Pathogens 2017, 6, 20. [Google Scholar] [CrossRef]
- Filipic, B.; Malesevic, M.; Vasiljevic, Z.; Lukic, J.; Novovic, K.; Kojic, M.; Jovcic, B. Uncovering differences in virulence markers associated with Achromobacter species of CF and non-CF origin. Front. Cell. Infect. Microbiol. 2017, 7, 224. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Prosseda, G.; Del Chierico, F.; Cannavacciuolo, S.; Cipriani, P.; Petrucca, A.; Superti, F.; Ammendolia, M.G.; Concato, C.; Fiscarelli, E.; et al. Molecular characterization of virulence determinants of Stenotrophomonas maltophilia strains isolated from patients affected by cystic fibrosis. Int. J. Immunopathol. Pharmacol. 2007, 20, 529–537. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; Confalone, P.; Nicoletti, M.; Petrucca, A.; Guarnieri, S.; Fiscarelli, E.; Savini, V.; Piccolomini, R.; Di Bonaventura, G. Adhesion to and biofilm formation on IB3-1 bronchial cells by Stenotrophomonas maltophilia isolates from cystic fibrosis patients. BMC Microbiol. 2010, 10, 102. [Google Scholar] [CrossRef]
- de Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Alcántara, N.; Martinez, M.B.; Girón, J.A. Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell Microbiol. 2003, 5, 625–636. [Google Scholar] [CrossRef]
- Gallo, S.W.; Figueiredo, T.P.; Bessa, M.C.; Pagnussatti, V.E.; Ferreira, C.A.S.; Oliveira, S.D. Isolation and characterization of Stenotrophomonas maltophilia isolates from a Brazilian hospital. Microb. Drug Resist. N 2016, 22, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.H.V.; Leão, R.S.; Carvalho-Assef, A.P.; Albano, R.M.; Rodrigues, E.R.A.; Firmida, M.C.; Folescu, T.W.; Plotkowski, M.C.; Bernardo, V.G.; Marques, E.A. Patterns of virulence factor expression and antimicrobial resistance in Achromobacter xylosoxidans and Achromobacter ruhlandii isolates from patients with cystic fibrosis. Epidemiol. Infect. 2017, 145, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Madi, H.; Lukić, J.; Vasiljević, Z.; Biočanin, M.; Kojić, M.; Jovčić, B.; Lozo, J. Genotypic and phenotypic characterization of Stenotrophomonas maltophilia strains from a pediatric tertiary care hospital in Serbia. PLoS ONE 2016, 11, e0165660. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Penstoft, L.N.; Nørskov-Lauritsen, N. Motility, biofilm formation and antimicrobial efflux of sessile and planktonic cells of Achromobacter xylosoxidans. Pathogens 2019, 8, 14. [Google Scholar] [CrossRef]
- Trancassini, M.; Iebba, V.; Citerà, N.; Tuccio, V.; Magni, A.; Varesi, P.; De Biase, R.V.; Totino, V.; Santangelo, F.; Gagliardi, A.; et al. Outbreak of Achromobacter xylosoxidans in an italian cystic fibrosis center: Genome variability, biofilm production, antibiotic resistance, and motility in isolated strains. Front. Microbiol. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Firmida, M.C.; Marques, E.A.; Leão, R.S.; Pereira, R.H.V.; Rodrigues, E.R.A.; Albano, R.M.; Folescu, T.W.; Bernardo, V.; Daltro, P.; Capone, D.; et al. Achromobacter xylosoxidans infection in cystic fibrosis siblings with different outcomes: Case reports. Respir. Med. Case Rep. 2017, 20, 98–103. [Google Scholar] [CrossRef]
- Flores-Treviño, S.; Bocanegra-Ibarias, P.; Camacho-Ortiz, A.; Morfín-Otero, R.; Salazar-Sesatty, H.A.; Garza-González, E. Stenotrophomonas maltophilia biofilm: Its role in infectious diseases. Expert Rev. Anti Infect. Ther. 2019, 17, 877–893. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Nørskov-Lauritsen, N.; Bjarnsholt, T.; Meyer, R.L. Achromobacter species Isolated from cystic fibrosis patients reveal distinctly different biofilm morphotypes. Microorganisms 2016, 4, 33. [Google Scholar] [CrossRef]
- Pompilio, A.; Pomponio, S.; Crocetta, V.; Gherardi, G.; Verginelli, F.; Fiscarelli, E.; Dicuonzo, G.; Savini, V.; D’Antonio, D.; Di Bonaventura, G. Phenotypic and genotypic characterization of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis: Genome diversity, biofilm formation, and virulence. BMC Microbiol. 2011, 11, 159. [Google Scholar] [CrossRef]
- Pompilio, A.; Savini, V.; Fiscarelli, E.; Gherardi, G.; Di Bonaventura, G. Clonal diversity, biofilm formation, and antimicrobial resistance among Stenotrophomonas maltophilia strains from cystic fibrosis and Non-cystic fibrosis patients. Antibiotics 2020, 9, 15. [Google Scholar] [CrossRef]
- Alio, I.; Gudzuhn, M.; Pérez García, P.; Danso, D.; Schoelmerich, M.C.; Mamat, U.; Schaible, U.E.; Steinmann, J.; Yero, D.; Gibert, I.; et al. Phenotypic and transcriptomic analyses of seven clinical Stenotrophomonas maltophilia isolates identify a small set of shared and commonly regulated genes involved in the biofilm lifestyle. Appl. Environ. Microbiol. 2020, 86, e02038-20. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J. Ecological determinants of biofilm formation. Biofouling 1996, 10, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007, 10, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.A.; Woods, D.E.; MacDonald, K.L.; Poole, K. Role of phosphoglucomutase of Stenotrophomonas maltophilia in lipopolysaccharide biosynthesis, virulence, and antibiotic resistance. Infect. Immun. 2003, 71, 3068–3075. [Google Scholar] [CrossRef]
- Chester, B.; Cooper, L.H. Achromobacter species (CDC Group VD): Morphological and biochemical characterization. J. Clin. Microbiol. 1979, 9, 425–436. [Google Scholar]
- Irifune, K.; Ishida, T.; Shimoguchi, K.; Ohtake, J.; Tanaka, T.; Morikawa, N.; Kaku, M.; Koga, H.; Kohno, S.; Hara, K. Pneumonia caused by Stenotrophomonas maltophilia with a mucoid phenotype. J. Clin. Microbiol. 1994, 32, 2856–2857. [Google Scholar] [CrossRef]
- Cescutti, P.; Cuzzi, B.; Liut, G.; Segonds, C.; Di Bonaventura, G.; Rizzo, R. A novel highly charged exopolysaccharide produced by two Strains of Stenotrophomonas maltophilia recovered from patients with cystic fibrosis. Carbohydr. Res. 2011, 346, 1916–1923. [Google Scholar] [CrossRef]
- Cameron, L.C.; Bonis, B.; Phan, C.Q.; Kent, L.A.; Lee, A.K.; Hunter, R.C. A putative enoyl-CoA hydratase contributes to biofilm formation and the antibiotic tolerance of Achromobacter xylosoxidans. NPJ Biofilms Microbiomes 2019, 5, 20. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Huang, Y.-W.; Chen, S.-J.; Chang, C.-W.; Yang, T.-C. The SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics, and virulence in mice. Antimicrob. Agents Chemother. 2015, 59, 4067–4073. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, F.-F.; Ren, B.-Z.; Liu, W.; Liu, Z.; Qian, W. Systematic mutational analysis of histidine kinase genes in the nosocomial pathogen Stenotrophomonas maltophilia identifies BfmAK system control of biofilm development. Appl. Environ. Microbiol. 2016, 82, 2444–2456. [Google Scholar] [CrossRef]
- Wu, K.; Yau, Y.C.W.; Matukas, L.; Waters, V. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 2013, 57, 1546–1548. [Google Scholar] [CrossRef]
- Tom, S.K.; Yau, Y.C.W.; Beaudoin, T.; LiPuma, J.J.; Waters, V. Effect of high-dose antimicrobials on biofilm growth of Achromobacter species isolated from cystic fibrosis patients. Antimicrob. Agents Chemother. 2016, 60, 650–652. [Google Scholar] [CrossRef]
- Cabak, A.; Hovold, G.; Petersson, A.-C.; Ramstedt, M.; Påhlman, L.I. Activity of airway antimicrobial peptides against cystic fibrosis pathogens. Pathog. Dis. 2020, 78, ftaa048. [Google Scholar] [CrossRef]
- Bador, J.; Amoureux, L.; Duez, J.-M.; Drabowicz, A.; Siebor, E.; Llanes, C.; Neuwirth, C. First description of an RND-type multidrug efflux pump in Achromobacter xylosoxidans, AxyABM. Antimicrob. Agents Chemother. 2011, 55, 4912–4914. [Google Scholar] [CrossRef]
- Bador, J.; Amoureux, L.; Blanc, E.; Neuwirth, C. Innate aminoglycoside resistance of Achromobacter xylosoxidans is due to AxyXY-OprZ, an RND-type multidrug efflux pump. Antimicrob. Agents Chemother. 2013, 57, 603–605. [Google Scholar] [CrossRef]
- Bador, J.; Neuwirth, C.; Liszczynski, P.; Mézier, M.-C.; Chrétiennot, M.; Grenot, E.; Chapuis, A.; de Curraize, C.; Amoureux, L. Distribution of innate efflux-mediated aminoglycoside resistance among different Achromobacter species. New Microbes New Infect. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Doi, Y.; Poirel, L.; Paterson, D.L.; Nordmann, P. Characterization of a naturally occurring class D β-lactamase from Achromobacter xylosoxidans. Antimicrob. Agents Chemother. 2008, 52, 1952–1956. [Google Scholar] [CrossRef]
- Neuwirth, C.; Freby, C.; Ogier-Desserrey, A.; Perez-Martin, S.; Houzel, A.; Péchinot, A.; Duez, J.-M.; Huet, F.; Siebor, E. VEB-1 in Achromobacter xylosoxidans from cystic fibrosis patient, France. Emerg. Infect. Dis. 2006, 12, 1737–1739. [Google Scholar] [CrossRef] [PubMed]
- Traglia, G.M.; Almuzara, M.; Merkier, A.K.; Adams, C.; Galanternik, L.; Vay, C.; Centrón, D.; Ramírez, M.S. Achromobacter xylosoxidans: An emerging pathogen carrying different elements involved in horizontal genetic transfer. Curr. Microbiol. 2012, 65, 673–678. [Google Scholar] [CrossRef]
- Vali, P.; Shahcheraghi, F.; Seyfipour, M.; Zamani, M.A.; Allahyar, M.R.; Feizabadi, M.M. Phenotypic and genetic characterization of carbapenemase and ESBLs producing Gram-Negative Bacteria (GNB) isolated from patients with Cystic Fibrosis (CF) in Tehran hospitals. J. Clin. Diagn. Res. 2014, 8, 26–30. [Google Scholar] [CrossRef]
- Riccio, M.L.; Pallecchi, L.; Fontana, R.; Rossolini, G.M. In70 of plasmid PAX22, a bla(VIM-1)-containing integron carrying a new aminoglycoside phosphotransferase gene cassette. Antimicrob. Agents Chemother. 2001, 45, 1249–1253. [Google Scholar] [CrossRef]
- Shin, K.S.; Han, K.; Lee, J.; Hong, S.B.; Son, B.R.; Youn, S.J.; Kim, J.; Shin, H.S. Imipenem-resistant Achromobacter xylosoxidans carrying blaVIM-2-containing class 1 integron. Diagn. Microbiol. Infect. Dis. 2005, 53, 215–220. [Google Scholar] [CrossRef]
- Sofianou, D.; Markogiannakis, A.; Metzidie, E.; Pournaras, S.; Tsakris, A. VIM-2 metallo-β-lactamase in Achromobacter xylosoxidans in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 854–855. [Google Scholar] [CrossRef]
- El Salabi, A.; Borra, P.S.; Toleman, M.A.; Samuelsen, Ø.; Walsh, T.R. Genetic and biochemical characterization of a novel metallo-β-lactamase, TMB-1, from an Achromobacter xylosoxidans strain isolated in Tripoli, Libya. Antimicrob. Agents Chemother. 2012, 56, 2241–2245. [Google Scholar] [CrossRef]
- Lilić, B.; Filipić, B.; Malešević, M.; Novović, K.; Vasiljević, Z.; Kojić, M.; Jovčić, B. Fluoroquinolone-resistant Achromobacter xylosoxidans clinical isolates from Serbia: High prevalence of the aac-(6′)-Ib-cr gene among resistant isolates. Folia Microbiol. 2019, 64, 153–159. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Sanchez, D.G.; Gallo, I.F.L.; Stehling, E.G. Replicon typing of plasmids in environmental Achromobacter sp. producing quinolone-resistant determinants. APMIS 2018, 126, 864–869. [Google Scholar] [CrossRef]
- Chang, L.-L.; Chen, H.-F.; Chang, C.-Y.; Lee, T.-M.; Wu, W.-J. Contribution of integrons, and SmeABC and SmeDEF efflux pumps to multidrug resistance in clinical isolates of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2004, 53, 518–521. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.Z.; Poole, K. SmeDEF multidrug efflux pump contributes to intrinsic multidrug resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2001, 45, 3497–3503. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Liu, Y.; Wang, D.; Ni, W.; Wang, R.; Liu, Y.; Zhang, B. Frequency and genetic determinants of tigecycline resistance in clinically isolated Stenotrophomonas maltophilia in Beijing, China. Front. Microbiol. 2018, 9, 549. [Google Scholar] [CrossRef]
- Wu, C.-J.; Lu, H.-F.; Lin, Y.-T.; Zhang, M.-S.; Li, L.-H.; Yang, T.-C. Substantial contribution of SmeDEF, SmeVWX, SmQnr, and heat shock response to fluoroquinolone resistance in clinical isolates of Stenotrophomonas maltophilia. Front. Microbiol. 2019, 10, 822. [Google Scholar] [CrossRef]
- Li, L.-H.; Zhang, M.-S.; Wu, C.-J.; Lin, Y.-T.; Yang, T.-C. Overexpression of SmeGH contributes to the acquired MDR of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2019, 74, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Liou, R.-S.; Lin, Y.-T.; Huang, H.-H.; Yang, T.-C. A linkage between SmeIJK efflux pump, cell envelope integrity, and σE-mediated envelope stress response in Stenotrophomonas maltophilia. PLoS ONE 2014, 9, e111784. [Google Scholar] [CrossRef]
- Lin, C.-W.; Huang, Y.-W.; Hu, R.-M.; Yang, T.-C. SmeOP-TolCSm efflux pump contributes to the multidrug resistance of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2014, 58, 2405–2408. [Google Scholar] [CrossRef]
- García-León, G.; Ruiz de Alegría Puig, C.; García de la Fuente, C.; Martínez-Martínez, L.; Martínez, J.L.; Sánchez, M.B. High-level quinolone resistance is associated with the overexpression of SmeVWX in Stenotrophomonas maltophilia clinical isolates. Clin. Microbiol. Infect. 2015, 21, 464–467. [Google Scholar] [CrossRef]
- Blanco, P.; Corona, F.; Martínez, J.L. Biolog phenotype microarray is a tool for the identification of multidrug resistance efflux pump inducers. Antimicrob. Agents Chemother. 2018, 62, e01263-18. [Google Scholar] [CrossRef]
- Wu, C.-J.; Huang, Y.-W.; Lin, Y.-T.; Ning, H.-C.; Yang, T.-C. Inactivation of SmeSyRy two-component regulatory system inversely regulates the expression of SmeYZ and SmeDEF efflux pumps in Stenotrophomonas maltophilia. PLoS ONE 2016, 11, e0160943. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Huang, Y.-W.; Liou, R.-S.; Chang, Y.-C.; Yang, T.-C. MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J. Antimicrob. Chemother. 2014, 69, 3221–3226. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Hu, R.-M.; Chu, F.-Y.; Lin, H.-R.; Yang, T.-C. Characterization of a major facilitator superfamily (MFS) tripartite efflux pump EmrCABsm from Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2013, 68, 2498–2505. [Google Scholar] [CrossRef]
- Hu, R.-M.; Liao, S.-T.; Huang, C.-C.; Huang, Y.-W.; Yang, T.-C. An inducible fusaric acid tripartite efflux pump contributes to the fusaric acid resistance in Stenotrophomonas maltophilia. PLoS ONE 2012, 7, e51053. [Google Scholar] [CrossRef]
- Al-Hamad, A.; Upton, M.; Burnie, J. Molecular cloning and characterization of SmrA, a novel ABC multidrug efflux pump from Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2009, 64, 731–734. [Google Scholar] [CrossRef][Green Version]
- Okazaki, A.; Avison, M.B. Induction of L1 and L2 β-lactamase production in Stenotrophomonas maltophilia is dependent on an AmpR-type regulator. Antimicrob. Agents Chemother. 2008, 52, 1525–1528. [Google Scholar] [CrossRef]
- Crowder, M.W.; Walsh, T.R.; Banovic, L.; Pettit, M.; Spencer, J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1998, 42, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; MacGowan, A.P.; Bennett, P.M. Sequence analysis and enzyme kinetics of the L2 serine β-lactamase from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1997, 41, 1460–1464. [Google Scholar] [CrossRef]
- Avison, M.B.; von Heldreich, C.J.; Higgins, C.S.; Bennett, P.M.; Walsh, T.R. A TEM-2 β-lactamase encoded on an active Tn1-like transposon in the genome of a clinical isolate of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2000, 46, 879–884. [Google Scholar] [CrossRef]
- Al Naiemi, N.; Duim, B.; Bart, A. A CTX-M extended-spectrum β-lactamase in Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Med. Microbiol. 2006, 55, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.; Ploy, M.C.; Denis, F.; Courvalin, P. Characterization of the chromosomal aac(6′)-Iz gene of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1999, 43, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Zhang, L.; McKay, G.A.; Poole, K. Role of the acetyltransferase aac(6′)-Iz modifying enzyme in iminoglycoside resistance in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2003, 51, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, A.; Avison, M.B. Aph(3′)-IIc, an aminoglycoside resistance determinant from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2007, 51, 359–360. [Google Scholar] [CrossRef]
- Tada, T.; Miyoshi-Akiyama, T.; Dahal, R.K.; Mishra, S.K.; Shimada, K.; Ohara, H.; Kirikae, T.; Pokhrel, B.M. Identification of a novel 6′-N-aminoglycoside acetyltransferase, AAC(6′)-Iak, from a multidrug-resistant clinical isolate of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2014, 58, 6324–6327. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kikuchi, K.; Sasaki, T.; Takahashi, N.; Ohtsuka, M.; Ono, Y.; Hiramatsu, K. Smqnr, a new chromosome-carried quinolone resistance gene in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2008, 52, 3823–3825. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.B.; Martínez, J.L. SmQnr contributes to intrinsic resistance to quinolones in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2010, 54, 580–581. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tsai, M.-J.; Huang, Y.-W.; Chung, T.-C.; Yang, T.-C. SmQnrR, a DeoR-type transcriptional regulator, negatively regulates the expression of Smqnr and SmtcrA in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2011, 66, 1024–1028. [Google Scholar] [CrossRef]
- Abbott, I.J.; Peleg, A.Y. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: Antimicrobial resistance and therapeutic strategies. Semin. Respir. Crit. Care Med. 2015, 36, 99–110. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Lin, C.-Y.; Chen, Y.-H.; Hsueh, P.-R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 2015, 6, 893. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: Looking beyond contemporary antibiotic therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef]
- Amoureux, L.; Sauge, J.; Sarret, B.; Lhoumeau, M.; Bajard, A.; Tetu, J.; Bador, J.; Neuwirth, C.; MucoMicrobes Group. Study of 109 Achromobacter spp. isolates from 9 French CF centres reveals the circulation of a multiresistant clone of A. xylosoxidans belonging to ST137. J. Cyst. Fibros. 2019, 18, 804–807. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Ekkelenkamp, M.; Morosini, M.-I.; Merino, I.; de Dios Caballero, J.; Jones, M.; van Westreenen, M.; Tunney, M.M.; Cantón, R.; Fluit, A.C. Antimicrobial susceptibility of non-fermenting Gram-negative pathogens isolated from cystic fibrosis patients. Int. J. Antimicrob. Agents 2019, 53, 84–88. [Google Scholar] [CrossRef]
- Okoliegbe, I.N.; Hijazi, K.; Cooper, K.; Ironside, C.; Gould, I.M. Longitudinal surveillance and combination antimicrobial susceptibility testing of multidrug-resistant Achromobacter species from cystic fibrosis patients. Antimicrob. Agents Chemother. 2020, 64, e01467-20. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Kidd, T.J.; Stewart, A.G.; Harris, P.; Paterson, D.L. Achromobacter infections and treatment options. Antimicrob. Agents Chemother. 2020, 64, e01025-20. [Google Scholar] [CrossRef]
- Anderson, S.W.; Stapp, J.R.; Burns, J.L.; Qin, X. Characterization of small-colony-variant Stenotrophomonas maltophilia isolated from the sputum specimens of five patients with cystic fibrosis. J. Clin. Microbiol. 2007, 45, 529–535. [Google Scholar] [CrossRef]
- Magalhães, A.P.; Lopes, S.P.; Pereira, M.O. Insights into cystic fibrosis polymicrobial consortia: The role of species interactions in biofilm development, phenotype, and response to in-use antibiotics. Front. Microbiol. 2017, 7, 2146. [Google Scholar] [CrossRef]
- O’Brien, S.; Fothergill, J.L. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol. Lett. 2017, 364, fnx128. [Google Scholar] [CrossRef]
- Menetrey, Q.; Dupont, C.; Chiron, R.; Jumas-Bilak, E.; Marchandin, H. High occurrence of bacterial competition among clinically documented opportunistic pathogens including Achromobacter xylosoxidans in cystic fibrosis. Front. Microbiol. 2020, 11, 558160. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; De Nicola, S.; Verginelli, F.; Fiscarelli, E.; Di Bonaventura, G. Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 2015, 6, 951. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 2013, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Melloul, E.; Luiggi, S.; Anaïs, L.; Arné, P.; Costa, J.-M.; Fihman, V.; Briard, B.; Dannaoui, E.; Guillot, J.; Decousser, J.-W.; et al. Characteristics of Aspergillus fumigatus in association with Stenotrophomonas maltophilia in an in vitro model of mixed biofilm. PLoS ONE 2016, 11, e0166325. [Google Scholar] [CrossRef]
- Melloul, E.; Roisin, L.; Durieux, M.-F.; Woerther, P.-L.; Jenot, D.; Risco, V.; Guillot, J.; Dannaoui, E.; Decousser, J.-W.; Botterel, F. Interactions of Aspergillus fumigatus and Stenotrophomonas maltophilia in an in vitro mixed biofilm model: Does the strain matter? Front. Microbiol. 2018, 9, 2850. [Google Scholar] [CrossRef]
- Roisin, L.; Melloul, E.; Woerther, P.-L.; Royer, G.; Decousser, J.-W.; Guillot, J.; Dannaoui, E.; Botterel, F. Modulated response of Aspergillus fumigatus and Stenotrophomonas maltophilia to antimicrobial agents in polymicrobial biofilm. Front. Cell. Infect. Microbiol. 2020, 10, 574028. [Google Scholar] [CrossRef] [PubMed]
- Bernier, S.P.; Workentine, M.L.; Li, X.; Magarvey, N.A.; O’Toole, G.A.; Surette, M.G. Cyanide toxicity to Burkholderia cenocepacia is modulated by polymicrobial communities and environmental factors. Front. Microbiol. 2016, 7, 725. [Google Scholar] [CrossRef] [PubMed]
- de Rossi, B.P.; García, C.; Alcaraz, E.; Franco, M. Stenotrophomonas maltophilia interferes via the DSF-mediated quorum sensing system with Candida albicans filamentation and its planktonic and biofilm modes of growth. Rev. Argent. Microbiol. 2014, 46, 288–297. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-P.; Wong, A.C.L. A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia. Appl. Environ. Microbiol. 2007, 73, 5034–5040. [Google Scholar] [CrossRef]
- Huedo, P.; Yero, D.; Martínez-Servat, S.; Estibariz, I.; Planell, R.; Martínez, P.; Ruyra, A.; Roher, N.; Roca, I.; Vila, J.; et al. Two different Rpf clusters distributed among a population of Stenotrophomonas maltophilia clinical strains display differential diffusible signal factor production and virulence regulation. J. Bacteriol. 2014, 196, 2431–2442. [Google Scholar] [CrossRef]
- Yero, D.; Huedo, P.; Conchillo-Solé, O.; Martínez-Servat, S.; Mamat, U.; Coves, X.; Llanas, F.; Roca, I.; Vila, J.; Schaible, U.E.; et al. Genetic variants of the DSF quorum sensing system in Stenotrophomonas maltophilia influence virulence and resistance phenotypes among genotypically diverse clinical isolates. Front. Microbiol. 2020, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Huedo, P.; Kumar, V.P.; Horgan, C.; Yero, D.; Daura, X.; Gibert, I.; O’Sullivan, T.P. Sulfonamide-based diffusible signal factor analogs interfere with quorum sensing in Stenotrophomonas maltophilia and Burkholderia cepacia. Future Med. Chem. 2019, 11, 1565–1582. [Google Scholar] [CrossRef]
- Alcaraz, E.; García, C.; Friedman, L.; de Rossi, B.P. The Rpf/DSF signalling system of Stenotrophomonas maltophilia positively regulates biofilm formation, production of virulence-associated factors and β-Lactamase induction. FEMS Microbiol. Lett. 2019, 366, fnz069. [Google Scholar] [CrossRef]
- Huedo, P.; Yero, D.; Martinez-Servat, S.; Ruyra, À.; Roher, N.; Daura, X.; Gibert, I. Decoding the genetic and functional diversity of the DSF quorum-sensing system in Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 761. [Google Scholar] [CrossRef]
- Ferrer-Navarro, M.; Planell, R.; Yero, D.; Mongiardini, E.; Torrent, G.; Huedo, P.; Martínez, P.; Roher, N.; Mackenzie, S.; Gibert, I.; et al. Abundance of the quorum-sensing factor Ax21 in four strains of Stenotrophomonas maltophilia correlates with mortality rate in a new zebrafish model of infection. PLoS ONE 2013, 8, e67207. [Google Scholar] [CrossRef]
- Han, S.-W.; Lee, S.-W.; Ronald, P.C. Secretion, modification, and regulation of AxCurr. Opin. Microbiol. 2011, 14, 62–67. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Q.; Tang, J.-L. The Ax21 Protein influences virulence and biofilm formation in Stenotrophomonas maltophilia. Arch. Microbiol. 2018, 200, 183–187. [Google Scholar] [CrossRef]
- Devos, S.; Van Oudenhove, L.; Stremersch, S.; Van Putte, W.; De Rycke, R.; Van Driessche, G.; Vitse, J.; Raemdonck, K.; Devreese, B. The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 298. [Google Scholar] [CrossRef]
- Martínez, P.; Huedo, P.; Martinez-Servat, S.; Planell, R.; Ferrer-Navarro, M.; Daura, X.; Yero, D.; Gibert, I. Stenotrophomonas maltophilia responds to exogenous AHL signals through the LuxR Solo SmoR (Smlt1839). Front. Cell. Infect. Microbiol. 2015, 5, 41. [Google Scholar] [CrossRef]
- Huedo, P.; Coves, X.; Daura, X.; Gibert, I.; Yero, D. Quorum sensing signaling and quenching in the multidrug-resistant pathogen Stenotrophomonas maltophilia. Front. Cell Infect. Microbiol. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, D.; Eom, Y.B. Anti-biofilm and anti-virulence efficacy of celastrol against Stenotrophomonas maltophilia. Int. J. Med. Sci. 2018, 15, 617–627. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Chiarelli, L.R.; Barbieri, G.; Buroni, S. Quorum sensing as antivirulence target in cystic fibrosis pathogens. Int. J. Mol. Sci. 2019, 20, 1838. [Google Scholar] [CrossRef] [PubMed]
| Bacteria/Types of Mechanisms | Resistance Mechanisms | Spectrum | Type of Resistance | Reference |
|---|---|---|---|---|
| A. xylosoxidans complex | ||||
| Efflux pumps | AxyABM | Cephalosporins (except cefuroxime and cefepime), aztreonam | Int | [108] |
| AxyXY-OprZ | Aminoglycosides, tetracyclines including tigecycline, fluoroquinolones, erythromycin, cefepime, carbapenems | Int | [109,110] | |
| AxyEF-OprN | Some fluoroquinolones, tetracyclines, carpabenems | Int? | [86] | |
| β-lactamases | OXA-114 | Piperacillin, ticarcillin, benzylpenicillin, cephalothin | Int | [111] |
| ESBL (CTX-M, VEB-1); AmpC (CMY-2, AmpC) | All β-lactams except carbapenems | Acq | [112,113,114] | |
| Plasmidic (IMP, VIM and KPC) and chromosomal carbapenemase (TMB-1) | All β-lactams except aztreonam (VIM-2 strains resistant to aztreonam) | Int and Acq | [115,116,117,118] | |
| Others | aac(6′)Ib-cr, qnrA, oqxA, oqxB | Fluoroquinolones, aminoglycosides | Acq | [113,119] |
| gyrA, parC | Fluoroquinolones | Acq | [120] | |
| S. maltophilia | ||||
| Efflux pumps | SmeABC | Aminoglycosides, β-lactams, fluoroquinolones | Acq | [121] |
| SmeDEF | Tetracycline, chloramphenicol, macrolides, fluoroquinolones, sulfamethoxazole, trimethoprim, trimethoprim/sulfamethoxazole, tigecycline | Int and Acq | [121,122,123,124] | |
| SmeGH | Fluoroquinolones, β-lactams, tetracycline, polymyxin B | Int and Acq | [71,125] | |
| SmeIJK | Aminoglycosides, tetracycline, minocycline, ciprofloxacin, levofloxacin | Int and Acq | [126] | |
| SmeOP | Nalidixic acid, doxycycline, aminoglycosides, macrolides | Int | [127] | |
| SmeVWZ | Quinolones, chloramphenicol, trimethoprim/sulfamethoxazole | Acq | [124,128,129] | |
| SmeYZ | Aminoglycosides, tetracycline, trimethoprim/sulfamethoxazole | Int and Acq | [103,129,130] | |
| MacABCsm | Aminoglycosides, macrolides, polymyxins | Int | [131] | |
| EmrCABsm | Nalidixic acid, erythromycin | Int | [132] | |
| FuaABC | Fusaric acid | Int | [133] | |
| SmrA | Fluoroquinolones, tetracycline | Int and Acq? | [123,134] | |
| β-lactamases | L1 Class B3 Zn2+ -dependent metallo-β-lactamase | β-lactams (except monobactams) | Int | [135,136] |
| L2 Class A clavulanic acid- susceptible cephalosporinase | β-lactams | Int | [135,137] | |
| TEM-2 penicillinase | Ampicillin, piperacillin | Int | [138] | |
| CTX-M-1 β-lactamase (ESBL) | β-lactams | Acq | [139] | |
| Others | aac(6′)-Iz, aph(3′)-IIc, aac(6′)-Iak | Aminoglycosides | Int | [140,141,142,143] |
| Smqnr | Quinolones | Int and Acq | [124,144,145,146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menetrey, Q.; Sorlin, P.; Jumas-Bilak, E.; Chiron, R.; Dupont, C.; Marchandin, H. Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung. Genes 2021, 12, 610. https://doi.org/10.3390/genes12050610
Menetrey Q, Sorlin P, Jumas-Bilak E, Chiron R, Dupont C, Marchandin H. Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung. Genes. 2021; 12(5):610. https://doi.org/10.3390/genes12050610
Chicago/Turabian StyleMenetrey, Quentin, Pauline Sorlin, Estelle Jumas-Bilak, Raphaël Chiron, Chloé Dupont, and Hélène Marchandin. 2021. "Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung" Genes 12, no. 5: 610. https://doi.org/10.3390/genes12050610
APA StyleMenetrey, Q., Sorlin, P., Jumas-Bilak, E., Chiron, R., Dupont, C., & Marchandin, H. (2021). Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung. Genes, 12(5), 610. https://doi.org/10.3390/genes12050610






